Abstract
The Internet is a potentially important medium for communication about public health programs including newborn screening. This study explores whether the information available on official newborn screening program websites is consistent with existing guidelines regarding educational content for parents. We conducted a systematic search of the public websites of newborn screening programs in the US and Canada, identifying web pages and downloadable brochures that contained educational information. Two researchers independently reviewed all documents to determine the extent to which they included 14 key recommended educational messages. We identified 85 documents containing educational information on 46 US and 6 Canadian newborn screening program websites. The documents contained from 1 to 14 of the recommended messages. The majority of identified materials emphasized the importance and benefits of screening. The differences between US and Canadian materials were related to the importance of parental involvement in follow-up and issues of consent and storage of blood spots. Our findings are consistent with studies of non-web-based newborn screening education materials. The results emphasize the need for further evaluation of newborn screening education, including internet-based resources, particularly in terms of the impact of particular messages on parental attitudes and behaviors.
Keywords: Newborn screening, Education, Internet
Introduction
The internet is widely used by consumers to obtain health-related information (Bernhardt et al. 2002; Cline and Haynes 2001; Cotten and Gupta 2004; Hesse et al. 2005), including information about parenting, pregnancy, and newborn care (Bernhardt and Felter 2004). From a consumer perspective, the appeal of online information may relate to factors such as anonymity, availability, immediacy, and convenience, particularly in response to perceptions of limited opportunities for direct contact with healthcare providers (Bernhardt et al. 2002; Cotten and Gupta 2004; Zhao 2009). While this trend has provoked legitimate concern over the potential for widespread misinformation communicated from online sources (Bernhardt et al. 2002), the internet as a medium of communication also provides an important opportunity for health education, particularly in relation to public health topics, which typically do not require personalized messaging (Brodie et al. 2000; Tian et al. 2009).
Newborn blood spot screening (NBS) is a population-level public health intervention that aims to identify newborns who are at a relatively higher risk for certain rare diseases where early diagnosis and treatment is likely to have clinical benefits for those affected. NBS in many jurisdictions, including most US states and many Canadian provinces, has expanded rapidly in the past decade to include numerous additional conditions, particularly metabolic diseases (Therrell and Adams 2007). Currently, NBS protocols and practices vary considerably across and within nations and while several countries have developed or are developing national guidelines (Watson et al. 2006), there is no international standard. The rapid pace of expansion of NBS programs coupled with variability across programs has created challenges for education about NBS to parents and prospective parents whose infants form the screened population.
The need for high-quality communication with parents about newborn screening is well recognized (Arnold et al. 2006; Clayton 2005; Davis et al. 2006; Stewart et al. 2005; Tluczek et al. 2009). To date, a few sets of guidelines or standard recommendations have provided advice about the content of NBS educational materials designed for parents (Davis et al. 2006; American Academy of Pediatrics 2000; UK Newborn Screening Programme Centre 2005). Here, we focus on two recently published North American guidelines (where our study was based), both of which were developed in the US. The more recent set of guidelines developed content recommendations based on focus groups and interviews with parents, healthcare providers, and state NBS program professionals, as well as a review of the literature and existing brochures (Davis et al. 2006; Table 1). These guidelines have been supported by the Health Resources and Services Administration and made widely available to US state NBS programs. An earlier set of guidelines was developed by a multidisciplinary Taskforce on Newborn Screening led by the American Academy of Pediatrics (AAP 2000), based on views of task force members and results of their consultation processes (Table 1).
Table 1.
Key messages recommended in US guidelines
| Purpose: NBS detects health problems that would not or might not be apparent without testinga |
| Benefit: NBS may prevent serious health problemsa,b |
| Protocol: how testing is donea |
| Results: how you will receive resultsa,b |
| You may ask your healthcare provider for the resultsa |
| Your child may require retesting/follow-up testinga,b |
| The purpose of retesting/follow-up testinga,b |
| The importance of responding quickly to a request for follow-up testinga,b |
| How to contact the NBS programa |
| The risk of receiving a false-positive resultb |
| The risk of receiving a false-negative resultb |
| The risk of pain/infection from the actual heel prickb |
| A list of the conditions screenedb |
| Information about policies and practices related to the storage and use of bloodspot samplesb |
There has been little empirical research regarding the relevance or uptake of the advice provided by either of these guidelines. Reasoning that parents and prospective parents would likely turn to the internet for information about NBS and, specifically, seek out information provided by NBS programs, the purpose of this study was to explore to what extent information available on public NBS program websites included key recommended messages as outlined in these North American guidelines.
Methods
Collection of educational materials
We conducted a systematic search of the public websites of NBS programs in the US and Canada in November 2008; NBS is governed at the state level in the US and at the provincial level in Canada (although some smaller provinces have joint programs or have contracts with the programs of larger jurisdictions). NBS program websites were identified by one researcher (MA) using the Google search engine and the National Newborn Screening and Genetics Resource Center (NNSGRC) website. A comprehensive list of websites was compiled. Each state and provincial website was visited by one researcher (MA), who printed all educational materials that were available directly on the website or as downloadable brochures. We limited our study to English language educational materials about NBS screening that were identified on the program websites. Materials that addressed only newborn hearing screening were excluded from the study.
Content abstraction
A standardized data collection instrument for content evaluation was developed and pilot tested by both authors (MA, BP). A coding system was used to categorize materials according to the type of document and the presence or absence of a set of 14 key messages (Table 1). With respect to the “type” of document, NBS materials were placed in one of four categories by one author (MA):
Type A—Educational materials directly accessible on the website that were clearly intended for parents;
Type B—Educational materials directly accessible on the website where the intended audience was unclear;
Type C—Downloadable educational brochures that were clearly intended for parents; and
Type D—Downloadable educational brochures where the intended audience was unclear.
Materials were considered to be clearly intended for parents if they made statements such as “Your baby will be tested two times” or “A few drops of blood will be taken from your baby’s heel”.
With respect to key messages, we focused on 14 messages identified in the two existing US guidelines for NBS education (Davis et al. 2006; AAP 2000; Table 1). To our knowledge, there are no existing guidelines for NBS educational content in Canada, and we therefore used the above US guidelines to assess all North American NBS educational materials. In addition to these 14 key recommended messages, we recorded whether educational materials included descriptions of the screened conditions (i.e., beyond simply listing them), whether they provided information on policies around consent or refusal for NBS, and whether they provided external website addresses or external website links for parents who wished to seek further information.
The presence or absence of the 14 key messages and the three additional pieces of information was determined by two reviewers (MA, BP) working independently for each document. Inter-rater agreement was evaluated using kappa for each key message. Disagreements were resolved by discussion. The data were then summarized across all documents overall, by country, and restricted to materials clearly intended for parents. Data were analyzed using SAS (version 9.1).
Results
We identified a public website for 46 US state NBS programs and 6 Canadian provincial programs. In total, from these 52 websites, we identified 85 documents containing educational materials (76 documents from US websites and 9 documents from Canadian websites). Of these, 37 documents were “type A” (educational materials directly accessible on the website and clearly intended for parents), 11 were “type B” (educational materials directly accessible on the website where the intended audience was unclear), and 37 were “type C” (downloadable educational brochures that were clearly intended for parents). No documents were “type D” (downloadable educational brochure where the intended audience was unclear).
The inter-rater agreement between the two reviewers for coding key messages ranged from k = 0.73–1.00. All kappa values were above 0.80 with the exception of “The risk of receiving a false-positive result” (k = 0.73).
Key messages—all programs
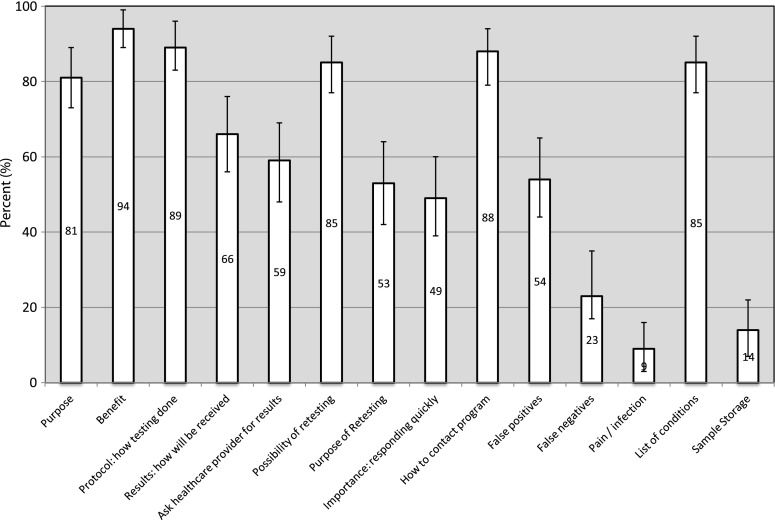
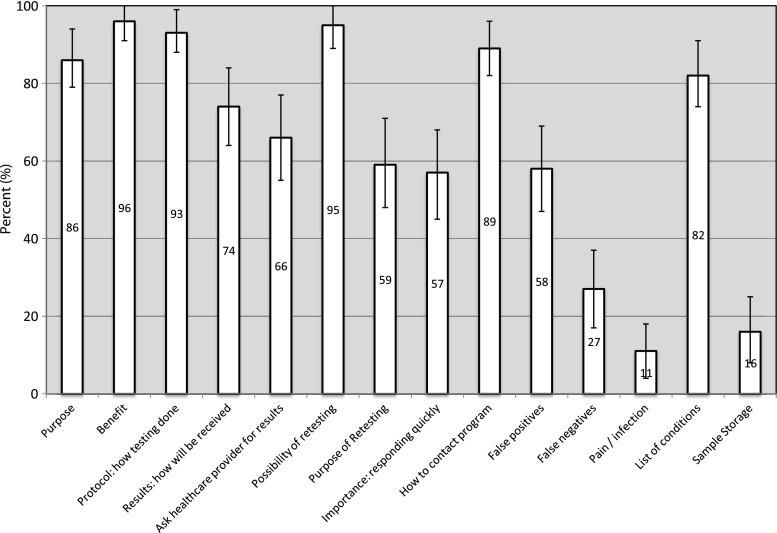
Across all documents, materials included from 1 to 14 of the 14 key recommended messages (only two documents included all 14), with a mean of 8.5 messages (SD = 2.77). A large majority of documents mentioned the purpose and benefits of NBS (81% and 94%, respectively), provided some description of the screening protocol (89%), identified the possibility of a need for retesting (85%), provided contact information for the program (88%), and listed the screened conditions (85%; Fig. 1). About two thirds of documents also identified how results would be communicated (66%) and 59% recommended or offered the option of asking a healthcare provider for the results. A smaller proportion of documents mentioned the risk of receiving a false-positive (54%) or false-negative (23%) result, identified a risk of pain and/or infection from the procedure (9%), described the purpose of and importance of responding to the need for further testing (53% and 49%, respectively), or identified how the blood spots would be stored and/or used for other purposes (14%; Fig. 1). In terms of the additional three messages or pieces of information that we recorded, a majority of documents stated whether consent was required and/or whether refusal was allowed (71%), described (rather than simply listing) the conditions screened (62%), and provided external website addresses or links for further information (53%). Similar results to the above were obtained when the analysis was restricted to materials specifically intended for parents (Fig. 2).
Fig. 1.
The proportion of all NBS educational materials that included key messages (N = 85)
Fig. 2.
The proportion of all NBS educational materials for parents that included key messages (N = 74)
Key messages—US and Canada separately
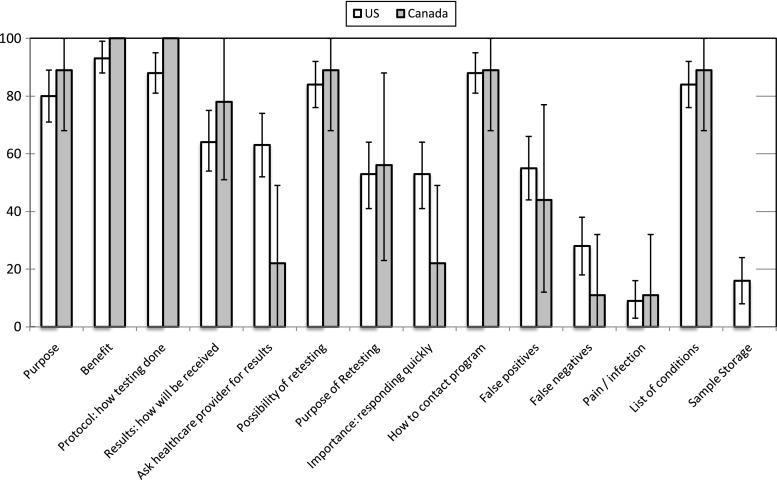
Over 80% of all educational materials in Canada and the US included information on the purpose/benefits of screening, testing protocol, possibility that retesting would be needed, how to contact the NBS program, and the conditions screened (Fig. 3). Similarly, fewer than 60% of documents from both countries included information about the risks associated with screening (false positives, false negatives, and pain/infection), the purpose of follow-up testing, and the importance of responding quickly to a request for further testing. However, while 16% of US documents included information about policies on the storage and secondary use of blood spot samples, this information was entirely absent from Canadian materials. A higher proportion of US documents (53%) compared with Canadian documents (22%) also emphasized the importance of responding quickly in the event that follow-up testing was required, and US documents were also more likely (63% vs. 22%) to recommend or offer the option of asking a healthcare provider for screening results. Finally, information on consent/refusal was present in a much greater proportion of US documents (78%) relative to Canadian documents (11%; not shown in figure).
Fig. 3.
The proportion of all NBS educational materials that included key messages by country (N = 76 US documents; N = 9 Canadian documents)
Discussion
Most North American state and provincial newborn screening programs provided a public website that was easily identifiable. A large proportion of the educational documents obtained from these websites (both directly on the web or as a downloadable brochure) were targeted specifically for parents (74 of 85 documents). Most of the materials we identified did not include all of the elements recommended in NBS education guidelines, and less than half (44%) of the documents included at least 10 of the 14 recommended messages. Our findings are thus in concordance with those of Fant and colleagues (2005), who found that while 98% (46 out of 47) of US state NBS programs reported providing standardized materials for parents, none of the program materials they obtained (not necessarily web-based) contained more than five of the seven elements recommended by AAP (2000). The more recent US guidelines were developed through an evidence-informed process (a review of existing brochures, a review of existing literature, and focus groups with parents and professionals; Davis et al. 2006), and as such, it may be useful to compare our findings to these guidelines more specifically. While many of the recommended messages from the model brochure and guidelines for health professionals were widely included, the following messages were mentioned in less than two thirds of the documents we reviewed: advice or offer to ask a healthcare provider for the newborn screening result; the possible reasons for a need for retesting or follow-up testing; and the need to respond quickly to a request for retesting or follow-up testing.
Although both sets of guidelines used in our study were developed by experts in the field and those developed by Davis et al. (2006) were explicitly informed by available evidence, the relative importance of different messages about NBS in parental educational materials remains uncertain. Proposed benefits of effective prescreening education about NBS include the potential for minimizing negative psychosocial outcomes as a result of receiving either a true- or false-positive result (Gurian et al. 2006; Hewlett and Waisbren 2006) and the potential to improve follow-up rates for children identified as having a positive screening result by providing parents the opportunity to understand the purpose and importance of screening (Kemper et al. 2005). This suggests that at a minimum, prescreening education should address the purpose of newborn screening and explain the possible results and their meaning. But what other content is most important, and on what basis should this be determined?
One potentially important influence on the relative importance of educational messages may be the way in which a NBS program is offered to parents (Hargreaves et al. 2005). For example, in North America, NBS is typically either mandated (in most US states, although refusal is often allowed on religious grounds) or implemented on a “presumed consent” or opt-out basis (in most Canadian provinces; Hanley 2005; Therrell and Adams 2007; Therrell et al. 2006). This is in contrast with the situation in the UK, for example, which has moved toward a policy of offering all screening programs, including newborn screening, on the basis of informed choice (UK Newborn Screening Programme Centre 2005). In communication with the public about screening programs, there is a recognized tension between the goals of encouraging high uptake (through provision of information that emphasizes benefits) and facilitating informed decision-making (through provision of information that covers potential harms as well as benefits; Raffle 2001). We found that North American NBS program websites tended to focus on the benefits of screening (mentioned in 94% of documents) and were less likely to highlight the potential harms (e.g., 54% mentioned the possibility of false-positive results, and 23% mentioned the possibility of false-negative results). This concurs with an earlier international review of written educational materials for parents regarding newborn screening, which concluded that such materials supported high uptake rather than informed choice (Hargreaves et al. 2005) and it may reflect the way in which newborn screening programs are delivered in the US and Canada (parents are not expected to explicitly make a choice). There is a need for continued debate about issues of choice and consent in the field of NBS and their implications for education, particularly in light of the continuing expansion of screening panels to include diseases for which the evidence supporting the benefits of screening is relatively less certain (Clayton 2005; Grosse et al. 2006; Moyer et al. 2008).
A potential explanation for the emphasis on benefits rather than potential harms in the online educational materials we reviewed is that NBS programs may be concerned about the possibility of decreasing screening uptake by identifying the potential drawbacks of NBS. As such, a useful direction for further research would be to explore empirically how educational messages influence parental attitudes and their behaviors with respect to screening. It is also possible that the lack of emphasis on the possibility of false-positive and false-negative results reflects their absence as recommended educational messages in the most recent US guidelines (Davis et al. 2006), suggesting that these messages were not prioritized by parents and professionals in the focus group research used to inform the guidelines. Future research could further investigate the relative importance of different messages from the perspectives of broad groups of both parents and professionals and, in particular, whether the potential harms of screening are considered to be an essential part of educational content. For example, including information about the risk of pain or infection from the heel prick was recommended in the earlier US guidelines (AAP 2000). From a professional perspective, it is unlikely that this would be viewed as a significant risk associated with NBS; it would be useful to know whether parents believe this information is important for prescreening education and whether it impacts their views toward screening.
Although it was not part of either US guideline, we investigated whether the documents we reviewed contained descriptions of the diseases included in NBS program panels. Including information about the diseases was recommended by some parents in a recent qualitative study on NBS education (Tluczek et al. 2009). Some participants also noted that a brochure from the NBS program was the first information source they turned to upon receiving a positive NBS result, strengthening the argument for including some discussion about the diseases screened. However, there is a risk that parents may become overwhelmed by detailed information about the diseases or that such information could be anxiety-provoking (Nijsingh 2007); given that the screened diseases are extremely rare, it may be hard to justify exposing all parents in the screened population to their descriptions. Although we focused only on educational information and downloadable brochures from NBS program websites, in practice, programs may resolve the differing information needs of different parents by relying on a layered approach to education, providing basic information to all parents and then directing those who want or need additional details to other sources. We found that just over half (53%) of the documents we reviewed included external website addresses or links for those seeking more information.
Educational materials on US and Canadian NBS program websites generally included similar messages. Exceptions were descriptions of policies with respect to consent or right of refusal and related to the storage and secondary use of blood spots, which were more likely to be included on US websites. NBS programs in the US appear more likely than their Canadian counterparts to have legislation or state/provincial policies in place with respect to these issues (Therrell and Adams 2007; Therrell et al. 2006; Hanley 2005), which would help to explain these differences. The storage and potential secondary uses (particularly research uses) of dried blood spots from NBS programs have been the subject of recent debate internationally (Couzin-Frankel 2009; Tinidad et al. 2011), and there is considerable variation in such policies across programs within both Canada and the US (Avard et al. 2006; Tinidad et al. 2011). We found that only 16 of US documents and no Canadian documents included information about storage and secondary use of dried blood spots, but we suspect that in light of recent attention to the issue, this is likely changing rapidly.
Limitations
An important limitation of this study is that it provides only a snapshot in time of the status of online NBS educational materials, when such materials are evolving in response to the expansion of programs, the increasing attention being paid to harmonization of guidelines and protocols, and the increasing attention to issues such as the storage and secondary use of blood spots, as mentioned. Although we evaluated all educational materials available online from NBS programs in the US and Canada, we focused only on written materials available either directly on public websites or as downloadable brochures. Messages lacking in these materials may be included in other sources of NBS information provided directly to parents, for example, in discussions with health professionals. We also focused mainly on messages recommended in recent North American guidelines, excluding some additional messages that may be interesting to explore (e.g., related to the possibility of identifying heterozygous mutation carriers for conditions such as cystic fibrosis and sickle cell disease). Finally, we were concerned only with the content of educational materials about newborn screening; previous research has demonstrated a need for improvement in readability and user friendliness of such materials, another important influence on the effectiveness of communication (Arnold et al. 2006).
Conclusion
There is general agreement that prescreening parental education is an important part of NBS programs. While there are guidelines for the content of NBS educational materials, our findings concur with others that such guidelines are often not followed in practice (Fant et al. 2005; Hargreaves et al. 2005). We found that most educational documents portrayed screening in a positive light, describing the purpose and benefits of screening. Fewer documents described the potential risks of screening. NBS is recognized as an important public health program that delivers benefits to infants affected by the screened diseases. However, the lack of emphasis on potential harms may represent a missed opportunity in terms of fully realizing the proposed benefits of effective parental education about NBS, particularly the opportunity to prepare parents for the possibility of receiving a false-positive result. Further research is needed to evaluate how parents and prospective parents respond to particular messages about NBS, both in terms of attitudes and behaviors. There is also a need for further reflection about the relative importance of different messages about NBS that may be included in parental educational and about the interaction between communication with families and policies related to consent and choice. To maximize effectiveness, communication strategies must be sensitive to ways in which parents currently seek health information, emphasizing the need for NBS programs to consider online resources when developing or refining educational initiatives.
Acknowledgments
Conflicts of Interest
None.
References
- American Academy of Pediatrics (AAP), Newborn Screening Task Force Serving the family from birth to the medical home. Newborn screening: a blueprint for the future. A call for a national agenda on state newborn screening programs. Pediatrics. 2000;106:389–427. [PubMed] [Google Scholar]
- Arnold CL, Davis TC, Frempong JO, Humiston SG, Bocchini A, Kennen EM, Lloyd-Puryear M. Assessment of newborn screening parent education materials. Pediatrics. 2006;117:S320–S325. doi: 10.1542/peds.2005-1934. [DOI] [PubMed] [Google Scholar]
- Avard D, Vallance H, Greenberg C, Laberge C, Kharaboyan L, Plant M. Variability in the storage and use of newborn dried bloodspots in Canada: is it time for national standards? Genomics Soc Policy. 2006;2:80–95. [Google Scholar]
- Bernhardt JM, Felter EM. Online pediatric information seeking among mothers of young children: results from a qualitative study using focus groups. J Med Internet Res. 2004;6:e7. doi: 10.2196/jmir.6.1.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt JM, Lariscy RA, Parrott RL, Silk KJ, Felter EM. Perceived barriers to internet-based health communication on human genetics. J Health Commun. 2002;7:325–340. doi: 10.1080/10810730290088166. [DOI] [PubMed] [Google Scholar]
- Brodie M, Flournoy RE, Altman DE, Blendon RJ, Benson JM, Rosenbaum MD. Health information, the internet, and the digital divide. Health Aff (Millwood, Va) 2000;19:255–265. doi: 10.1377/hlthaff.19.6.255. [DOI] [PubMed] [Google Scholar]
- Clayton EW. Talking with parents before newborn screening. J Pediatr. 2005;147(3 Suppl):S26–S29. doi: 10.1016/j.jpeds.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Cline RJ, Haynes KM. Consumer health information seeking on the internet: the state of the art. Health Educ Res. 2001;16:671–692. doi: 10.1093/her/16.6.671. [DOI] [PubMed] [Google Scholar]
- Cotten SR, Gupta SS. Characteristics of online and offline health information seekers and factors that discriminate between them. Soc Sci Med. 2004;59:1795–1806. doi: 10.1016/j.socscimed.2004.02.020. [DOI] [PubMed] [Google Scholar]
- Couzin-Frankel J. Science gold mine, ethical minefield. Science. 2009;324:166–168. doi: 10.1126/science.324.5924.166. [DOI] [PubMed] [Google Scholar]
- Davis TC, Humiston SG, Arnold CL, Bocchini JA, Jr, Bass PF, III, Kennen EM, Bocchini A, Kyler P, Lloyd-Puryear M. Recommendations for effective newborn-screening communication: results of focus groups with parents, providers, and experts. Pediatrics. 2006;117:S326–S340. doi: 10.1542/peds.2005-2633M. [DOI] [PubMed] [Google Scholar]
- Fant KE, Clark SJ, Kemper AR. Completeness and complexity of information available to parents from newborn screening programs. Pediatrics. 2005;115:1268–1272. doi: 10.1542/peds.2004-0834. [DOI] [PubMed] [Google Scholar]
- Grosse SD, Boyle CA, Kenneson A, Khoury MJ, Wilfond BS. From public health emergency to public health service: the implications of evolving criteria for newborn screening panels. Pediatrics. 2006;117:923–929. doi: 10.1542/peds.2005-0553. [DOI] [PubMed] [Google Scholar]
- Gurian EA, Kinnamon DD, Henry JJ, Waisbren SE. Expanded newborn screening for biochemical disorders: the effect of a false-positive result. Pediatrics. 2006;117:1915–1921. doi: 10.1542/peds.2005-2294. [DOI] [PubMed] [Google Scholar]
- Hanley WB. Newborn screening in Canada—are we out of step? Paediatr Child Health. 2005;10:203–207. doi: 10.1093/pch/10.4.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves K, Stewart R, Oliver S. Newborn screening information supports public health more than informed choice. Health Educ J. 2005;64:110–119. doi: 10.1177/001789690506400203. [DOI] [Google Scholar]
- Hesse BW, Nelson DE, Kreps GL, Croyle RT, Arora NK, Rimer BK, Viswanath K. Trust and sources of health information: the impact of the internet and its implications for health care providers: findings from the first Health Information National Trends Survey. Arch Intern Med. 2005;165:2618–2624. doi: 10.1001/archinte.165.22.2618. [DOI] [PubMed] [Google Scholar]
- Hewlett J, Waisbren SE. A review of the psychosocial effects of false-positive results on parents and current communication practices in newborn screening. J Inherit Metab Dis. 2006;29:677–682. doi: 10.1007/s10545-006-0381-1. [DOI] [PubMed] [Google Scholar]
- Kemper AR, Fant KE, Clark SJ. Informing parents about newborn screening. Public Health Nurs. 2005;22:332–338. doi: 10.1111/j.0737-1209.2005.220408.x. [DOI] [PubMed] [Google Scholar]
- Moyer VA, Calonge N, Teutsch SM, Botkin JR. Expanding newborn screening: process, policy, and priorities. Hastings Cent Rep. 2008;38:32–39. doi: 10.1353/hcr.0.0011. [DOI] [PubMed] [Google Scholar]
- Nijsingh N. Informed consent and the expansion of newborn screening. In: Dawson A, Verweij M, editors. Ethics, prevention, and public health. UK: Oxford University Press; 2007. pp. 198–212. [Google Scholar]
- Raffle AE. Information about screening—is it to achieve high uptake or to ensure informed choice? Health Expect. 2001;4:92–98. doi: 10.1046/j.1369-6513.2001.00138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart R, Hargreaves K, Oliver S. Evidence informed policy making for health communication. Health Educ J. 2005;64:120–128. doi: 10.1177/001789690506400204. [DOI] [Google Scholar]
- Therrell BL, Adams J. Newborn screening in North America. J Inherit Metab Dis. 2007;30:447–465. doi: 10.1007/s10545-007-0690-z. [DOI] [PubMed] [Google Scholar]
- Therrell BL, Johnson A, Williams D. Status of newborn screening programs in the United States. Pediatrics. 2006;117:S212–S252. doi: 10.1542/peds.2005-1432. [DOI] [PubMed] [Google Scholar]
- Tian H, Brimmer DJ, Lin JM, Tumpey AJ, Reeves WC. Web usage data as a means of evaluating public health messaging and outreach. J Med Internet Res. 2009;11:e52. doi: 10.2196/jmir.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinidad SB, Fullerton SM, Ludman EJ, Jarvik GP, Larson EB, Burke W. Research practice and participant preferences: the growing gulf. Science. 2011;331:287–288. doi: 10.1126/science.1199000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tluczek A, Orland KM, Nick SW, Brown RL. Newborn screening: an appeal for improved parent education. J Perinat Neonatal Nurs. 2009;23:326–334. doi: 10.1097/JPN.0b013e3181a1bc1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UK Newborn Screening Programme Centre (2005) Newborn blood spot screening in the UK. Policies and standards
- Watson MS, Lloyd-Puryear MA, Mann MY, Rinaldo P, Howell RR (eds) (2006) Newborn screening: toward a uniform screening panel and system. Genet Med 8(Suppl 1):1S–252S [DOI] [PMC free article] [PubMed]
- Zhao S. Parental education and children’s online health information seeking: beyond the digital divide debate. Soc Sci Med. 2009;69:1501–1505. doi: 10.1016/j.socscimed.2009.08.039. [DOI] [PubMed] [Google Scholar]