Abstract

To evaluate previously proposed functions of renal caveolar Na+/K+-ATPase, we modified the standard procedures for the preparation of the purified membrane-bound kidney enzyme, separated the caveolar and noncaveolar pools, and compared their properties. While the subunits of Na+/K+-ATPase (α,β,γ) constituted most of the protein content of the noncaveolar pool, the caveolar pool also contained caveolins and major caveolar proteins annexin-2 tetramer and E-cadherin. Ouabain-sensitive Na+/K+-ATPase activities of the two pools had similar properties and equal molar activities, indicating that the caveolar enzyme retains its ion transport function and does not contain nonpumping enzyme. As minor constituents, both caveolar and noncaveolar pools also contained Src, EGFR, PI3K, and several other proteins known to be involved in stimulous-induced signaling by Na+/K+-ATPase, indicating that signaling function is not limited to the caveolar pool. Endogenous Src was active in both pools but was not further activated by ouabain, calling into question direct interaction of Src with native Na+/K+-ATPase. Chemical cross-linking, co-immunoprecipitation, and immunodetection studies showed that in the caveolar pool, caveolin-1 oligomers, annexin-2 tetramers, and oligomers of the α,β,γ-protomers of Na+/K+-ATPase form a large multiprotein complex. In conjunction with known roles of E-cadherin and the β-subunit of Na+/K+-ATPase in cell adhesion and noted intercellular β,β-contacts within the structure of Na+/K+-ATPase, our findings suggest that interacting caveolar Na+/K+-ATPases located at renal adherens junctions maintain contact of two adjacent cells, conduct essential ion pumping, and are capable of locus-specific signaling in junctional cells.
Na+/K+-ATPase is the energy-transducing enzyme that maintains the normal physiological gradients of Na+ and K+ across the plasma membrane of most higher eukaryotic cells.1,2 Two subunits of the enzyme (α and β) are essential for catalytic and transport functions, and some of the preparations contain other subunits (FXYD proteins; e.g., the γ-subunit of the kidney enzyme) that regulate function.(2) Na+/K+-ATPase is also a signal transducer; i.e., in response to some stimuli (drugs, hormones, and putative hormones), it interacts with neighboring membrane proteins to activate multiple growth-related signal transduction pathways, leading to a host of cell-specific downstream effects.3−5 More recently, we showed that in several different cell types, a significant fraction of Na+/K+-ATPase resides in the caveolar microdomains of the plasma membrane, and we suggested that this pool of the enzyme may be responsible for its signal transducing function because of its proximity to its signaling partners that are colocalized in caveolae.(6) Although our subsequent studies in cardiac myocytes and smooth muscle cells confirmed the presence of Na+/K+-ATPase in caveolar microdomains, they also indicated diverse and cell-specific signaling events linked to the enzyme and even suggested that signaling by Na+/K+-ATPase may not be limited to the caveolar pool.7,8 On the other hand, a number of other studies of the signaling function of Na+/K+-ATPase of renal epithelial cells9−12 have suggested that the enzyme-linked signaling in these cells occurs through a pool of caveolar enzyme that does not pump even in the absence of a stimulus, and that ouabain-induced signaling is solely due to a specific interaction among the α-subunit of Na+/K+-ATPase, Src, and caveolin-1. Attempting to clarify these apparent discrepancies with respect to the role of the caveolar enzyme in the signaling and the pumping functions of Na+/K+-ATPase, we deemed it necessary to separate the caveolar and the noncaveolar pools of the renal enzyme for comparative structure–function studies.
The commonly used biochemical procedures for the separation of caveolar and noncaveolar membranes6,7 that are applicable to only small quantities of cultured cells or tissue samples yield such limited samples of these membranes that are not suitable for further purification or extensive studies. Therefore, we used the ample prior experience in the field on the purification of the membrane-bound Na+/K+-ATPase from kidney(13) to develop procedures for the separation and large-scale preparations of the caveolar and noncaveolar pools of the kidney enzyme. These procedures and the initial studies on the comparative properties of the two pools are presented here. The findings not only resolve some of the uncertainties mentioned above but also provide previously unavailable information about (a) the characteristics of the enzymic activities of the caveolar and noncaveolar pools of kidney Na+/K+-ATPase, (b) the nature and relative quantities of the signaling proteins that accompany the subunits of Na+/K+-ATPase in the caveolar and noncaveolar pools, (c) the established and potential sites of interaction among Na+/K+-ATPase oligomers, caveolin oligomers, and the other two major caveolar proteins, E-cadherin and annexin-2 tetramer, and (d) how the interactions between the caveolar Na+/K+-ATPases of two adjacent renal epithelial cells occur at adherens junctions.
Experimental Procedures
Preparation of the Caveolar and Noncaveolar Pools of the Enzyme
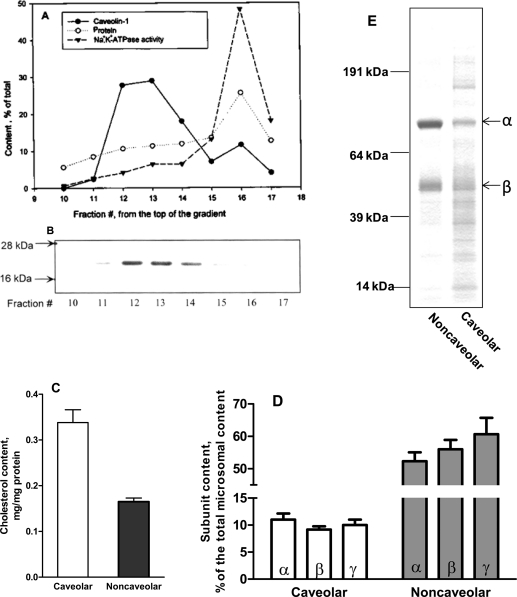
Microsomes from pig kidney outer medulla were prepared as described previously13−15 and suspended (1.4 mg of protein/mL) in a solution containing 3 mM ATP, 2 mM EDTA, and 50 mM imidazole (pH 7.5). A concentrated solution of SDS was added to the suspension dropwise with constant stirring over 30 min, at 24 °C, yielding a final SDS concentration of 0.056%. Sucrose was then added to this microsomal suspension to a final concentration of 12%. Discontinuous glycerol gradients, each containing 25 mM imidazole and 1 mM EDTA (pH 7.5), were set up as follows, from bottom to top, in a 100 mL centrifuge tube (Beckman model 345778): 11 mL of 64% glycerol and 26 mL of 44% glycerol. On top of these were overlaid 26 mL of the SDS-treated microsomes in sucrose, and then 32 mL of 25 mM imidazole and 1 mM EDTA (pH 7.5). The tubes were centrifuged in a Beckman Ti-45 rotor at 45000 rpm and 4 °C for 165 min. To obtain the two clearly separated opaque bands, successive 5 mL fractions were collected from each tube. Each fraction was diluted 7-fold in 25 mM imidazole and 1 mM EDTA (pH 7.5) and centrifuged in a Beckman Ti-70 rotor at 55000 rpm and 4 °C for 1 h. The resulting membrane pellets were suspended in 0.25 M sucrose, 30 mM histidine, and 1 mM EDTA (pH 7.4) and used. As indicated in Results (Figure 1), the pellets from fractions 12 and 13 of each tube usually contained most of the lighter caveolar enzyme, and that of fraction 16 contained most of the heavier noncaveolar enzyme.
Figure 1.
Separation of caveolar and noncaveolar pools of the kidney Na+/K+-ATPase. Microsomal membranes were purified and subjected to density gradient fractionation as described in Experimental Procedures. (A) Distribution of caveolin-1, Na+/K+-ATPase activity, and protein in the fractions. The specific Na+/K+-ATPase activity of fraction 16 was 980 μmol of Pi mg–1 h–1. (B) Distribution of the immunoreactive caveolin-1. From each fraction, 1 μg of protein was subjected to SDS–PAGE and immunostaining. (C) Cholesterol contents of the caveolar (fractions 12 and 13) and noncaveolar (fraction 16) pools. Means ± SE (n = 4). (D) Relative distributions of the subunits of Na+/K+-ATPase (α,β,γ) in the caveolar and noncaveolar pools. The two pools and the microsomal preparation used for the fractionation were subjected to quantitative immunoassays for the subunits. No significant differences between the subunit contents were noted in either pool (n = 4). (E) Equal amounts of protein (5 μg) from each pool were subjected to SDS–PAGE, and the gels were stained.
Na+/K+-ATPase and Other Assays
The steady-state ATPase activity was assayed at 37 °C by measuring the initial rate of release of Pi from ATP in a medium containing optimal concentrations of the required ligands: 100 mM NaCl, 25 mM KCl, 2 mM ATP, 3 mM MgCl2, 1 mM EGTA, and 20 mM Tris-HCl (pH 7.4). Each assay was conducted in the presence and absence of 1 mM ouabain, and the ouabain-sensitive component was considered as the Na+/K+-ATPase activity. The K0.5 values and the Hill coefficient of the essential ligands were determined as indicated previously.(16) Pi was assayed with Malachite Green as described previously.(7) The maximal level of the phosphoenzyme intermediate of Na+/K+-ATPase was measured using [γ-32P]ATP as described previously,14−16 and the molar activity of the enzyme was calculated as indicated.(17) Cholesterol and protein assays were conducted as described previously.(7)
To assay for Src activity in the membrane preparation or purified Src, we incubated samples in a buffer containing 2 mM ATP, 125 mM MgCl2, 25 mM MnCl2, 2 mM EGTA, 250 μM sodium orthovanadate, 2 mM dithiothreitol, and 100 mM Tris-HCl (pH 7.2). After termination of the reaction by the addition of SDS and boiling (see the section below), samples were subjected to SDS–PAGE and immunoassayed for an increase in the level of Src pY418 or total tyrosine-phosphorylated Src with the appropriate antibodies.
Immunoblot and Immunoprecipitation
Samples were solubilized in a buffer containing 2% SDS and 5% β-mercaptoethanol, boiled for 5 min, and then subjected to SDS–PAGE and probed with appropriate antibodies for detection or for quantitative immunoblot analyses as described previously.6,7 In some experiments, boiling of samples prior to SDS–PAGE was avoided as specified. For immunoprecipitation experiments, membrane samples were first treated with a lysis buffer containing the detergents Triton X-100, octyl glucoside, and other components.(7) The detergent-solubilized proteins were then subjected to immunoprecipitation, SDS–PAGE, and immunodetection using appropriate antibodies and procedures as we described previously.(7)
Cross-Linking Experiments
Membrane samples were reacted with BS3 and DDS as described previously(14) and then subjected to SDS–PAGE and immunodetection with the indicated antibodies.
Materials
Pig kidneys were purchased from the slaughterhouse. ATP, ouabain, and routine chemicals of the highest available purity were obtained from Sigma (St. Louis, MO). [γ-32P]ATP was bought from Perkin-Elmer Life Sciences (Boston, MA). Antibodies against the indicated antigens were purchased from the following vendors: β1-subunit of Na+/K+-ATPase and EGFR from Upstate Biotechnology (Lake Placid, NY); ERK 1/2, Src (sc-8056), phosphotyrosine, PY99 (sc-7020), goat anti-rabbit IgG-bound horseradish peroxidase (HRP), and goat anti-mouse IgG-bound HRP from Santa Cruz Biotechnology (Santa Cruz, CA); caveolin-1 (610059), caveolin-2 (610684), annexin-2 p36 (610068), annexin-2 p11 (610070), and E-cadherin (610181) from BD Transduction Laboratories (Lexington, KY); Na+/K+-ATPase α1 (α6F) from Developmental Studies Hybridoma Bank, University of Iowa (Iowa City, IA); Akt and PI3K p85 from Cell Signaling Technology (Danvers, MA); and phospho-Src and occludin from Invitrogen (Carlsbad, CA).
The customer-designed antibody against the 14 C-terminal residues of the rat γ-subunit of Na+/K+-ATPase(14) was produced by Sigma-Genosys (The Woodlands, TX). BS3 and DSS were purchased from Thermo Fisher Scientific (Rockford, IL). Purified (>90%) Src (p60c-src) (14–117) was purchased from Millipore (Billerica, MA). Purified (>90%) human EGFR (BML-SE116) was purchased from Enzo Life Sciences International, Inc. (formerly BIOMOL International, L.P., Plymouth Meeting, PA).
Analysis of Data
Unless stated otherwise, data are means ± the standard error (SE) of the results of a minimum of three experiments. A Student’s t test was used, and a p < 0.05 significance was accepted.
Results
Identification and Separation of the Caveolar and Noncaveolar Pools of the Enzyme
Most of the Na+/K+-ATPase content of the kidney outer medulla (∼70%) is found in the crude microsomal membranes prepared from this tissue homogenate,(13) and such microsomes of various mammalian kidneys have been standard starting materials for the well-established purification procedures of this enzyme. We used one such procedure that we and others had used before,14,15 but in the final step involving density gradient centrifugation of membrane fragments, we looked not only for fractions enriched with Na+/K+-ATPase but also for those enriched with caveolin-1. The results shown for a typical experiment (Figure 1A,B) indicated clear separation of light membrane fragments containing most of caveolin-1 from heavy fragments containing the major peak of Na+/K+-ATPase activity. The results also showed a smaller shoulder of caveolin-1 in the heavy fragments and a smaller shoulder of Na+/K+-ATPase activity in the light membranes (Figure 1A,B). The distribution of caveolin-2 in the various fractions was the same as that of caveolin-1 (not shown). The patterns of numerous runs similar to that described above were the same, but with minor shifts in the positions of the caveolin-1 and Na+/K+-ATPase activity peaks in different runs. For comparison of the properties of the light and heavy membrane fragments presented below, we usually used combinations of two fractions, 12 and 13, for the light caveolar membranes, and one peak fraction, 16, for the heavy noncaveolar membranes. As expected, the caveolar membranes contained significantly more cholesterol than the noncaveolar membranes (Figure 1C).
Na+/K+-ATPase Activities and Subunit Compositions of the Caveolar and Noncaveolar Pools of the Enzyme
Because in cardiac myocytes the different subunits of Na+/K+-ATPase are unevenly distributed among caveolar and noncaveolar membranes,(7) it was important to determine the relative distributions of the enzyme subunits in the caveolar and noncaveolar kidney membranes prepared here. The immunoassays of the α, β, and γ subunit contents of the fractionated microsomes (Figure 1D) clearly showed that the three subunits of the kidney enzyme are evenly distributed between the purified caveolar and noncaveolar membranes. Because the three subunits have already been shown to exist in 1:1:1 ratio in the purified noncaveolar kidney enzyme,(14) the combined data of panels A and D of Figure 1 indicate that the kidney caveolar enzyme also contains the three subunits in the same ratio.
The specific Na+/K+-ATPase activity of the purified noncaveolar enzyme was routinely 5–6 times higher than that of the caveolar enzyme. In six runs similar to Figure 1A, the specific activities (micromoles of Pi per milligram of protein per hour) were 211 ± 40 for the caveolar pool and 1118 ± 124 for the noncaveolar pool. Experiments aimed at the enzyme activities of the two pools as functions of varying ATP, Na+, and K+ concentrations showed no significant differences between the kinetic parameters of the two pools (not shown). The K0.5 values and Hill coefficients of the three essential ligands were nearly the same for the caveolar and noncaveolar pools and similar to the values presented previously for the purified canine kidney enzyme.(16) The K0.5 values for the standard inhibitors of Na+/K+-ATPase activity (ouabain, vanadate, and oligomycin) were also the same for the two pools (not shown), and as presented previously for the purified canine kidney enzyme.(16)
The molar activities of the caveolar and noncaveolar pools, based on the determination of the maximal phosphoenzyme intermediate capacity, were found not to be significantly different [7124 ± 382 min–1 (n = 3); 7510 ± 500 min–1 (n = 3)], and in agreement with the molar activities of all undamaged Na+/K+-ATPases.(17)
Taken together, our findings indicate that the caveolar and noncaveolar pools of the kidney Na+/K+-ATPase have similar catalytic properties, but that the caveolar pool is simply less pure. Comparison of the stained SDS gels of the two pools confirms the latter conclusion (Figure 1E).
Other Proteins of the Caveolar and Noncaveolar Enzymes
In the highly purified preparations of the kidney enzyme studied previously (similar to the noncaveolar membrane enzyme used here), the subunits of Na+/K+-ATPase make up most of the protein content of the preparation.13−15 Because the caveolar enzyme is less pure (Figure 1A,E), it was of interest to identify the other major proteins of the caveolar preparation. Of particular interest were E-cadherin and the annexin-2 heterotetramer that are known to be major proteins of epithelial caveolae and rafts.18−21 Immunoassays showed that E-cadherin and p36 and p11 of annexin-2 (two components of the annexin-2 heterotetramer) are indeed highly enriched in kidney caveolar Na+/K+-ATPase relative to the noncaveolar enzyme (Figure 2A,B). In experiments depicted in Figure 2B, the detected components of the annexin-2 tetramer were the p11 monomer and a complex of p11 and p36 that was stable on the SDS gel. To explore further the various known association states of annexin-220,22 in the caveolar enzyme, we immunoassayed samples that were boiled or not boiled (Figure 3A). While in the boiled sample the major detected species was the dimer of p11 and p36, in the unboiled sample there seemed to be only one complex (>100 kDa) that could be detected by the antibody to either p11 or p36. These results suggest that in the caveolar enzyme, the annexin-2 proteins exist as oligomers with the minimum structure of the heterotetramer. Because caveolin-1 oligomers are also known to be resistant to SDS,(23) we probed the caveolar enzyme with the anti-caveolin-1 antibody before and after boiling (Figure 3B). The results confirmed that caveolin-1 of this enzyme pool also consists of large homo-oligomers of caveolin-1.
Figure 2.
Relative contents of E-cadherin and annexin-2 in caveolar and noncaveolar pools of the enzyme. (A) Caveolar and noncaveolar enzymes were prepared as described in the legend of Figure 1, and equal amounts of protein from each pool were subjected to a quantitative immunoassay for E-cadherin (n = 4). (B) Representative blots of pairs of caveolar and noncaveolar pools were probed with antibody to p11 of annexin-2.
Figure 3.
Oligomeric states of annexin-2 proteins and caveolin-1 in the caveolar Na+/K+-ATPase. (A) Samples of the caveolar enzyme were either boiled in the standard sample buffer containing SDS and β-mercaptoethanol (Experimental Procedures) or not boiled and then subjected to SDS–PAGE and probed with antibodies to p11 and p36 of annexin-2. (B) Boiled and unboiled samples as described for panel A were probed with the caveolin-1 antibody.
We also compared the relative contents of a number of proteins that have been implicated in the signaling functions of Na+/K+-ATPase5,24 in the caveolar and noncaveolar enzymes. Somewhat surprisingly, Src, EGFR, p85 of PI3K, Akt, and ERK 1/2 were all enriched in the noncaveolar relative to the caveolar pool (Figure 4). Using highly purified preparations of Src and EGFR as standards, and three different preparations of caveolar and noncaveolar enzymes, we determined the molar ratios of the α-subunit of Na+/K+-ATPase to these signaling proteins to be as follows: α:Src ratio of 220 ± 12.5 (n = 3) and α:EGFR ratio of 324 ± 105 (n = 3) for the caveolar enzyme and α:Src ratio of 1701 ± 133 (n = 3) and α:EGFR ratio of 604 ± 36 (n = 3) for the noncaveolar enzyme. Thus, in both caveolar and noncaveolar preparations of the kidney enzyme, the signaling proteins exist as minor constituents relative to Na+/K+-ATPase proteins. Nevertheless, their presence has important functional implications (see Discussion).
Figure 4.
Relative contents of some proteins related to the signaling functions of Na+/K+-ATPase in the caveolar and noncaveolar pools of the kidney enzyme. Equal amounts of protein from each pool were immunoassayed for the indicated proteins. The result for each protein is expressed as a percentage of the total caveolar and noncaveolar contents of that protein (n = 4–8).
Protein–Protein Interactions within the Caveolar Na+/K+-ATPase
For further exploration of the previously suggested interactions between the subunits of Na+/K+-ATPase and other proteins, we first conducted co-immunoprecipitation studies. Using detergent-solubilized caveolar Na+/K+-ATPase and the indicated antibodies, experiments depicted in Figure 5 suggested interactions between the α,β,γ-protomers of Na+/K+-ATPase and (a) caveolin-1 oligomers and (b) the annexin-2 tetramer. The data do not show which subunit(s) of Na+/K+-ATPase or the annexin-2 tetramer is involved in such interactions. Similar experiments in which immmunoprecipitations were conducted with antibodies against the p36 or the β-subunit (not shown) led to the same conclusions.
Figure 5.
Protein–protein interactions of caveolar Na+/K+-ATPase detected by co-immunoprecipitation. The membrane-bound caveolar enzyme as purified in Figure 1 was solubilized by a mixture of octyl glucoside and Triton X-100 (Experimental Procedures) in which caveolin-1 retains its native oligomeric structure.7,23 Immunoprecipitation was conducted using a polyclonal anticaveolin-1 antibody, and immunoblots were created with a monoclonal anticaveolin-1 and the other indicated antibodies.
Because any interactions detected by co-immunoprecipitation may have been induced by the solubilizing detergent used, we also conducted chemical cross-linking studies using the native purified caveolar Na+/K+-ATPase and the cross-linking reagents DSS and BS3, which have been used before to study the oligomeric structures of caveolins and Na+/K+-ATPase.14,15,23 The results summarized in Figure 6 supported the suggestions of Figure 5, indicating that in native caveolar membranes there are large oligomers of Na+/K+-ATPase, the annexin-2 tetramer, and caveolin-1. No attempt was made to identify specific cross-linked products.
Figure 6.
Cross-linking of caveolar Na+/K+-ATPase. Samples were incubated with the amino-reactive cross-linking reagents for 15 min at 24 °C as indicated in Experimental Procedures. BS3 and DSS concentrations were 0.5 mM, unless indicated otherwise. After termination of the reactions, samples were subjected to SDS–PAGE and probed with the indicated antibodies.
Activity of the Endogenous Src of the Caveolar and Noncaveolar Pools
Because extensive studies of the functional consequences of presumed direct interaction of Src with Na+/K+-ATPase have been reported,9−12 it was important to explore the functional status of the endogenous Src of the two kidney pools of the Na+/K+-ATPase. Under conditions where purified Src exhibits kinase activity, the endogenous Src forms of both caveolar and noncaveolar pools of Na+/K+-ATPase were also active, and sensitive to a Src inhibitor, PP2 (Figure 7). When the effects of ouabain on PP2-sensitive phosphotyrosine formation were examined, there was no ouabain-induced increase in either the caveolar or the noncaveolar preparation (Figure 8). In fact, significant ouabain-induced inhibition was noted in the noncaveolar pool (Figure 8). In experiments similar to those depicted in Figure 6, no immunoreactive cross-linked products of Src were noted (not shown). These findings indicate that interactions of endogenous Src with Na+/K+-ATPase, if any, are different from the previously reported interactions of excess Src added to an isolated preparation of Na+/K+-ATPase.(9)
Figure 7.
Endogenous active Src in caveolar and noncaveolar pools of Na+/K+-ATPase. Five pairs of caveolar and noncaveolar enzymes prepared as described in the legend of Figure 1 were incubated at 30 °C for 10 min in the Src kinase buffer as indicated in Experimental Procedures. ATP was absent from controls. Reactions were terminated by the addition of SDS-containing sample buffer, and samples were subjected to SDS–PAGE and immunoassayed for p-Src also as described in Experimental Procedures. *p < 0.05 compared to no ATP control. #p < 0.05 compared to ATP alone.
Figure 8.
Effects of ouabain on the endogenous Src of the caveolar and noncaveolar Na+/K+-ATPase. Experiments were conducted as described in the legend of Figure 7 on the same five pairs of enzymes in the absence and presence of 10 μM ouabain. *p < 0.05 compared to no ouabain.
Discussion
Large-Scale Preparation of the Membrane-Bound Caveolar Enzyme
Since the discovery of Na+/K+-ATPase,(25) most of the classical studies on the biochemistry of this enzyme (e.g., its reaction mechanism) have been conducted with highly purified but still membrane-bound preparations to allow the enzyme to retain its natural environment and minimize functional perturbations induced by purification.1,2,13,14 With the realization of the existence of the caveolar microdomains of the plasma membrane,(26) the existing knowledge that both caveolae and Na+/K+-ATPase are located at the basolateral membranes of polarized renal epithelial cells,(27) and our unambiguous demonstration that some Na+/K+-ATPase is present in caveolae of the cell lines originating from renal epithelium,(6) we expected the existence of two distinct caveolar and noncaveolar pools of kidney Na+/K+-ATPase. In this study, therefore, we have used the same procedures that have been widely used before for the large-scale preparation of the highly purified membrane-bound enzyme to show that a caveolar pool of the enzyme, albeit less pure, may easily be separated and also obtained in large quantities for further structure–function studies. It is important to note that in the purification procedures utilized here, SDS is used at concentrations that do not denature the membrane-bound Na+/K+-ATPase but remove significant amounts of other proteins from the microsomal membranes.13,14
Na+/K+-ATPase Activities of the Caveolar and Noncaveolar Pools
The availability of sufficient quantities of the two pools of the kidney enzyme allowed us to compare their steady-state catalytic activities and conclude (Results) that there are no apparent differences between the two pools for the K0.5 values of the essential ligands, and in their molar activities (turnover numbers). These findings clearly suggest that the ion transport function of the enzyme is retained within the kidney caveolae as it is within the cardiac caveolae.(7) It is important to note, however, that subtle but significant differences between the catalytic and transport functions of the two pools are not ruled out by these limited initial studies. There are major differences between the lipid compositions of the caveolae–raft microdomains and the remainder of the plasma membrane (Figure 1B and ref (26)), and previous studies have clearly indicated effects of lipid on the catalytic activities and quaternary structure of Na+/K+-ATPase.28−30 It is also known that quaternary structure has profound effects on the reaction mechanism of the enzyme.31−33 Hence, we suggest the need for more rigorous investigations, e.g., those using transient kinetics, on the potential differences between the turnover cycles of the caveolar and noncaveolar preparations, and the possible physiological implications of such differences.33,34
Our findings that the turnover numbers of the caveolar and noncaveolar pools do not differ and are within the range of all previously reported kidney enzyme preparations(17) are inconsistent with the proposed existence of a nonpumping caveolar Na+/K+-ATPase.(11) This suggestion was made on the basis of differing turnover numbers in stable cell lines that were generated with various degrees of the α-subunit knockdown. Because of the likely possibility of unidentified changes in a multitude of gene products other than those of the α-subunit or caveolins in these knockdown cells, it is difficult to identify the causes of the damaged turnover numbers of the cell lines. In view of the normal turnover numbers of the caveolar and noncaveolar pools found here (Results), we suggest that the hasty generalization11,12 of the existence of a nonpumping pool of Na+/K+-ATPase in normal renal epithelia or other cell types needs to be re-examined.
Signaling Functions of Caveolar and Noncaveolar Na+/K+-ATPases
Because at least two parallel cell signaling pathways (EGFR/Src-Ras-Raf-ERK and PI3K-PDK-Akt) are known to be linked to Na+/K+-ATPase and activated by ouabain,(24) we looked for and found several components of the two pathways in both pools of the kidney enzyme. The fact that the levels of some are even higher in the noncaveolar pool (Figure 4) is inconsistent with the previous suggestions that only the caveolar enzyme may signal6,9,12 but favors the later suggestion7,8 that in any membrane domain of an intact cell, Na+/K+-ATPase may exhibit different signaling functions depending on the nature of the existing neighboring proteins and those that are recruited upon stimulation in a cell-specific, locus-specific, and stimulus-specific manner.
The demonstration of the presence of signaling proteins in both pools of the kidney enzyme also has special relevance to previous cell-free studies of the signaling function of the enzyme. The EGFR/Src-Ras-Raf-ERK pathway was the first cell signaling sequence that was shown to be linked to Na+/K+-ATPase.(5) Though early studies showed the diversity of the downstream events of this ouabain-activated pathway in different cells, the postulated proximal steps seemed amenable to cell-free studies using purified Na+/K+-ATPase. Therefore, Tian et al.(10) added purified Src to the same purified kidney enzyme used here (the noncaveolar pool), with and without ouabain, and interpreted the resulting changes in the phosphorylation of Y418 of Src in the context of the direct interaction of Src with the α-subunit of Na+/K+-ATPase. Our findings, however, suggest that even in this cell-free system, ouabain is interacting with a Na+/K+-ATPase that may be associating with EGFR, Src, ERK, and a host of other signaling proteins, some of which are yet to be identified. In such a complex, it would be difficult to justify the choice of the specific pair of Src and Na+/K+-ATPase as the receptor for ouabain-induced signaling.10,12
The complexity of assessing the role of Src in ouabain-activated signaling in cell-free experiments is compounded by our finding of endogenous Src in both pools of the isolated kidney enzyme (Figure 7), and the absence of ouabain activation of this Src (Figure 8), in apparent conflict with previous findings for cases in which Src was added to isolated Na+/K+-ATPase.(10) It is important to note that while Src is indeed activated when ouabain is added to a variety of intact cell types,5,8,12 there is no compelling reason to assume that such Src activation must be mediated through ouabain’s effect on a direct contact between Src and Na+/K+-ATPase subunits. Perhaps in some intact cells direct contact between the α-subunit and Src, or other Src family kinases, does occur as postulated previously,10,35,36 but the existing in vitro experiments do not provide unambiguous support for this postulate (Figures 7 and 8 and ref (36)). In this regard, it is also appropriate to note that the best previous evidence of direct interaction between the Na+/K+-ATPase α-subunit and Src is provided by co-immunoprecipitation experiments in which Na+/K+-ATPase was denatured by the detergents used to solubilize membranes prior to the addition of antibodies.9,10,35,36 That denatured Na+/K+-ATPase subunits, but not those of the native enzyme, directly interact with Src and related kinases was also reported long ago(37) as a conclusion to serious attempts by Racker’s laboratory to clarify if Na+/K+-ATPase is regulated by tyrosine phosphorylation of its subunits. We suggest that the possible direct contact of Src with native Na+/K+-ATPase is yet to be established.
Physiological Role of the Kidney Caveolar Na+/K+-ATPase
If the renal caveolar Na+/K+-ATPase has no monopoly on the signaling function of the enzyme, what is its physiological role? Our findings clearly indicate that it is in the regulation of renal epithelial adherens junctions.
The fact that E-cadherin and the annexin-2 tetramer are involved in cell–cell adhesion in renal epithelial cells at adherens junctions and tight junctions is well-established.40−42 More importantly, extensive previous studies in renal and other epithelial cells have implicated the β-subunit of Na+/K+-ATPase in the regulation of cell adhesion in partnership with E-cadherin and annexin-2.40−45 This evidence and prior studies showing that E-cadherin and annexin-2 are major proteins of the caveolae/rafts,18−21 coupled with our findings about the enrichment of both E-cadherin and the annexin-2 tetramer in the caveolar pool (Figures 2 and 3), lead us to conclude that the role of the kidney caveolar Na+/K+-ATPase must be at the specialized junctional domains of the renal epithelia. However, because we have not been able to clearly identify immunoreactive occludin (a marker of tight junctions) in the caveolar Na+/K+-ATPase (not shown), we favor the hypothesis that the caveolar enzyme regulates adherens junctions through the pumping and signaling functions of the enzyme.5,24,40,43
Association States of the Major Proteins of the Caveolar Enzyme
The fact that in renal epithelial cells caveolin-1 exists primarily as large caveolin-1 oligomers is well-established.23,38 The findings presented here (Figures 3, 5, and 6) show that in the kidney caveolar Na+/K+-ATPase caveolin-1 also exists as homo-oligomers that interact with annexin-2 oligomers and oligomers of the α,β,γ-protomer of Na+/K+-ATPase. Neither in co-immunoprecipitation nor in cross-linking studies, however, do we find evidence of direct contact between caveolin-1 and any specific subunits of the other two oligomers. Our findings, in conjunction with previous extensive evidence of the higher oligomeric states of Na+/K+-ATPase promoters14,15,29,30,32,39 and caveolin-1,23,38 clearly indicate that interaction of caveolin-1 with kidney caveolar Na+/K+-ATPase occurs within a large oligomeric complex of caveolin-1, the annexin-2 tetramer, and Na+/K+-ATPase (Figure 9A).
Figure 9.
Schematic presentation of the arrangement of the components of the caveolar Na+/K+-ATPase at renal epithelial adherens junctions. (A) Large multiprotein complex of the caveolar enzyme, with identified interactions among caveolin-1 oligomers, Na+/K+-ATPase oligomers, and the annexin-2 tetramer, but with unidentified nearest-neighbor interactions. (B) Cell–cell contact at the lateral side of the polarized cells maintained by E-cadherin and the multiprotein complexes, with the essential role of the association of the extracellular domains of the β-subunits of two adjacent cells, possibly through some of several GXXXG dimerizing motifs of the extracellular parts of the β-subunits. See Discussion.
Though the nearest-neighbor interactions in the multiprotein complex of Figure 9A need to be established in future studies, the available structural information about the components allows some suggested leads. The α-subunit of Na+/K+-ATPase has two caveolin binding motifs, and the possibility of its interaction with caveolin-1 has been considered.(9) p36 of the annexin-2 tetramer also has a caveolin binding motif and, hence, is a likely neighbor of caveolins within the multiprotein complex. p11 of the annexin-2 tetramer has a well-defined TM helix, and recent evidence(46) suggests the presence of two highly ordered TM helices in caveolins. Therefore, considering the abilities of several TM domains of the α-subunit to interact with those of other integral membrane proteins,(4) both p11 and caveolins are potential partners of the α-subunit through TM–TM interactions.
We have not included E-cadherin in the multiprotein complex of Figure 9A because we have no experimental data that establish its contact with the complex. E-Cadherin’s enrichment in the caveolar pool of the enzyme may be due to its preferential binding to caveolar lipids.(21) On the other hand, E-cadherins also contain highly ordered TM helices with dimerizing motifs,(47) thus having the potential of interacting with TM domains of the other components of the complex depicted in Figure 9A.
Interactions of the Caveolar Na+/K+-ATPase of Two Adjacent Cells
Though there is ample prior evidence of a role of β,β-interactions in cell adhesion, the major difference between the previously proposed models is whether the relevant β,β-interactions are intracellular44,45 or intercellular.40,41,43 The latter is favored by our findings and the available information about the structure of the kidney enzyme as discussed below.
The existence of β,β-interactions was demonstrated previously14,15 in purified noncaveolar kidney enzyme preparations by cross-linking experiments. The cross-linking experiments presented here are also consistent with the existence of β,β-interactions in the caveolar pool of the enzyme. These observations on purified enzyme preparations raise an important question. If the β-subunits of two adjacent junctional cells are in contact as has been suggested,40,43 is it reasonable to expect that such noncovalent intercellular β,β-contacts would survive the purification process? The detergents used in the purification procedure are known not to disrupt the strong noncovalent α,β,γ-associations;13,14 hence, there is no a priori reason for thinking that β,β-associations (intracellular or intercellular) would be disrupted. We conclude, therefore, that protein–protein interactions noted in both caveolar and noncaveolar preparations include those that originate from cell–cell contacts.
We may now ask if the information surmised from the crystal structure of the purified pig kidney enzyme(48) is helpful in deciding whether the β,β-associations relevant to cell adhesion are intercellular or intracellular in origin. We suggest that the answer is yes in favor of the former. Morth et al.(48) noted that the only interactions between two β-subunits occurred outside of the plane of the membrane, through the extracellular segments of β (up to residue 73) that are well-resolved in the electron density maps. We consider this clear evidence of intercellular β,β-interactions indicating the absence of intracellular β,β-interactions, at least in the crystals of the kidney enzyme containing bound Rb+.(48)
In the caveolar kidney Na+/K+-ATPase, the β,β-interactions of the enzymes of two adjacent cells must cooperate with known and potential intercellular contacts provided by E-cadherin and the annexin-2 tetramer, as discussed in the previous section and depicted in Figure 9B. Because caveolae were discovered as inpocketings of the plasma membrane(26) and are usually shown as such, it may be awkward to envision contacts of adjacent cells as indicated in Figure 9B. There is ample evidence, however, to show that caveolin-1 is located mostly at the caveolar necks and that caveolin-1 clusters also exist in the flat parts of the plasma membrane.38,49−51
An important issue related to the β,β-interactions of Na+/K+-ATPase is the matter of the GXXXG amino acid sequence motifs of the β-subunit TM. With the realization that this motif stabilizes TM helix interactions in many membrane proteins,(52) a number of studies of the structure of Na+/K+-ATPase have focused on the existence of two such motifs in the β-subunit TM, and their possible participation in α,β- and β,β-interactions (refs (15), (45), (48), and (53) and references cited therein). With respect to the significance of these oligomerization motifs to intercellular β,β-interactions, we emphasize the following. First, GXXXG and related AXXXA motifs have also been noted to stabilize helix–helix interactions and the folded states outside the lipid bilayer and in soluble proteins.54,55 Second, the pig kidney β-subunit contains eight GXXXG and AXXXA motifs, with four of the former being extracellular. These facts, the extensive previous studies of Creijido and colleagues,40,41,43 and the findings presented here provide strong support for the contacts of the caveolar Na+/K+-ATPase of two adjacent cells at adherens junctions (Figure 9B).
Glossary
Abbreviations
- BS3
bis(sulfosuccinimidyl)suberate
- EGFR
epidermal growth factor receptor
- ERK
extracellular signal-regulated kinase
- DSS
disuccinimidyl suberate
- PI3K
phosphatidylinositol 3-kinase
- PP2
4-amino-5-(4-chlorophenyl)-7-(dimethylethyl)pyrazola[3,4-d]pyridine
- SDS–PAGE
sodium dodecyl sulfate–polyacrylamide gel electrophoresis
- TM
transmembrane domain.
Supported by Grant HL-36573 from the National Heart, Lung and Blood Institute.
Funding Statement
National Institutes of Health, United States
References
- Skou J. C.; Esmann M. (1992) The Na,K-ATPase. J. Bioenerg. Biomembr. 24, 249–261. [DOI] [PubMed] [Google Scholar]
- Kaplan J. H. (2002) Biochemistry of Na,K-ATPase. Annu. Rev. Biochem. 71, 511–535. [DOI] [PubMed] [Google Scholar]
- Nesher M.; Shpolansky U.; Rosen H.; Lichstein D. (2007) The digitalis-like steroid hormones: New mechanisms of action and biological significance. Life Sci. 80, 2093–2107. [DOI] [PubMed] [Google Scholar]
- Morrill G. A.; Kostellow A. B.; Askari A. (2010) Progesterone modulation of transmembrane helix-helix interactions between the α-subunit of Na/K-ATPase and phospholipid N-methyltransferase in the oocyte plasma membrane. BMC Struct. Biol. 10, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z.; Askari A. (2002) Na+/K+-ATPase as a signal transducer. Eur. J. Biochem. 269, 2434–2439. [DOI] [PubMed] [Google Scholar]
- Liu L.; Mohammadi K.; Aynafshar B.; Wang H.; Li D.; Liu J.; Ivanov A. V.; Xie Z.; Askari A. (2003) Role of caveolae in signal-transducing function of cardiac Na+/K+-ATPase. Am. J. Physiol. 284, C1550–C1560. [DOI] [PubMed] [Google Scholar]
- Liu L.; Askari A. (2006) B-subunit of cardiac Na+-K+-ATPase dictates the concentration of the functional enzyme in caveolae. Am. J. Physiol. 291, C569–C578. [DOI] [PubMed] [Google Scholar]
- Liu L.; Abramowitz J.; Askari A.; Allen J. C. (2004) Role of caveolae in ouabain-induced proliferation of cultured vascular smooth muscle cells of the synthetic phenotype. Am. J. Physiol. 287, H2173–H2182. [DOI] [PubMed] [Google Scholar]
- Wang H.; Haas M.; Liang M.; Cai T.; Tian J.; Li S.; Xie Z. (2004) Ouabain assembles signaling cascades through the caveolar Na+/K+-ATPase. J. Biol. Chem. 279, 17250–17259. [DOI] [PubMed] [Google Scholar]
- Tian J.; Cai T.; Yuan Z.; Wang H.; Liu L.; Haas M.; Maksimova E.; Huang X. Y.; Xie Z. J. (2006) Binding of Src to Na+/K+-ATPase forms a functional signaling complex. Mol. Biol. Cell 17, 317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang M.; Tian J.; Liu L.; Pierre S.; Liu J.; Shapiro J.; Xie Z. J. (2007) Identification of a pool of a non-pumping Na/K-ATPase. J. Biol. Chem. 282, 10585–10593. [DOI] [PubMed] [Google Scholar]
- Li Z.; Xie Z. (2009) The Na/K-ATPase/Src complex and cardiotonic steroid-activated protein kinase cascades. Pfluegers Arch. 457, 635–644. [DOI] [PubMed] [Google Scholar]
- Jørgensen P. L. (1988) Purification of Na+,K+-ATPase: Enzyme sources, preparative problems, and preparation from mammalian kidney. Methods Enzymol. 156, 29–43. [DOI] [PubMed] [Google Scholar]
- Ivanov A. V.; Gable M. E.; Askari A. (2004) Interaction of SDS with Na+/K+-ATPase: SDS-solubilized enzyme retains partial structure and function. J. Biol. Chem. 279, 29832–29840. [DOI] [PubMed] [Google Scholar]
- Ivanov A. V.; Modyanov N. N.; Askari A. (2002) Role of the self-association of β subunits in the oligomeric structure of Na+/K+-ATPase. Biochem. J. 364, 293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Periyasamy S. M.; Huang W. H.; Askari A. (1983) Origins of the different sensitivities of (Na+ + K+)-dependent adenosine triphosphatase preparations to ouabain. Comp. Biochem. Physiol., Part B: Biochem. Mol. Biol. 76, 449–454. [DOI] [PubMed] [Google Scholar]
- Esmann M. (1988) ATPase and phosphatase activity of Na+,K+-ATPase: Molar and specific activity, protein determination. Methods Enzymol. 156, 105–115. [DOI] [PubMed] [Google Scholar]
- Miotti S.; Tomassetti A.; Facetti I.; Sanna E.; Berno V.; Canevari S. (2005) Simultaneous expression of caveolin-1 and E-cadherin in ovarian carcinoma cells stabilizes adherens junctions through inhibition of Src-related kinases. Am. J. Pathol. 167, 1411–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallo-Medved D.; Dosescu J.; Linebaugh B. E.; Sameni M.; Rudy D.; Sloane B. F. (2003) Mutant K-ras regulates cathepsin B localization on the surface of human colorectal carcinoma cells. Neoplasia 5, 507–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W. Q.; Waisman D. M.; Grimaldi M. (2004) Specific localization of the annexin II heterotetramer in brain lipid raft fractions and its changes in spatial learning. J. Neurochem. 90, 609–620. [DOI] [PubMed] [Google Scholar]
- Harder T.; Gerke V. (1994) The annexin II2p11(2) complex is the major protein component of the triton X-100-insoluble low-density fraction prepared from MDCK cells in the presence of Ca2. Biochim. Biophys. Acta 1223, 375–382. [DOI] [PubMed] [Google Scholar]
- Illien F.; Finet S.; Lambert D.; Ayala-Sammartin J. (2010) Different molecular arrangements of the tetrameric annexin 2 modulate the size and dynamics of membrane aggregation. Biochim. Biophys. Acta 1798, 1790–1796. [DOI] [PubMed] [Google Scholar]
- Sargiacomo M.; Scherer P. E.; Tang Z.; Kübler E.; Song K. S.; Sanders M. C.; Lisanti M. P. (1995) Oligomeric structure of caveolin: Implications for caveolae membrane organization. Proc. Natl. Acad. Sci. U.S.A. 92, 9407–9411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L.; Zhao X.; Pierre S. V.; Askari A. (2007) Association of PI3K-Akt signaling pathway with digitalis-induced hypertrophy of cardiac myocytes. Am. J. Physiol. 293, C1489–C1497. [DOI] [PubMed] [Google Scholar]
- Skou J. C. (1957) The influence of some cations on an adenosine triphosphatase from peripheral nerves. Biochim. Biophys. Acta 23, 394–401. [DOI] [PubMed] [Google Scholar]
- Anderson R. G. (1998) The caveolae membrane system. Annu. Rev. Biochem. 67, 199–225. [DOI] [PubMed] [Google Scholar]
- Scheiffele P.; Verkade P.; Fra A. M.; Virta H.; Simons K.; Ikonen E. (1998) Caveolin-1 and -2 in the exocytic pathway of MDCK cells. J. Cell Biol. 140, 795–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius F.; Turner N.; Christensen H. R. (2003) Modulation of Na,K-ATPase by phospholipids and cholesterol. II. Steady-state and presteady-state kinetics. Biochemistry 42, 8541–8549. [DOI] [PubMed] [Google Scholar]
- Kobayashi T.; Tahara Y.; Takenaka H.; Mimura K.; Hayashi Y. (2007) Na+- and K+-dependent oligomeric interconversion among αβ-protomers, diprotomers and higher oligomers in solubilized Na+/K+-ATPase. J. Biochem. 142, 157–173. [DOI] [PubMed] [Google Scholar]
- Mimura K.; Tahara Y.; Shinji N.; Tokuda E.; Takenaka H.; Hayashi Y. (2008) Isolation of stable (αβ)4-tetraprotomer from Na+/K+-ATPase solubilized in the presence of short-chain fatty acids. Biochemistry 47, 6039–6051. [DOI] [PubMed] [Google Scholar]
- Clarke R. J.; Apell H. J.; Kong B. Y. (2007) Allosteric effect of ATP on Na+,K+-ATPase conformational kinetics. Biochemistry 46, 7034–7044. [DOI] [PubMed] [Google Scholar]
- Clarke R. J.; Kane D. J. (2007) Two gears of pumping by the sodium pump. Biophys. J. 93, 4187–4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalid M.; Cornelius F.; Clarke R. J. (2010) Dual mechanisms of allosteric acceleration of the Na+,K+-ATPase by ATP. Biophys. J. 98, 2290–2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke R. J. (2009) Mechanism of allosteric effects of ATP on the kinetics of P-type ATPases. Eur. Biophys. J. 39, 3–17. [DOI] [PubMed] [Google Scholar]
- Bozulic L. D.; Dean W. L.; Delamere N. A. (2004) The influence of Lyn kinase on Na,K-ATPase in porcine lens epithelium. Am. J. Physiol. 286, C90–C96. [DOI] [PubMed] [Google Scholar]
- Bozulic L. D.; Dean W. L.; Delamere N. A. (2005) The influence of SRC-family tyrosine kinases on Na,K-ATPase activity in lens epithelium. Invest. Ophthalmol. Visual Sci. 46, 618–622. [DOI] [PubMed] [Google Scholar]
- Nakamura S.; Braun S.; Racker E. (1987) A tyrosine-specific protein kinase from Ehrlich ascites tumor cells. Arch. Biochem. Biophys. 252, 538–548. [DOI] [PubMed] [Google Scholar]
- Liu P.; Rudick M.; Anderson R. G. (2002) Multiple functions of caveolin-1. J. Biol. Chem. 277, 41295–41298. [DOI] [PubMed] [Google Scholar]
- Laughery M.; Todd M.; Kaplan J. H. (2004) Oligomerization of the Na,K-ATPase in cell membranes. J. Biol. Chem. 279, 36339–36348. [DOI] [PubMed] [Google Scholar]
- Cereijido M.; Contreras R. G.; Shoshani L. (2004) Cell adhesion, polarity, and epithelia in the dawn of metazoans. Physiol. Rev. 84, 1229–1262. [DOI] [PubMed] [Google Scholar]
- Cereijido M.; Contreras R. G.; Shoshani L.; Flores-Benitez D.; Larre I. (2008) Tight junction and polarity interaction in the transporting epithelial phenotype. Biochim. Biophys. Acta 1778, 770–793. [DOI] [PubMed] [Google Scholar]
- Lee D. B.; Jamgotchian N.; Allen S. G.; Kan F. W.; Hale I. L. (2004) Annexin A2 heterotetramer: Role in tight junction assembly. Am. J. Physiol. 287, F481–F491. [DOI] [PubMed] [Google Scholar]
- Padilla-Benavides T.; Roldán M. L.; Larre I.; Flores-Benitez D.; Villegas-Sepúlveda N.; Contreras R. G.; Cereijido M.; Shoshani L. (2010) The polarized distribution of Na+,K+-ATPase: Role of the interaction between β subunits. Mol. Biol. Cell 21, 2217–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barwe S. P.; Anilkumar G.; Moon S. Y.; Zheng Y.; Whitelegge J. P.; Rajasekaran S. A.; Rajasekaran A. K. (2005) Novel role for Na,K-ATPase in phosphatidylinositol 3-kinase signaling and suppression of cell motility. Mol. Biol. Cell 16, 1082–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barwe S. P.; Kim S.; Rajasekaran S. A.; Bowie J. U.; Rajasekaran A. K. (2007) Janus model of the Na,K-ATPase β-subunit transmembrane domain: Distinct faces mediate α/β assembly and β-β homo-oligomerization. J. Mol. Biol. 365, 706–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrill G. A.; Kostellow A. B. (2011) Plasma membrane topology of caveolins: Insights from computational modeling. Biophys. J. 100, Suppl. 1204a. [Google Scholar]
- Huber O.; Kemler R.; Langosch D. (1999) Mutations affecting transmembrane segment interactions impair adhesiveness of E-cadherin. J. Cell Sci. 112, 4415–4423. [DOI] [PubMed] [Google Scholar]
- Morth J. P.; Pedersen B. P.; Toustrup-Jensen M. S.; Sørensen T. L.; Petersen J.; Andersen J. P.; Vilsen B.; Nissen P. (2007) Crystal structure of the sodium-potassium pump. Nature 450, 1043–1049. [DOI] [PubMed] [Google Scholar]
- Thorn H.; Stenkula K. G.; Karlsson M.; Örtegren U.; Nystrom F. H.; Gustavsson J.; Strålfors P. (2003) Cell surface orifices of caveolae and localization of caveolin to the necks of caveolae in adipocytes. Mol. Biol. Cell 10, 3967–3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermann M.; Steiniger F.; Richter W. (2005) Belt-like localization of caveolin in deep caveolae and its re-distribution after cholesterol depletion. Histochem. Cell Biol. 123, 613–620. [DOI] [PubMed] [Google Scholar]
- Robenek H.; Weissen-Plenz G.; Severs N. J. (2008) Freeze-fracture replica immunolabelling reveals caveolin-1 in the human cardiomyocyte plasma membrane. J. Cell. Mol. Med. 12, 2519–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russ W. P.; Engelman D. M. (2000) The GxxxG motif: A framework for transmembrane helix-helix association. J. Mol. Biol. 296, 911–919. [DOI] [PubMed] [Google Scholar]
- Clifford R. J.; Kaplan J. H. (2008) β-Subunit overexpression alters the stoicheometry of assembled Na-K-ATPase subunits in MDCK cells. Am. J. Physiol. 295, F1314–F1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiger G.; Grothe R.; Mallick P.; Eisenberg D. (2002) GXXXG and AXXXA: Common α-helical interaction motifs in proteins, particularly in extremophiles. Biochemistry 41, 5990–5997. [DOI] [PubMed] [Google Scholar]
- Gimpelev M.; Forrest L. R.; Murray D.; Honig B. (2004) Helical Packing Patterns in Membrane and Soluble Proteins. Biophys. J. 87, 4075–4086. [DOI] [PMC free article] [PubMed] [Google Scholar]