Abstract
Valid experimental evidence has recently shown that progression of malignant tumors does not depend exclusively on cell-autonomous properties of the cancer cells, but is also deeply influenced by tumor stroma reactivity and undergoes a strict microenvironmental control. Beside structural environmental components as extracellular matrix (ECM) or hypoxia, stromal cells as macrophages, endothelial cells, and cancer-associated fibroblasts (CAFs) play a definite role in cancer progression. This review summarizes our current knowledge on the role of CAFs in tumor progression towards an aggressive phenotype, with particular emphasis on invasiveness, stemness, and preparation of metastatic niche. The controversial origins of CAFs as well as the therapeutical implications of targeting CAFs for anticancer therapy are discussed.
Keywords: Cancer associated fibroblasts (CAFs), tumor stroma, cancer microenvironment, extracellular matrix (ECM), progression, therapy
Activation of fibroblasts in cancer stroma
Fibroblasts are the most abundant cell type in connective tissues and form the structural framework of tissues through their secretion of ECM components [1]. Quiescent fibroblasts undergo activation during wound healing and fibrosis, both conditions sharing the requirement for tissue remodelling, and become myofibroblasts (MFs), as originally described by Giulio Gabbiani in 1971 [1]. MFs acquire contractile stress fibers, de novo express α-smooth muscle actin (α -SMA) and the ED-A splice variant of fibronectin, and form cell-cell contacts through gap junctions [2]. Upon completion of the wound healing process, activated fibroblasts undergo a particular type of programmed cell death, called nemosis, and are removed by the granulation tissue [3,4].
Considering that “tumors are wounds that do not heal” [5], CAFs share some similarities with MFs, including expression of SMA and ED-A fibronectin, but greatly differ for their duration (they are not removed by apoptosis) and their activation is not reversible. CAFs are the most prominent cell type within the tumor stroma of many cancers, most notably breast, prostate and pancreatic carcinoma [6,7]. Recent studies underscore several subpopulations of stromal fibroblasts within different tumors. These populations share some properties collectively leading to their “activation state”, although their expression of acknowledged activation markers is only partial. The main activation markers are α-SMA and fibroblast specific protein (FSP), although platelet-derived growth factor (PDGF) receptors-β and fibroblast activation protein (FAP) have been found overexpressed in stromal fibroblasts of solid tumors [6,8,9]. Beside these molecular markers of fibroblasts activation, some other proteins expressed by stromal fibroblasts are recognised to have a prognostic value for solid tumors. In particular, a poor prognosis has been associated with expression in CAFs of the hypoxia marker carbonic anidrase IX in human lung adenocarcinoma [10], or perio-stin in cholangiocarcinoma [11], or p53 tumor suppressor in ductal carcinoma [12]. On the contrary expression in CAFs of Caveolin-1, PTEN or podoplanin correlates with a favourable prognosis for several carcinomas [13,14,15]. Indeed, recent studies have reported a tumor promoter effect of p53 inactivation in the stromal fibroblasts, as well as that genetic inactivation of PTEN in CAFs accelerates both onset and progression of breast carcinoma [16,17,13]. This large heterogeneity in marker expression for CAFs originating from different tumors may be explained by their possible diverse origin. Indeed, CAFs are variously reported to stem from resident local fibroblasts, bone marrow-derived progenitor cells or trans-differentiating epithelial/endothelial cells through epigenetic transitions (see below) [18,19,20,21].
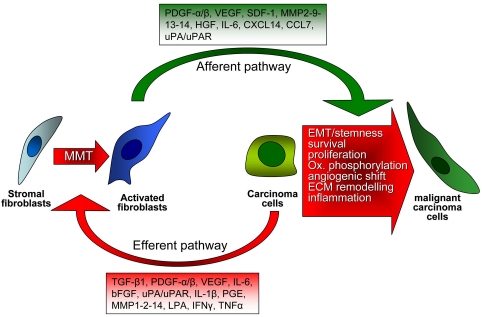
The role of CAFs in tumor progression is multi-faceted. Similarly to immune cells, which initially repress malignant growth, CAFs inhibit early stages of tumor progression, mainly through the formation of gap junctions between activated fibroblasts [19, 20]. Conversely, later on CAFs become activated by several tumor-secreted factors and promote both tumor growth and progression. Two closely interactive pathways are established in the crosstalk between cancer and stromal cells: a) in the “efferent” pathway, cancer cells trigger a reactive response in the stroma, and b) in the “afferent” pathway, the modified stromal cells in the surrounding microenvironment affect cancer cell responses [22,23] (Figure 1). The trans-differentiation of CAFs, a process commonly called mesenchymal-mesenchymal transition (MMT) [6], is currently poorly understood. TGF-β1 has been largely acknowledged to be one of the major tumor-cell derived factors affecting CAF activation [24]. Nevertheless other pro-fibrotic factors can be released by cancer cells and act on CAFs inducing their activation, including PDGF-α/β [25,26], basic fibroblast growth factor (b-FGF) [27] or interleukin (IL)-6 [23]. Several data indicate that activation of CAFs is under a clear redox control. Tumor growth factor (TGF)-β1 causes an increase in reactive oxygen species (ROS) in CAFs, which is responsible for downregulation of gap junctions between CAFs, for their achievement of MF-phenotype, as well as for their tumor promoting activity in skin tumors [28,29]. Antioxidant treatments, or the micronutrient selenite, prevent CAF activation and their enhancement of tumor invasion [28]. In keeping, the activation of prostate CAFs by tumor-secreted IL-6 is again redox-dependent [30], and the oxidative stress due to JunD genetic inactivation promotes myofibroblast differentiation and tumour spreading in breast adenocarcinoma [31]. Again antioxidant treatments blocks secretion by CAFs of matrix metalloproteases (MMPs) or stromal-derived factor (SDF)-1, thereby affecting the CAF “efferent” pathway. In resident human mammary fibroblasts progressively converting into CAFs, SDF-1 and TGF-β1 have been involved in the acquisition of two autocrine signaling loops, which initiate and maintain the differentiation of fibroblasts into myofibroblasts and the concurrent tumor-promoting phenotype [32].
Figure 1.
Interplay between CAFs and tumor cells. Tumor progression needs a positive and reciprocal feedback between CAFs and cancer cells. Cancer cells induce and maintain the fibroblasts activated phenotype which, in turn, produce a series of growth factors and cytokines that sustain tumor progression by promoting ECM remodelling, cell proliferation, angiogenesis and EMT.
Origins of CAFs
A key unsolved question on CAFs is their possible multiple origin. It is becoming evident that CAFs origin can vary both between different tumor hystotypes and within different areas of individual tumors. In keeping with the idea to develop an effective therapeutic stromal strategy (see below), extensive information about the taxonomy of CAFs in different tumor is mandatory.
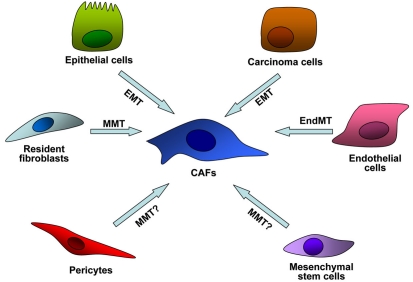
We can roughly classify the line of evidence about CAFs origin in: i) resident; ii) mesenchymal stem cell (MSC)-derived; iii) mutational (Figure 2).
Figure 2.
Multiple origins of CAFs within tumor microenvironment. CAFs can stem from trans-differentiation of resting resident fibroblasts or pericytes within tumor microenvironment, through mesenchymal mesenchymal transition (MMT). Alternatively CAFs could derive from bone marrow mesenchymal stem cells (MSCs), or from epithelial normal or transformed cells via epithelial to mesenchymal trasnsition (EMT), or finally from endothelial cells via endothelial to mesenchymal transition (EndMT).
Resident CAFs originate primarily by activation of local fibroblasts by cancer-derived growth factor. Indeed, tumor cells produce high levels of growth factors like as TGF-β, PDGF and bFGF that activate stromal cells including resting fibroblasts, as well as smooth muscle cells, pericytes, adipocytes or inflammatory cells (Figure 2). This trans-differentiation MMT process is accompanied by the expression of CAF-specific genes in fibroblasts [33,34] such as αSMA, MMP1, MMP3, collagens etc [35,36]. An “in vivo” confirmation of this model come from data showing that human mammary fibroblasts convert into CAFs during the course of tumor progression in a breast tumor xenograft model, and that this effect is due to autocrine activating signalling loops, mediated by TGF-β and SDF-1 cytokines [37]. Again, Toullec reported that SDF-1 is the key factor involved in activation of resident fibroblast of human adenocarcinoma [31].
A second kind of CAF source is represented by bone marrow-derived MSCs. MSCs are able to differentiate into bone, fat, cartilage and muscle cells in many physiological and pathological processes [38,39]. MSCs display homing and engraftment at injury sites in many conditions such as tissue repair, inflammation and neoplasia [40,41,42]. The recruitment of MSCs at tumor sites, similarly to activation of inflammatory cells in tissue repair processes, is mediated by many cytokines and growth factor produced by tumor cells or by activated stroma such as VEGF, EGF, HGF, bFGF, PDGF and CCL2 [43,44,45]. In vivo labeled MSCs have been shown to localize within tumor mass and to differentiate into pericytes and CAFs, acquiring de novo expression of characteristic markers such as α-SMA, FAP, tenascin-c and thrombospondin-1, markers phenotypically associated with aggressiveness [46]. However, the role of recruited MSCs within tumor microenvironment is still controversial. Indeed, MSCs have been both negatively or positively involved in tumor progression, through immunomodulatory and pro-angiogenic properties, depending on of the source of MSC and the tumor model used [47].
A third proposed source of CAFs origin are epithelial cells that, through an EMT process, achieve mesenchymal characteristic and become fibroblasts [6,48]. This hypothesis arises from the evidence that epithelial cells exposed to MMP-driven oxidative stress undergo DNA oxidation and experience mutations, thereby undergoing to specialized EMT in which they transdifferentiate into activated myofibroblasts [6,49]. In addition, genetic studies mainly carried out in breast cancers reported CAF somatic mutations in TP53 and PTEN, as well as gene copy number alteration at other loci in tumor stroma [50,51,52]. In keeping with this idea, p53 inactivation in stromal fibroblasts, as well as that genetic inactivation of PTEN in CAFs enhances tumor progression in breast carcinoma models [16,17,13]. These studies collectively elicit the idea that the tumor promoting activity of CAFs may be mainly based on these somatic mutations in key tumor suppressor genes. In addition, somatic alterations were consistently observed at a high frequency (>30%) in tumor juxtaposed fibroblasts [51,52]. On the contrary, more recent studies have stated that genetic alterations were detected only in cancer epithelial cells and not in the stroma [53], and copy number and loss of heterozygosity analysis of CAFs derived from breast and ovarian carcinomas showed that somatic genetic alterations in CAFs are extremely rare and cannot be the basis of the carcinoma-promoting phenotypes of these cancers [54].
In addition, CAFs may arise directly from carcinoma cells through EMT [6,48], which allows cancer cells to adopt a mesenchymal cell phenotype, characterized by an enhanced migratory capacity and invasiveness [55]. EMT is induced by many factors (i.e. PDGF, TGFβ, EGF, etc) and is mediated by the activation of typical transcription factors like Snail, Slug, Twist and FOXC2 [55,56]. Similarly, proliferating endothelial cells might contribute to CAF via endothelial to mesenchymal transition (EndMT). This process is characterized by the loss of endothelial markers like CD31, the expression of mesenchymal markers like FSP-1 and SMA under the stimulation of TGFβ, a growth factor abundantly present within tumor microenvironment [57].
Although the relative contribution of each of these models in carcinogenesis needs to be further elucidated, it is worth notice that the models described above are not mutually exclusive either for a given cancer type or for CAFs constituting the stroma of a single tumor.
Role of CAFs in cancer progression
Secretion of GFs, cytokines and proteases
CAFs directly stimulate tumor cell proliferation by contributing various growth factors, hormones and cytokines (Figure 1). Classical mitogens for epithelial cancer cells, such as hepatocyte growth factor (HGF), epidermal growth factor (EGF), b-FGF, as well as cytokines such as SDF-1 and IL-6, are all vastly expressed by CAFs contacting different tumor types. For example, CAFs obtained from lung cancer tissue produced HGF, thereby activating the c-Met pathway in neighbouring cancer cells. Of note, the secretion of HGF by CAFs leads to resistance of lung cancer cells to conventional tyrosine kinase inhibitors against EGF receptor and blocking antibodies against HGF circumvent this acquired resistance [58]. In addition, HGF secreted by CAFs enhanced secretion of uPA and uPAR in breast cancer cells. SU11274, an inhibitor of c-Met decreased both the invasion of the cancer cells and secretion of urokinase type plasminogen activator (uPA) and its receptor (uPAR), thereby validating the anti-HGF treatment plans for antimetastatic purposes [59]. It has also been reported that several GFs secreted by cancer cells themselves act on neighbouring CAFs leading them to activation and secretion of HGF [60]. In particular, cancer cells may affect HGF secretion by CAFs through interleukin IL-1β, IL-6 [23], prostaglandins [61], PDGF-β, or β-FGF. Interestingly, secretion of TGF -β1 by cancer cells suppresses HGF expression in CAFs [62].
Beside growth factors, pro-inflammatory cytokines, such as interleukins, interferons and members of the tumor necrosis factor family, are produced both by stromal and cancer cells, and exert tumor-modulating effects [4]. Expression by CAFs of cytokines and chemokines leads to immune cell infiltration that in turn promotes angiogenesis and metastasis [63]. Fibroblast-derived SDF-1 enhanced invasiveness of pancreatic cancer cells, showing a synergy with IL-8 in the promotion of a complete angiogenic response in recruited endothelial cells [64]. SDF-1 secreted by breast cancer CAFs has been involved in mobilization of endothelial precursor cells from bone marrow, thereby inducing de novo angiogenesis, as well as in tumor growth through a paracrine effect on CXCR4 expressing cancer cells [65]. In addition, increased secretion of CXCL14 chemokine by CAFs has been reported in prostate cancer stromal fibroblasts. CXCL14 increases both growth and migration of fibroblasts, which in turn increased their activity on tumor cells affecting their growth, angiogenesis and macrophage infiltration [66]. In a similar manner, CCL7 secreted by CAFs associated to oral squamous cell carcinoma leads to paracrine IL-1α secretion from cancer cells [67]. A recent paper demonstrated that CAFs associated to incipient neoplasia exhibit a pro-inflammatory signature, leading them to mainly overexpress SDF-1, IL-6 and IL-1β, as well as to recruit proangiogenic macrophages and promote tumor growth. This gene set is under the transcriptional control of nuclear factor-κB (NF-κB) and cyclooxygenase 2 (COX-2), thereby strengthening the link between CAFs and inflammatory mediators in tumor progression [68]. Furthermore, in breast adenocarcinoma CAFs have been found affected by oxidative stress-mediated activation of hypoxia-inducible factor-1 (HIF-1), which in turn activates the secretion of SDF-1 [31]. Interestingly we have recently reported that in prostate carcinoma CAF contact leads cancer cells to activate the same pro-inflammatory gene signature (NF-κB, COX-2 and HIF-1), leading them to achieve a motile phenotype, and confirming that stromal and tumor cells share common key pathways during tumor progression [31].
CAFs are also able to secrete plasminogen activators as well as several members of the MMP family. These enzymes may be exploited essentially for two purposes: 1) direct degradation of ECM, obviously associated with tumor expansion, invasion and angiogenesis, 2) cleavage of growth factors, pro-inflammatory cytokines and their receptors, commonly associated with their activation, or cleavage of cell adhesion molecules, leading to increase motility and epithelial -mesenchymal transition (EMT) [69,70]. Expression of tumor (MMP -1, -2 and -14) and stromal (MMP -9, -13 and -14) matrix metallo-proteinases is mandatory for squamous cell carcinoma progression [71]. MMP-13 secreted by CAFs promotes tumor angiogenesis by releasing vascular endothelial growth factor (VEGF) from ECM, thereby leading to increased invasion of squamous cell carcinoma or in melanoma [72]. We have recently reported that in prostate carcinoma CAFs secrete large amount of MMP-2 and MMP-9, which in turn induce a clear EMT in prostate carcinoma cells, likely through E-cadherin downregulation [23]. Cancer cells close the circuitry engaged with their CAFs through secretion of IL-6, the main responsible of activation of stroma fibroblasts in prostate cancers. Of note, sensitivity of CAFs to IL-6 has been associated with senescence, a known prognostic factor for prostate cancer aggressiveness [73].
Beside MMPs, CAFs from colon and breast carcinoma express uPA and its receptor uPAR, key components of the activation of plasminogen to the serine protease plasmin [74]. In ovarian carcinoma cancer cells engage with their stromal fibroblasts a specific feed-forward loop mediated by cancer cell-derived paracrine bFGF and EGF inducing uPA transcription in the fibroblasts. In turn the serine protease system helps in activating these paracrine factors from tumor cell surface/matrix [75].
Regulation of motility and stemness
Cancer progression towards a malignant state involves the achievement of ability to invade surrounding tissues through extensive remodeling of the surrounding ECM, therefore seeding metastases elsewhere [76]. Clinical and experimental data sustain the hypothesis that CAFs regulate cell motility and the metastatic spread towards secondary organs. The cancer cell-derived efferent signals triggers a stromal response initiating a vicious cycle of paracrine afferent signals leading to tumor invasion and loss of tissue integrity (see Figure 1). Efferent signals that have an impact on fibroblast attraction, differentiation, proliferation and production of proinvasive signals have been identified as transient heterotypic cell-cell contacts or secreted growth factors, chemochines or lipid products as PDGF-α/β, TGFβ1, bFGF, IL-6, LPA, eicosanoids [22,77]. Once stromal cells are attracted, they differentiate into CAFs and elicit cancer cells exit from dormancy, affecting cancer cell motility and aggressiveness. Activated CAFs then induce invasive growth by cell-cell contacts or by paracrine diffusible signals. The afferent proinvasive growth signals that are identified by screening the secretome from MFs or CAFs include TGFβ, HGF, VEGF, FGF, SDF-1, as well as various types of protease activity, including matrix metalloproteases, cathepsins and plasminogen activators (Figure 1) [77,76].
The proinvasive activity of human CAFs in vitro was shown by De Wever et al. using human colon cancer cells and stroma fibroblasts isolated from surgical colon cancer fragments [78]. The involvement of soluble mediators has been suggested by the maintenance of this proinvasive activity in conditioned media from CAFs. A co-implantation tumor xenograft mouse model showed that CAFs are able to stimulate invasive growth of breast and colon cancer cells [78,65]. In vitro co-culture experiments showed that TGF-β secreted by colon, breast and squamous carcinoma cells modulates myofibroblast differentiation and promotes HGF dependent invasion [79,80]. A similar motogenic and pro-metastatic effect was also shown for CAFs isolated from surgical prostate carcinoma specimens [23]. In this model CAFs exert a very powerful metastatic spur as they prompt spontaneous lung metastases after heterotopic co-injection of cancer and stromal cells.
CAFs mainly contribute to the invasive and metastatic process by inducing EMT of tumor cells, a known epigenetic program leading cells to engage a motile and proteolytic phenotype [81,82]. In addition to the pro-migratory spur, EMT has also been correlated with the induction of a cancer stem cells phenotype. Indeed, in both breast and prostate cancer cells the generation of cancer stem cells has been shown to be driven by EMT through overexpression of Snail or Twist transcription factors [83,84]. In keeping with this, Giannoni reported that CAFs isolated by prostate carcinoma specimens, by means of activating the EMT epigenetic program, promote/select the generation of cancer stem cells [23]. Indeed CAFs affect clonogenicity and self-renewal ability of carcinoma cells, lead to increase in their expression of acknowledged cancer stem cell markers (CD133+ and high CD44/CD24 ratio) and the formation of non-adherent prostaspheres, a property associated with prostate stem cells [23,85,84,86]. Finally, the analysis of tumor-forming ability, as well as spontaneous lung metastasis formation of carcinoma cells after contact with CAFs, revealed that the diabolic interplay between cancer cells and their activated stroma contributes to generate a population of prostate cancer stem cells with defined ability to form primary tumors and distant metastases [23]. The role of CAFs in prostate cancer stem cell biology has been further stressed in a conditional Pten deletion mouse model of prostate adenocarcinoma [87]. Indeed, compared with mouse urogenital sinus mesenchyme or normal prostate fibroblasts, CAFs enhance spheroid formation, as well as prostatic glandular structures with lesions, high proliferative index and tumor-like histopathology.
Conversely to embryonic EMT, EMT serving tissue repair or metastatic dissemination is often correlated with tissue inflammation and persists until the provoking spur is eliminated [81]. In this context CAFs play a key role in sustaining a chronic pro-inflammatory stimulus, giving rise to multiple effects and culminating in prompting the escape from the hostile tumor microenvironment. In incipient tumors CAFs have been shown to orchestrate macrophage recruitment and neovascularization in strict dependence on NF-B activation [68,88]. In keeping with the idea that metastasis is a phenomenon reminiscent of the migratory/invasive behaviour of inflammatory cells, Giannoni reported that CAF-mediated EMT undergoing cells share with inflammatory cells the same signals. Indeed, CAFs exert their propelling role for EMT by eliciting pro -inflammatory pathways in metastatic cells, exploiting oxidative stress and involving activation of COX-2, NF-kB and HIF-1 [30].
In addition to epigenetic mechanisms influencing cross-signalling between CAFs and cancer cells, the motile spur may also be initiated by stromal mutations. Mouse prostate carcinoma cells induce upregulation of p53 in stromal CAFs, mainly through a paracrine mechanism [16], thereby creating a selective pressure leading to the expansion of a p53-lacking subpopulation of CAFs. These CAFs lacking p53 contribute to cancer invasion and to the eventual loss of p53 in the epithelium. Moreover, in several sporadic breast cancers CAFs carry p53 mutations and this is significantly associated with lymph-node metastases [89]. EMT induced by CAFs in cancer cells may be the main responsible for the achievement of mutations by tumor stroma. Indeed stromal-derived MMP-3, that is frequently upregulated in breast cancer, induces genomic instability through upregulation of reactive oxygen species (ROS) [49]. Alternatively, mutated stromal cells might directly derive from cancer cells that have undergone EMT and achieve the characteristics of CAFs [90].
Regulation of tumor metabolism
One of the main differences observed among cancer and normal cells is their glucose metabolism. Indeed, cancer cells primarily use glucose by aerobic glycolysis, producing lactate (the so-called Warburg effect), while normal cells completely catabolize glucose by oxidative phosphorylation [91]. The Warburg effect, coupled with increased glucose uptake due to incomplete glucose oxidation, facilitates in cancer/proliferating cells the efficient anabolism of macromolecules needed to construct a new cell from glicolytic intermediates [92]. The M1 or M2 splice isoforms of pyruvate kinase (PK), a key regulatory glycolytic enzyme, drives glucose metabolism towards aerobic glycolysis (PKM2) or oxidative phosphorylation (PKM1) [93]. Of note, all cancer cells studied to date exclusively express PKM2, an enzyme with lower catalytic activity with respect to PKM1, whereas cells in many normal differentiated tissues express PKM1 [93]. Recently Heiden clarifies that cancer cells uses PKM2 to short-circuit ATP production during glycolysis, thereby producing pyruvate for lactate production without ATP-mediated inhibition of glycolysis [94]. This landmark paper finally clarifies the molecular basis of the Warburg effect.
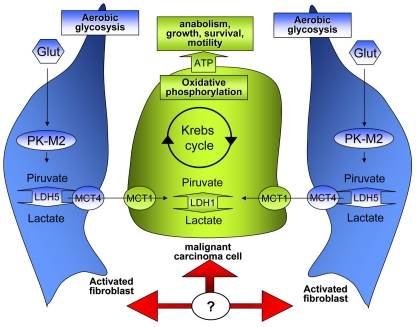
Stromal fibroblasts have been shown to participate in the complex metabolism of tumors, engaging a biunivocal relationship with cancer cells forcing them to respire and overcome energy depletion due to the Warburg effect. In particular, fibroblasts in contact with epithelial cancer cells undergo myo-fibroblast differentiation and produce lactate and pyruvate through aerobic glycolysis [95]. This “corrupted” stroma produces energy-rich metabolites which are used in cancer cells for TCA cycle and ATP production (Figure 3). Histopathological analysis reveals that PKM2 and lactate dehydrogenase are highly expressed in the stroma of breast cancer lacking caveolin-1 expression [95]. In addition, fibroblasts undergoing activation due to caveolin-1 deletion, or in response to down-regulation of caveolin-1 upon oxidative stress induced by contact with cancer cells, show a stabilization of HIF-1. The metabolic consequence in these CAFs is the HIF-1-mediated shift towards aerobic glycolysis and elimination of mitochondrial activity through mitofagy [96]. The advantages gained by cancer cells through CAFs contact are two: the upload of lactate/piruvate, i. e. energy rich metabolites to fuel their TCA cycle, and the protection from apoptosis induced by the hostile tumor microenvironment.
Figure 3.
Metabolic interplay between CAFs and cancer cells. Tumor cells are characterized by an high proliferation rate and consequently undergo a strong activation of anabolic pathways that allow rapid growing. In the model reported in this figure cancer cells force CAFs to undergo aerobic glycolysis, and to produce energy-rich nutrients (lactate or pyruvate) which are used in cancer cells for TCA cycle, ATP production, sustaining anabolism and growth.
Although these data are intriguing and pose the molecular basis for the role of CAFs in controlling the metabolism of tumor cells, for the moment they remain limited to CAFs activated by deletion/down-regulation of caveolin-1. In colorectal carcinoma, histological analyses suggested an opposite behaviour of the tumoral stroma. Indeed, the stroma infiltrating these tumors expresses aerobic glycolysis enzymes and the authors propose a role of these stromal cells to buffer and recycle products of anaerobic metabolism of cancer cells in order to sustain invasive cancer growth [97]. On the basis of these controversial findings, further confirmations in other experimental settings, as in ex-vivo CAFs, are therefore needed. However, in keeping with this role of CAFs as metabolic syn-ergistic bystanders, we recently observed that the contact between prostate CAFs and their carcinoma cells gives rise to an increase in their sensitivity to stresses as pH and hypoxia an sustains their proliferative rate (unpublished results).
Reorganization of tumor ECM
The tumor microenvironment is composed by both cellular and non-cellular components. The major cellular components include fibroblasts, endothelial and immune cells that, collectively, produce the variety of molecules that represent the non-cellular components of the tumor stroma. i.e. the extracellular matrix (ECM) proteins, proteases, cytokines and growth factors. Recent data are unveiling the relationship between the architecture of ECM and many important cell functions such as proliferation, differentiation and cancer [98,99]. The stiffness (or “compliance”) of the ECM affects cell morphology, integrin signalling, and the cellular actin cytoskeleton, thereby participating in the control of the cell cycle [100].
The ECM structure is profoundly altered in tumors and CAFs retain a major role in this process because they are the main responsible for production of ECM proteins, including collagens, fibronectin and many others, as well as proteases and other enzymes involved in post-transcriptional modification of ECM proteins themselves. Indeed, many cancers are associated with desmoplasia, a common fibrotic state, characterized by an accumulation of type I and III collagens, accompained by increased degradation of type IV collagen [101,102]. Of note, tumor desmoplasia has been associated with poor prognosis of cancers [103] and it is not exclusive of primary tumors, as it has been observed also in metastatic sites [104].
Tumor cells greatly affect their ECM, both from a quantitative point of view, leading progressively to increased matrix deposition, and qualitatively leading to a progressive stiffening of the 3D matrix. Both qualitative and quantitative changes in ECM have been reported to influence proliferation, survival and migration of cancer cells [105,106]. In particular, tumor fibrillar collagens are linearized and their maturation process is enhanced, as they show an increased number of covalent cross-link between collagen molecules [107]. Collagen crosslinking is predominantly catalyzed by lysyl oxidase (LOX), expressed in fibroblasts during the early stages of breast carcinogenesis, while in a later stage LOX is induced also in carcinoma cells exposed to hypoxic environment, a common feature in many aggressive cancers [108]. These over-matured collagen fibres produced by LOX activation positively affect tumor cell migration and invasion. Indeed, in a mouse model of breast carcinoma, the treatment with LOX inhibitors, leading to decrease of ECM crosslinks, prevents tissue stiffening and delay tumor progression [107]. It has been shown that ECM stiffening within tumor microenvironment, through activation of integrins and discoidin domain receptor-1 enhances growth factor-mediated cell migration, thereby contributing to the metastatic process [109,107].
Another important consequence of matrix rigidity in tumors is that it contributes, together with impaired vascular and lymphatic mesh, to increase interstitial fluid pressure. This phenomenon decreases drug delivery inside tumors contributing to reduce the efficacy of chemotherapy. In a mouse model of pancreatic adenocarcinoma the reduction of tumor-associated CAFs leads to an increased efficacy of chemotherapy, thereby suggesting a key role for CAFs also in intra-tumor drug delivery [110].
Besides collagen two other ECM members, mostly produced by fibroblasts and enhanced in CAFs, have been shown to play a role in tumor microenvironment: fibronectin and hyaluronan. Fibronectin mediates a wide variety of cellular interactions with ECM and plays important roles in cell adhesion, migration and growth [111]. It can be a ligand for a dozen members of the integrin receptor family [112], including the α5β1 receptor, and regulates collagen fibril structure [113]. Upon activation CAFs show enhanced expression of fibronectin, as well as de novo expression of its variant ED-A [6]. Stromal fibronectin is positively associated with human tumor metastatic potential and MMP secretion [104]. Indeed, up-regulation of fibronectin, together with tissue transglutaminase, facilitates the metastatic spreading of A431 tumor cells [114]. In addition, fibronectin regulates ovarian cancer metastatic potential by promoting a ligand-independent activation of the c-Met proto-oncogene through binding to its α5β1 receptor [115]. Finally, increased expression of hyaluronan by intratumoral fibroblasts plays a key role in the recruitment of tumor-associated macrophages, key regulators of tumor progression through CAF and endothelial cell recruitment [116].
Remodelling of the ECM by proteases conceivably enhances tumor invasion and metastasis by dissolving contacts between tumor cells and adjacent cells or matrix and by creating a physical path through degradation of the ECM. CAFs may also serve as guidance structures that direct the migration of epithelial cancer cells. Imaging of collective migration of squamous cell carcinoma cells and CAF demonstrated that CAFs behave as leading cells, degrading ECM and creating the path for cancer cells, moving within these tracks [117].
Preparation of metastatic niche
It is becoming increasingly clear that only a minority of malignant cells undertakes the metastatic route, and, of those, an even smaller fraction succeeds in this task [118]. Evidence also exists that these cells may share some properties with somatic or embryonic stem cells, which has led to the hypothesis that metastases are initiated by cell clones endowed of unique self-renewal and tumor propagating capacity like cancer stem cells [119]. In keeping with this idea CAFs within the primary tumors elicit achievement of stem-like traits (see above, [23]). Of note, the role of CAFs embraces also the preparation of the metastatic site in which the secondary tumor will grow up. It has been known since many years that metastatic cancer cells preferentially grow in secondary sites with particular and selected microenvironment [120]. Recently Duda et al. reported that metastatic cells bring their own soil, i. e. CAFs originating from the primary tumors, to the lung in order to modify the metastatic site and to colonize it [121]. The first effects that CAFs exert while they are circulating in the bloodstream are the protection of cancer cells from apoptosis. Once arrived in the metastatic niche the co-traveling CAFs provide and early growth advantage to the accompanying cancer cells that do not enter dormancy [121]. In addition prostate cancer and fibroblasts resident in the metastatic niche co-evolve, thereby accelerating growth of metastatic colonies [122]. It is therefore feasible that metastatic cells affect reactivity of these tissue resident stromal fibroblasts in a similar manner to the biunivocal interplay engaged within the primary tumor. In keeping with this, metastatic cells induce activation of resident fibroblasts and expression of ECM proteins and chemokines [122].
Therapeutic implications
Given the essential contribution of stroma in cancer progression, CAFs have recently emerged in recent years as new interesting therapeutic targets. The main reasons for choosing CAFs are targets are: i) CAFs are genetically stable, in comparison to cancer cells, and this is a guarantee for maintaining them sensitive to drugs; ii) CAFs are the main responsible for the structure of tumor ECM (collagens, proteoglycans, glycosaminoglycans, etc) that hampers the diffusion of anti-cancer agents through solid tumours; iii) The crosstalk between CAFs and tumor cells favours the survival, the proliferation and the invasive features of cancer cells.
Tumor stroma-directed therapies can target the growth factors/cytokines that mediate this crosstalk within tumor microenvironment promoting an anti-cancer effect. In a mouse model of cervical carcinogenesis, the block of PDGF receptor signaling in CAFs inhibits progression of premalignant lesions [123] while overexpression of trombospondin-1, an angiogenesis inhibitor, has been shown to inhibit cervical tumor growth accompanied by a decrease of two markers of CAFs, αSMA and desmin [124]. Pre-clinical studies using NK4, a competitive antagonist of Met, as well as anti-HGF monoclonal antibodies, showed a remarkable inhibition of tumor growth and metastasis [125,126]. In addition, inhibition of stromal cell proliferation in a pancreatic cancer model, using a specific inhibitor of the Hedgehog receptor, allows improved delivery of gemcitabine to the tumor and increased survival [110].
Interestingly, CAF-directed therapy can be used also to overcome loss of effects of VEGF inhibitors. In fact, it has been shown that PDGF-C produced by CAFs is able to elicit VEGF production of tumor cells, thereby sustaining the angiogenic shift. Hence, antibodies against PDGF-C can be used in order to inhibit angiogenesis in tumor refractory to anti VEGF treatment [127].
COX-2 is a protein involved in the inflammatory response whose expression is markedly increased when fibroblasts are co-cultured with cancer cells [128]. Up-regulation of COX-2 in a mouse xenograft model resulted in increased VEGF and MMP14 expression, which contribute to cancer progression and invasion of COX-2-expressing tumors [129]. The involvement of COX-2 in CAF-tumor interplay has been also reported for prostate carcinoma malignancy through EMT [23]. Along this line of evidence, anti-inflammatory drugs as selective COX-2 inhibitors as celecoxib or refecoxib, represent a promising therapeutic tool for targeting CAF-induced effects on tumor progression.
Another promising approach is the inhibition of CAF MMT in order to eliminate the efferent way affecting cancer aggressiveness. In vitro myofibroblast differentiation from hepatic stellate cells was halted by inhibition of DNA methyl -tranferase 1 by 5-aza-2 -deoxycytidine [130], while a monoclonal Ab against FAP, a protein involved in the MMT process, is extremely active in clinical trials [131]. Similarly, Cat et al reported that MMT of skin cancer fibroblasts in response to TGF-β is abolished by antioxidant treatment using trolox or selenite [28]. Again, MMT inhibition is effective in inhibiting skin cancer progression towards the most aggressive phenotype.
In conclusion, the large range of therapeutic approaches that are emerging from basic and applied research, are promising and encouraging. Unfortunately they need to be focused for each tumor histotype, in the light of the variability of the role played by CAFs in each tumor histotype, as well as considering the specific tissue source of their CAFs. Hence, every future therapy directed against CAFs, or tumor stroma in general, will be more effective in the presence of exhaustive studies on CAF taxonomy and accurate and detailed comprehension or CAF-tumor relationship.
References
- 1.Gabbiani G, Ryan GB, Majne G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia. 1971;27:549–550. doi: 10.1007/BF02147594. [DOI] [PubMed] [Google Scholar]
- 2.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mech-ano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 3.Desmouliere A, Redard M, Darby I, Gabbiani G. Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol. 1995;146:56–66. [PMC free article] [PubMed] [Google Scholar]
- 4.Rasanen K, Vaheri A. Activation of fibroblasts in cancer stroma. Exp Cell Res. 2010;316:2713–2722. doi: 10.1016/j.yexcr.2010.04.032. [DOI] [PubMed] [Google Scholar]
- 5.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 6.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 7.Pietras K, Ostman A. Hallmarks of cancer: interactions with the tumor stroma. Exp Cell Res. 2010;316:1324–1331. doi: 10.1016/j.yexcr.2010.02.045. [DOI] [PubMed] [Google Scholar]
- 8.Micke P, Ostman A. Tumour-stroma interaction: cancer-associated fibroblasts as novel targets in anti-cancer therapy? Lung Cancer. 2004;45(Suppl 2):S163–S175. doi: 10.1016/j.lungcan.2004.07.977. [DOI] [PubMed] [Google Scholar]
- 9.O'Brien P, O'Connor BF. Seprase: an overview of an important matrix serine protease. Biochim Biophys Acta. 2008;1784:1130–1145. doi: 10.1016/j.bbapap.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 10.Nakao M, Ishii G, Nagai K, Kawase A, Kenmotsu H, Kon-No H, Hishida T, Nishimura M, Yoshida J, Ochiai A. Prognostic significance of carbonic anhydrase IX expression by cancer-associated fibroblasts in lung adeno-carcinoma. Cancer. 2009;115:2732–2743. doi: 10.1002/cncr.24303. [DOI] [PubMed] [Google Scholar]
- 11.Utispan K, Thuwajit P, Abiko Y, Charngkaew K, Paupairoj A, Chauin S, Thuwajit C. Gene expression profiling of cholangiocarcinoma-derived fibroblast reveals alterations related to tumor progression and indicates periostin as a poor prognostic marker. Mol Cancer. 2010;9:13. doi: 10.1186/1476-4598-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hasebe T, Tamura N, Okada N, Hojo T, Akashi-Tanaka S, Shimizu C, Tsuda H, Shibata T, Sasajima Y, Iwasaki M, Kinoshita T. p53 expression in tumor-stromal fibroblasts is closely associated with the nodal metastasis and outcome of patients with invasive ductal carcinoma who received neoadjuvant therapy. Hum Pathol. 2010;41:262–270. doi: 10.1016/j.humpath.2009.07.021. [DOI] [PubMed] [Google Scholar]
- 13.Trimboli AJ, Cantemir-Stone CZ, Li F, Wallace JA, Merchant A, Creasap N, Thompson JC, Caserta E, Wang H, Chong JL, Naidu S, Wei G, Sharma SM, Stephens JA, Fernandez SA, Gurcan MN, Weinstein MB, Barsky SH, Yee L, Rosol TJ, Stromberg PC, Robinson ML, Pepin F, Hallett M, Park M, Ostrowski MC, Leone G. Pten in stromal fibroblasts suppresses mammary epithelial tumours. Nature. 2009;461:1084–1091. doi: 10.1038/nature08486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Witkiewicz AK, Dasgupta A, Sotgia F, Mercier I, Pestell RG, Sabel M, Kleer CG, Brody JR, Lisanti MP. An absence of stromal caveolin-1 expression predicts early tumor recurrence and poor clinical outcome in human breast cancers. Am J Pathol. 2009;174:2023–2034. doi: 10.2353/ajpath.2009.080873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamanashi T, Nakanishi Y, Fujii G, Akishima-Fukasawa Y, Moriya Y, Kanai Y, Watanabe M, Hirohashi S. Podoplanin expression identified in stromal fibroblasts as a favorable prognostic marker in patients with colorectal carcinoma. Oncology. 2009;77:53–62. doi: 10.1159/000226112. [DOI] [PubMed] [Google Scholar]
- 16.Hill R, Song Y, Cardiff RD, van DT. Selective evolution of stromal mesenchyme with p53 loss in response to epithelial tumorigenesis. Cell. 2005;123:1001–1011. doi: 10.1016/j.cell.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 17.Kiaris H, Chatzistamou I, Trimis G, Frangou-Plemmenou M, Pafiti-Kondi A, Kalofoutis A. Evidence for nonautonomous effect of p53 tumor suppressor in carcinogenesis. Cancer Res. 2005;65:1627–1630. doi: 10.1158/0008-5472.CAN-04-3791. [DOI] [PubMed] [Google Scholar]
- 18.Anderberg C, Pietras K. On the origin of cancer-associated fibroblasts. Cell Cycle. 2009;8:1461–1462. doi: 10.4161/cc.8.10.8557. [DOI] [PubMed] [Google Scholar]
- 19.Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofi-broblast: one function, multiple origins. Am J Pathol. 2007;170:1807–1816. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McAnulty RJ. Fibroblasts and myofibroblasts: their source, function and role in disease. IntJ Biochem Cell Biol. 2007;39:666–671. doi: 10.1016/j.biocel.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Ostman A, Augsten M. Cancer-associated fibroblasts and tumor growth–bystanders turning into key players. Curr Opin Genet Dev. 2009;19:67–73. doi: 10.1016/j.gde.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 22.De WO, Mareel M. Role of tissue stroma in cancer cell invasion. J Pathol. 2003;200(4):429–447. doi: 10.1002/path.1398. [DOI] [PubMed] [Google Scholar]
- 23.Giannoni E, Bianchini F, Masieri L, Serni S, Torre E, Calorini L, Chiarugi P. Reciprocal activation of prostate cancer cells and cancerassociated fibroblasts stimulates epithelialmesenchymal transition and cancer stemness. Cancer Res. 2010;70:6945–6956. doi: 10.1158/0008-5472.CAN-10-0785. [DOI] [PubMed] [Google Scholar]
- 24.Lohr M, Schmidt C, Ringel J, Kluth M, Muller P, Nizze H, Jesnowski R. Transforming growth factor-beta1 induces desmoplasia in an experimental model of human pancreatic carcinoma. Cancer Res. 2001;61:550–555. [PubMed] [Google Scholar]
- 25.Bronzert DA, Pantazis P, Antoniades HN, Kasid A, Davidson N, Dickson RB, Lippman ME. Synthesis and secretion of platelet-derived growth factor by human breast cancer cell lines. Proc Natl Acad Sci U S A. 1987;84:5763–5767. doi: 10.1073/pnas.84.16.5763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shao ZM, Nguyen M, Barsky SH. Human breast carcinoma desmoplasia is PDGF initiated. Oncogene. 2000;19:4337–4345. doi: 10.1038/sj.onc.1203785. [DOI] [PubMed] [Google Scholar]
- 27.Strutz F, Zeisberg M, Hemmerlein B, Sattler B, Hummel K, Becker V, Muller GA. Basic fibroblast growth factor expression is increased in human renal fibrogenesis and may mediate autocrine fibroblast proliferation. Kidney Int. 2000;57:1521–1538. doi: 10.1046/j.1523-1755.2000.00997.x. [DOI] [PubMed] [Google Scholar]
- 28.Cat B, Stuhlmann D, Steinbrenner H, Alili L, Holtkotter O, Sies H, Brenneisen P. Enhancement of tumor invasion depends on transdifferentiation of skin fibroblasts mediated by reactive oxygen species. J Cell Sci. 2006;119(Pt 13):2727–2738. doi: 10.1242/jcs.03011. [DOI] [PubMed] [Google Scholar]
- 29.Stuhlmann D, Steinbrenner H, Wendlandt B, Mitic D, Sies H, Brenneisen P. Paracrine effect of TGF-beta1 on downregulation of gap junctional intercellular communication between human dermal fibroblasts. Biochem Biophys Res Commun. 2004;319:321–326. doi: 10.1016/j.bbrc.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 30.Giannoni E, Bianchini F, Calorini L, Chiarugi P. Cancer associated fibroblasts exploit reactive oxygen species through a proinflammatory signature leading to emt and stemness. Antioxid Redox Signal. 2011 doi: 10.1089/ars.2010.3727. (in press) [DOI] [PubMed] [Google Scholar]
- 31.Toullec A, Gerald D, Despouy G, Bourachot B, Cardon M, Lefort S, Richardson M, Rigaill G, Parrini MC, Lucchesi C, Bellanger D, Stern MH, Dubois T, Sastre-Garau X, Delattre O, Vincent-Salomon A, Mechta-Grigoriou F. Oxidative stress promotes myofibroblast differentiation and tumour spreading. EMBO Mol Med. 2010;2:211–230. doi: 10.1002/emmm.201000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kojima Y, Acar A, Eaton EN, Mellody KT, Scheel C, Ben-Porath I, Onder TT, Wang ZC, Richardson AL, Weinberg RA, Orimo A. Autocrine TGF-beta and stromal cell-derived factor-1 (SDF-1) signaling drives the evolution of tumor-promoting mammary stromal myofibroblasts. Proc Natl Acad Sci U S A. 2010;107:20009–20014. doi: 10.1073/pnas.1013805107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gallagher PG, Bao Y, Prorock A, Zigrino P, Nischt R, Politi V, Mauch C, Dragulev B, Fox JW. Gene expression profiling reveals cross-talk between melanoma and fibroblasts: implications for host-tumor interactions in metastasis. Cancer Res. 2005;65:4134–4146. doi: 10.1158/0008-5472.CAN-04-0415. [DOI] [PubMed] [Google Scholar]
- 34.Buess M, Nuyten DS, Hastie T, Nielsen T, Pesich R, Brown PO. Characterization of heterotypic interaction effects in vitro to deconvolute global gene expression profiles in cancer. Genome Biol. 2007;8:R191. doi: 10.1186/gb-2007-8-9-r191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Denk PO, Hoppe J, Hoppe V, Knorr M. Effect of growth factors on the activation of human Tenon's capsule fibroblasts. Curr Eye Res. 2003;27:35–44. doi: 10.1076/ceyr.27.2.35.15456. [DOI] [PubMed] [Google Scholar]
- 36.Ronnov-Jessen L, Petersen OW. Induction of alpha-smooth muscle actin by transforming growth factor-beta 1 in quiescent human breast gland fibroblasts. Implications for myofibroblast generation in breast neoplasia. Lab Invest. 1993;68:696–707. [PubMed] [Google Scholar]
- 37.Kojima Y, Acar A, Eaton EN, Mellody KT, Scheel C, Ben-Porath I, Onder TT, Wang ZC, Richardson AL, Weinberg RA, Orimo A. Autocrine TGF-beta and stromal cell-derived factor-1 (SDF-1) signaling drives the evolution of tumor-promoting mammary stromal myofibroblasts. Proc Natl Acad Sci U S A. 2010;107:20009–20014. doi: 10.1073/pnas.1013805107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bergfeld SA, DeClerck YA. Bone marrowderived mesenchymal stem cells and the tumor microenvironment. Cancer Metastasis Rev. 2010;29:249–261. doi: 10.1007/s10555-010-9222-7. [DOI] [PubMed] [Google Scholar]
- 39.Dominici M, Le BK, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 40.Hung SC, Deng WP, Yang WK, Liu RS, Lee CC, Su TC, Lin RJ, Yang DM, Chang CW, Chen WH, Wei HJ, Gelovani JG. Mesenchymal stem cell targeting of microscopic tumors and tumor stroma development monitored by noninvasive in vivo positron emission tomography imaging. Clin Cancer Res. 2005;11:7749–7756. doi: 10.1158/1078-0432.CCR-05-0876. [DOI] [PubMed] [Google Scholar]
- 41.Kidd S, Spaeth E, Dembinski JL, Dietrich M, Watson K, Klopp A, Battula VL, Weil M, Andreeff M, Marini FC. Direct evidence of mesenchymal stem cell tropism for tumor and wounding microenvironments using in vivo bioluminescent imaging. Stem Cells. 2009;27:2614–2623. doi: 10.1002/stem.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hall B, Andreeff M, Marini F. The participation of mesenchymal stem cells in tumor stroma formation and their application as targeted-gene delivery vehicles. Handb Exp Pharmacol. 2007:263–283. doi: 10.1007/978-3-540-68976-8_12. [DOI] [PubMed] [Google Scholar]
- 43.Dwyer RM, Potter-Beirne SM, Harrington KA, Lowery AJ, Hennessy E, Murphy JM, Barry FP, O'Brien T, Kerin MJ. Monocyte chemotactic protein-1 secreted by primary breast tumors stimulates migration of mesenchymal stem cells. Clin Cancer Res. 2007;13:5020–5027. doi: 10.1158/1078-0432.CCR-07-0731. [DOI] [PubMed] [Google Scholar]
- 44.Spaeth E, Klopp A, Dembinski J, Andreeff M, Marini F. Inflammation and tumor microenvironments: defining the migratory itinerary of mesenchymal stem cells. Gene Ther. 2008;15:730–738. doi: 10.1038/gt.2008.39. [DOI] [PubMed] [Google Scholar]
- 45.Feng B, Chen L. Review of mesenchymal stem cells and tumors: executioner or coconspirator? Cancer Biother Radiopharm. 2009;24:717–721. doi: 10.1089/cbr.2009.0652. [DOI] [PubMed] [Google Scholar]
- 46.Spaeth EL, Dembinski JL, Sasser AK, Watson K, Klopp A, Hall B, Andreeff M, Marini F. Mesenchymal stem cell transition to tumorassociated fibroblasts contributes to fibrovascular network expansion and tumor progression. PLoS One. 2009;4:e4992. doi: 10.1371/journal.pone.0004992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kidd S, Spaeth E, Klopp A, Andreeff M, Hall B, Marini FC. The (in) auspicious role of mesenchymal stromal cells in cancer: be it friend or foe. Cytotherapy. 2008;10:657–667. doi: 10.1080/14653240802486517. [DOI] [PubMed] [Google Scholar]
- 48.Radisky DC, Kenny PA, Bissell MJ. Fibrosis and cancer: do myofibroblasts come also from epithelial cells via EMT? J Cell Biochem. 2007;101:830–839. doi: 10.1002/jcb.21186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Radisky DC, Levy DD, Littlepage LE, Liu H, Nelson CM, Fata JE, Leake D, Godden EL, Albertson DG, Nieto MA, Werb Z, Bissell MJ. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature. 2005;436:123–127. doi: 10.1038/nature03688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tuhkanen H, Anttila M, Kosma VM, Yla-Herttuala S, Heinonen S, Kuronen A, Juhola M, Tammi R, Tammi M, Mannermaa A. Genetic alterations in the peritumoral stromal cells of malignant and borderline epithelial ovarian tumors as indicated by allelic imbalance on chromosome 3p. Int J Cancer. 2004;109:247–252. doi: 10.1002/ijc.11733. [DOI] [PubMed] [Google Scholar]
- 51.Kurose K, Gilley K, Matsumoto S, Watson PH, Zhou XP, Eng C. Frequent somatic mutations in PTEN and TP53 are mutually exclusive in the stroma of breast carcinomas. Nat Genet. 2002;32:355–357. doi: 10.1038/ng1013. [DOI] [PubMed] [Google Scholar]
- 52.Moinfar F, Man YG, Arnould L, Bratthauer GL, Ratschek M, Tavassoli FA. Concurrent and independent genetic alterations in the stromal and epithelial cells of mammary carcinoma: implications for tumorigenesis. Cancer Res. 2000;60:2562–2566. [PubMed] [Google Scholar]
- 53.Allinen M, Beroukhim R, Cai L, Brennan C, Lahti-Domenici J, Huang H, Porter D, Hu M, Chin L, Richardson A, Schnitt S, Sellers WR, Polyak K. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell. 2004;6:17–32. doi: 10.1016/j.ccr.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 54.Qiu W, Hu M, Sridhar A, Opeskin K, Fox S, Shipitsin M, Trivett M, Thompson ER, Ramakrishna M, Gorringe KL, Polyak K, Haviv I, Campbell IG. No evidence of clonal somatic genetic alterations in cancer-associated fibroblasts from human breast and ovarian carcinomas. Nat Genet. 2008;40:650–655. doi: 10.1038/ng.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Medici D, Hay ED, Olsen BR. Snail and Slug promote epithelial-mesenchymal transition through beta-catenin-T-cell factor-4-dependent expression of transforming growth factor-beta3. Mol Biol Cell. 2008;19:4875–4887. doi: 10.1091/mbc.E08-05-0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zeisberg EM, Potenta S, Xie L, Zeisberg M, Kalluri R. Discovery of endothelial to mesenchymal transition as a source for carcinomaassociated fibroblasts. Cancer Res. 2007;67:10123–10128. doi: 10.1158/0008-5472.CAN-07-3127. [DOI] [PubMed] [Google Scholar]
- 58.Wang W, Li Q, Yamada T, Matsumoto K, Matsumoto I, Oda M, Watanabe G, Kayano Y, Nishioka Y, Sone S, Yano S. Crosstalk to stromal fibroblasts induces resistance of lung cancer to epidermal growth factor receptor tyrosine kinase inhibitors. Clin Cancer Res. 2009;15:6630–6638. doi: 10.1158/1078-0432.CCR-09-1001. [DOI] [PubMed] [Google Scholar]
- 59.Jedeszko C, Victor BC, Podgorski I, Sloane BF. Fibroblast hepatocyte growth factor promotes invasion of human mammary ductal carcinoma in situ. Cancer Res. 2009;69:9148–9155. doi: 10.1158/0008-5472.CAN-09-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Matsumoto K, Nakamura T. Hepatocyte growth factor and the Met system as a mediator of tumor-stromal interactions. Int J Cancer. 2006;119:477–483. doi: 10.1002/ijc.21808. [DOI] [PubMed] [Google Scholar]
- 61.Matsumoto K, Okazaki H, Nakamura T. Novel function of prostaglandins as inducers of gene expression of HGF and putative mediators of tissue regeneration. J Biochem. 1995;117:458–464. doi: 10.1093/jb/117.2.458. [DOI] [PubMed] [Google Scholar]
- 62.Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, Shappell S, Washington MK, Neilson EG, Moses HL. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303:848–851. doi: 10.1126/science.1090922. [DOI] [PubMed] [Google Scholar]
- 63.Gerber PA, Hippe A, Buhren BA, Muller A, Homey B. Chemokines in tumor-associated angiogenesis. Biol Chem. 2009;390:1213–1223. doi: 10.1515/BC.2009.144. [DOI] [PubMed] [Google Scholar]
- 64.Matsuo Y, Ochi N, Sawai H, Yasuda A, Takahashi H, Funahashi H, Takeyama H, Tong Z, Guha S. CXCL8/IL-8 and CXCL12/SDF-1alpha co-operatively promote invasiveness and angiogenesis in pancreatic cancer. Int J Cancer. 2009;124:853–861. doi: 10.1002/ijc.24040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 66.Augsten M, Hagglof C, Olsson E, Stolz C, Tsagozis P, Levchenko T, Frederick MJ, Borg A, Micke P, Egevad L, Ostman A. CXCL14 is an autocrine growth factor for fibroblasts and acts as a multi-modal stimulator of prostate tumor growth. Proc Natl Acad Sci U S A. 2009;106:3414–3419. doi: 10.1073/pnas.0813144106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jung DW, Che ZM, Kim J, Kim K, Kim KY, Williams D, Kim J. Tumor-stromal crosstalk in invasion of oral squamous cell carcinoma: a pivotal role of CCL7. Int J Cancer. 2010;127:332–344. doi: 10.1002/ijc.25060. [DOI] [PubMed] [Google Scholar]
- 68.Erez N, Truitt M, Olson P, Arron ST, Hanahan D. Cancer-Associated Fibroblasts Are Activated in Incipient Neoplasia to Orchestrate Tumor-Promoting Inflammation in an NFkappaB-Dependent Manner. Cancer Cell. 2010;17:135–147. doi: 10.1016/j.ccr.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 69.Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–1219. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Roy R, Yang J, Moses MA. Matrix metalloproteinases as novel biomarkers and potential therapeutic targets in human cancer. J Clin Oncol. 2009;27:5287–5297. doi: 10.1200/JCO.2009.23.5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vosseler S, Lederle W, Airola K, Obermueller E, Fusenig NE, Mueller MM. Distinct progression-associated expression of tumor and stromal MMPs in HaCaT skin SCCs correlates with onset of invasion. Int J Cancer. 2009;125:2296–2306. doi: 10.1002/ijc.24589. [DOI] [PubMed] [Google Scholar]
- 72.Lederle W, Hartenstein B, Meides A, Kunzelmann H, Werb Z, Angel P, Mueller MM. MMP13 as a stromal mediator in controlling persistent angiogenesis in skin carcinoma. Carcinogenesis. 2010;31:1175–1184. doi: 10.1093/carcin/bgp248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dean JP, Nelson PS. Profiling influences of senescent and aged fibroblasts on prostate carcinogenesis. Br J Cancer. 2008;98:245–249. doi: 10.1038/sj.bjc.6604087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Blasi F, Sidenius N. The urokinase receptor: focused cell surface proteolysis, cell adhesion and signaling. FEBS Lett. 2010;584:1923–1930. doi: 10.1016/j.febslet.2009.12.039. [DOI] [PubMed] [Google Scholar]
- 75.Noskova V, Ahmadi S, Asander E, Casslen B. Ovarian cancer cells stimulate uPA gene expression in fibroblastic stromal cells via multiple paracrine and autocrine mechanisms. Gynecol Oncol. 2009;115:121–126. doi: 10.1016/j.ygyno.2009.06.026. [DOI] [PubMed] [Google Scholar]
- 76.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.De WO, Demetter P, Mareel M, Bracke M. Stromal myofibroblasts are drivers of invasive cancer growth. Int J Cancer. 2008;123:2229–2238. doi: 10.1002/ijc.23925. [DOI] [PubMed] [Google Scholar]
- 78.De WO, Nguyen QD, Van HL, Bracke M, Bruyneel E, Gespach C, Mareel M. Tenascin-C and SF/HGF produced by myofibroblasts in vitro provide convergent pro-invasive signals to human colon cancer cells through RhoA and Rac. FASEB J. 2004;18:1016–1018. doi: 10.1096/fj.03-1110fje. [DOI] [PubMed] [Google Scholar]
- 79.Casey TM, Eneman J, Crocker A, White J, Tessitore J, Stanley M, Harlow S, Bunn JY, Weaver D, Muss H, Plaut K. Cancer associated fibroblasts stimulated by transforming growth factor beta1 (TGF-beta 1) increase invasion rate of tumor cells: a population study. Breast Cancer Res Treat. 2008;110:39–49. doi: 10.1007/s10549-007-9684-7. [DOI] [PubMed] [Google Scholar]
- 80.Lewis MP, Lygoe KA, Nystrom ML, Anderson WP, Speight PM, Marshall JF, Thomas GJ. Tumour-derived TGF-beta1 modulates myofibroblast differentiation and promotes HGF/SFdependent invasion of squamous carcinoma cells. Br J Cancer. 2004;90:822–832. doi: 10.1038/sj.bjc.6601611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kalluri R. EMT: when epithelial cells decide to become mesenchymal-like cells. J Clin Invest. 2009;119:1417–1419. doi: 10.1172/JCI39675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 83.Blick T, Hugo H, Widodo E, Waltham M, Pinto C, Mani SA, Weinberg RA, Neve RM, Lenburg ME, Thompson EW. Epithelial mesenchymal transition traits in human breast cancer cell lines parallel the CD44(hi/)CD24 (lo/-) stem cell phenotype in human breast cancer. J Mammary Gland Biol Neoplasia. 2010;15:235–252. doi: 10.1007/s10911-010-9175-z. [DOI] [PubMed] [Google Scholar]
- 84.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA. The epithelialmesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Klarmann GJ, Hurt EM, Mathews LA, Zhang X, Duhagon MA, Mistree T, Thomas SB, Farrar WL. Invasive prostate cancer cells are tumor initiating cells that have a stem cell-like genomic signature. Clin Exp Metastasis. 2009;26:433–446. doi: 10.1007/s10585-009-9242-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8(10):755–768. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 87.Liao CP, Adisetiyo H, Liang M, Roy-Burman P. Cancer-associated fibroblasts enhance the gland-forming capability of prostate cancer stem cells. Cancer Res. 2010;70:7294–7303. doi: 10.1158/0008-5472.CAN-09-3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wu Y, Deng J, Rychahou PG, Qiu S, Evers BM, Zhou BP. Stabilization of snail by NFkappaB is required for inflammation-induced cell migration and invasion. Cancer Cell. 2009;15:416–428. doi: 10.1016/j.ccr.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Patocs A, Zhang L, Xu Y, Weber F, Caldes T, Mutter GL, Platzer P, Eng C. Breast-cancer stromal cells with TP53 mutations and nodal metastases. N Engl J Med. 2007;357:2543–2551. doi: 10.1056/NEJMoa071825. [DOI] [PubMed] [Google Scholar]
- 90.De WO, Pauwels P, De CB, Sabbah M, Emami S, Redeuilh G, Gespach C, Bracke M, Berx G. Molecular and pathological signatures of epithelial-mesenchymal transitions at the cancer invasion front. Histochem Cell Biol. 2008;130:481–494. doi: 10.1007/s00418-008-0464-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jones RG, Thompson CB. Tumor suppressors and cell metabolism: a recipe for cancer growth. Genes Dev. 2009;23:537–548. doi: 10.1101/gad.1756509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, Fleming MD, Schreiber SL, Cantley LC. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452:230–233. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- 94.Vander Heiden MG, Locasale JW, Swanson KD, Sharfi H, Heffron GJ, Amador-Noguez D, Christofk HR, Wagner G, Rabinowitz JD, Asara JM, Cantley LC. Evidence for an alternative glycolytic pathway in rapidly proliferating cells. Science. 2010;329:1492–1499. doi: 10.1126/science.1188015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pavlides S, Whitaker-Menezes D, Castello-Cros R, Flomenberg N, Witkiewicz AK, Frank PG, Casimiro MC, Wang C, Fortina P, Addya S, Pestell RG, Martinez-Outschoorn UE, Sotgia F, Lisanti MP. The reverse Warburg effect: aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle. 2009;8:3984–4001. doi: 10.4161/cc.8.23.10238. [DOI] [PubMed] [Google Scholar]
- 96.Martinez-Outschoorn UE, Trimmer C, Lin Z, Whitaker-Menezes D, Chiavarina B, Zhou J, Wang C, Pavlides S, Martinez-Cantarin MP, Capozza F, Witkiewicz AK, Flomenberg N, Howell A, Pestell RG, Caro J, Lisanti MP, Sotgia F. Autophagy in cancer associated fibroblasts promotes tumor cell survival: Role of hypoxia, HIF1 induction and NFkappaB activation in the tumor stromal microenvironment. Cell Cycle. 2010;9:3515–3533. doi: 10.4161/cc.9.17.12928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Koukourakis MI, Giatromanolaki A, Harris AL, Sivridis E. Comparison of metabolic pathways between cancer cells and stromal cells in colorectal carcinomas: a metabolic survival role for tumor-associated stroma. Cancer Res. 2006;66:632–637. doi: 10.1158/0008-5472.CAN-05-3260. [DOI] [PubMed] [Google Scholar]
- 98.Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 99.Georges PC, Janmey PA. Cell type-specific response to growth on soft materials. J Appl Physiol. 2005;98:1547–1553. doi: 10.1152/japplphysiol.01121.2004. [DOI] [PubMed] [Google Scholar]
- 100.Assoian RK, Klein EA. Growth control by intracellular tension and extracellular stiffness. Trends Cell Biol. 2008;18:347–352. doi: 10.1016/j.tcb.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kauppila S, Stenback F, Risteli J, Jukkola A, Risteli L. Aberrant type I and type III collagen gene expression in human breast cancer in vivo. J Pathol. 1998;186:262–268. doi: 10.1002/(SICI)1096-9896(1998110)186:3<262::AID-PATH191>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 102.Huijbers IJ, Iravani M, Popov S, Robertson D, Al-Sarraj S, Jones C, Isacke CM. A role for fibrillar collagen deposition and the collagen internalization receptor endo180 in glioma invasion. PLoS One. 2010;5:e9808. doi: 10.1371/journal.pone.0009808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hasebe T, Sasaki S, Imoto S, Mukai K, Yokose T, Ochiai A. Prognostic significance of fibrotic focus in invasive ductal carcinoma of the breast: a prospective observational study. Mod Pathol. 2002;15:502–516. doi: 10.1038/modpathol.3880555. [DOI] [PubMed] [Google Scholar]
- 104.Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, Zhu Z, Hicklin D, Wu Y, Port JL, Altorki N, Port ER, Ruggero D, Shmelkov SV, Jensen KK, Rafii S, Lyden D. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chun TH, Hotary KB, Sabeh F, Saltiel AR, Allen ED, Weiss SJ. A pericellular collagenase directs the 3-dimensional development of white adipose tissue. Cell. 2006;125:577–591. doi: 10.1016/j.cell.2006.02.050. [DOI] [PubMed] [Google Scholar]
- 106.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA, Weaver VM. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 107.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, Yamauchi M, Gasser DL, Weaver VM. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Santhanam AN, Baker AR, Hegamyer G, Kirschmann DA, Colburn NH. Pdcd4 repression of lysyl oxidase inhibits hypoxiainduced breast cancer cell invasion. Oncogene. 2010;29:3921–3932. doi: 10.1038/onc.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zaman MH, Trapani LM, Sieminski AL, Mackellar D, Gong H, Kamm RD, Wells A, Lauffenburger DA, Matsudaira P. Migration of tumor cells in 3D matrices is governed by matrix stiffness along with cell-matrix adhesion and proteolysis. Proc Natl Acad Sci U S A. 2006;103:10889–10894. doi: 10.1073/pnas.0604460103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D, Frese KK, Denicola G, Feig C, Combs C, Winter SP, Ireland-Zecchini H, Reichelt S, Howat WJ, Chang A, Dhara M, Wang L, Ruckert F, Grutzmann R, Pilarsky C, Izeradjene K, Hingorani SR, Huang P, Davies SE, Plunkett W, Egorin M, Hruban RH, Whitebread N, McGovern K, Adams J, Iacobuzio-Donahue C, Griffiths J, Tuveson DA. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pankov R, Yamada KM. Fibronectin at a glance. J Cell Sci. 2002;115(Pt 20):3861–3863. doi: 10.1242/jcs.00059. [DOI] [PubMed] [Google Scholar]
- 112.Plow EF, Haas TA, Zhang L, Loftus J, Smith JW. Ligand binding to integrins. J Biol Chem. 2000;275:21785–21788. doi: 10.1074/jbc.R000003200. [DOI] [PubMed] [Google Scholar]
- 113.Velling T, Risteli J, Wennerberg K, Mosher DF, Johansson S. Polymerization of type I and III collagens is dependent on fibronectin and enhanced by integrins alpha 11beta 1 and alpha 2beta 1. J Biol Chem. 2002;277:37377–37381. doi: 10.1074/jbc.M206286200. [DOI] [PubMed] [Google Scholar]
- 114.Chen SH, Lin CY, Lee LT, Chang GD, Lee PP, Hung CC, Kao WT, Tsai PH, Schally AV, Hwang JJ, Lee MT. Up-regulation of fibronectin and tissue transglutaminase promotes cell invasion involving increased association with integrin and MMP expression in A431 cells. Anticancer Res. 2010;30:4177–4186. [PubMed] [Google Scholar]
- 115.Mitra AK, Sawada K, Tiwari P, Mui K, Gwin K, Lengyel E. Ligand-independent activation of c-Met by fibronectin and alpha(5)beta(1)-integrin regulates ovarian cancer invasion and metastasis. Oncogene. 2010 doi: 10.1038/onc.2010.532. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kobayashi N, Miyoshi S, Mikami T, Koyama H, Kitazawa M, Takeoka M, Sano K, Amano J, Isogai Z, Niida S, Oguri K, Okayama M, McDonald JA, Kimata K, Taniguchi S, Itano N. Hyaluronan deficiency in tumor stroma impairs macrophage trafficking and tumor neovascularization. Cancer Res. 2010;70:7073–7083. doi: 10.1158/0008-5472.CAN-09-4687. [DOI] [PubMed] [Google Scholar]
- 117.Gaggioli C, Hooper S, Hidalgo-Carcedo C, Grosse R, Marshall JF, Harrington K, Sahai E. Fibroblastled collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat Cell Biol. 2007;9:1392–1400. doi: 10.1038/ncb1658. [DOI] [PubMed] [Google Scholar]
- 118.Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–284. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 119.Tu SM, Lin SH, Logothetis CJ. Stem-cell origin of metastasis and heterogeneity in solid tumours. Lancet Oncol. 2002;3(8):508–513. doi: 10.1016/s1470-2045(02)00820-3. [DOI] [PubMed] [Google Scholar]
- 120.Psaila B, Lyden D. The metastatic niche: adapting the foreign soil. Nat Rev Cancer. 2009;9(4):285–293. doi: 10.1038/nrc2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Duda DG, Duyverman AM, Kohno M, Snuderl M, Steller EJ, Fukumura D, Jain RK. Malignant cells facilitate lung metastasis by bringing their own soil. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.1016234107. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sung SY, Hsieh CL, Law A, Zhau HE, Pathak S, Multani AS, Lim S, Coleman IM, Wu LC, Figg WD, Dahut WL, Nelson P, Lee JK, Amin MB, Lyles R, Johnstone PA, Marshall FF, Chung LW. Coevolution of prostate cancer and bone stroma in three-dimensional coculture: implications for cancer growth and metastasis. Cancer Res. 2008;68:9996–10003. doi: 10.1158/0008-5472.CAN-08-2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pietras K, Pahler J, Bergers G, Hanahan D. Functions of paracrine PDGF signaling in the proangiogenic tumor stroma revealed by pharmacological targeting. PLoS Med. 2008;5:e19. doi: 10.1371/journal.pmed.0050019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wu MP, Young MJ, Tzeng CC, Tzeng CR, Huang KF, Wu LW, Chou CY. A novel role of thrombospondin-1 in cervical carcinogenesis: inhibit stroma reaction by inhibiting activated fibroblasts from invading cancer. Carcinogenesis. 2008;29:1115–1123. doi: 10.1093/carcin/bgn077. [DOI] [PubMed] [Google Scholar]
- 125.Wen J, Matsumoto K, Taniura N, Tomioka D, Nakamura T. Hepatic gene expression of NK4, an HGF-antagonist/angiogenesis inhibitor, suppresses liver metastasis and invasive growth of colon cancer in mice. Cancer Gene Ther. 2004;11:419–430. doi: 10.1038/sj.cgt.7700705. [DOI] [PubMed] [Google Scholar]
- 126.Kim KJ, Wang L, Su YC, Gillespie GY, Salhotra A, Lal B, Laterra J. Systemic antihepatocyte growth factor monoclonal antibody therapy induces the regression of intracranial glioma xenografts. Clin Cancer Res. 2006;12:1292–1298. doi: 10.1158/1078-0432.CCR-05-1793. [DOI] [PubMed] [Google Scholar]
- 127.Crawford Y, Kasman I, Yu L, Zhong C, Wu X, Modrusan Z, Kaminker J, Ferrara N. PDGF -C mediates the angiogenic and tumorigenic properties of fibroblasts associated with tumors refractory to anti-VEGF treatment. Cancer Cell. 2009;15:21–34. doi: 10.1016/j.ccr.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 128.Sato N, Maehara N, Goggins M. Gene expression profiling of tumor-stromal interactions between pancreatic cancer cells and stromal fibroblasts. Cancer Res. 2004;64:6950–6956. doi: 10.1158/0008-5472.CAN-04-0677. [DOI] [PubMed] [Google Scholar]
- 129.Hu M, Peluffo G, Chen H, Gelman R, Schnitt S, Polyak K. Role of COX-2 in epithelialstromal cell interactions and progression of ductal carcinoma in situ of the breast. Proc Natl Acad Sci U S A. 2009;106:3372–3377. doi: 10.1073/pnas.0813306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mann J, Oakley F, Akiboye F, Elsharkawy A, Thorne AW, Mann DA. Regulation of myofibroblast transdifferentiation by DNA methylation and MeCP2: implications for wound healing and fibrogenesis. Cell Death Differ. 2007;14:275–285. doi: 10.1038/sj.cdd.4401979. [DOI] [PubMed] [Google Scholar]
- 131.Scott AM, Wiseman G, Welt S, Adjei A, Lee FT, Hopkins W, Divgi CR, Hanson LH, Mitchell P, Gansen DN, Larson SM, Ingle JN, Hoffman EW, Tanswell P, Ritter G, Cohen LS, Bette P, Arvay L, Amelsberg A, Vlock D, Rettig WJ, Old LJ. A Phase I dose-escalation study of sibrotuzumab in patients with advanced or metastatic fibroblast activation proteinpositive cancer. Clin Cancer Res. 2003;9:1639–164. [PubMed] [Google Scholar]