Abstract
To overcome problems of systemic toxicity associated with chemotherapy and enhance treatment resolution of cancer therapies, nanotechnology is increasingly providing many novel approaches, especially to energy-based cancer therapies. Enhancements to treatment targeting, the ability to facilitate combined therapies, and treatment imaging are but a few of the ongoing investigations in this ever growing field. This review briefly explores the modalities of energy-based cancer therapies, how nanotechnology has been allowed for improvements within them, and discusses potential future applications of combined therapies.
Keywords: Nanotechnology, energy-based therapies, nanoencapsulation, thermal ablation, combined modality therapy, drug delivery, chemotherapy, cryosurgery, tumor targeting, cancer treatment
1. Introduction
According to the American Cancer Society's 2010 statistical data, almost 1.5 million new cases of cancer were diagnosed in the United States and more than one third as many people were projected to succumb to their disease [1]. The data also lists cancer as the second leading cause of death behind heart disease with an expected total annual healthcare cost of $263.8 billion. Even though many advances have been made in cancer diagnosis and treatment, many of the current treatments still cause considerable harm and discomfort to the patient.
A common treatment, chemotherapy, attempts to systemically deliver anticancer agents to patients in order to eradicate the uncontrolled proliferation of cancer cells. Unfortunately, because of nonspecific targeting, healthy cells can also be damaged during the treatment. This systemic approach results in hair loss, pain, anemia, and other side effects [2, 3]. In addition to the toxicity problems associated with nonspecific systemic treatments, up to 50% of approved active molecules for cancer therapy have poor solubility in physiological conditions [4].
Another common treatment, radiation therapy, provides a more narrow treatment region, but still has side effects due to its indiscriminate nature. Moreover, this treatment modality has limited applications due to site specificity [5, 6]. However, using more direct methods such as the surgical removal of cancerous tissue may cause permanent disfigurement, is also location dependent, and may cause post operational infection or complications [3]. In an attempt to eliminate or reduce the disadvantages associated with traditional techniques, minimally invasive energy-based therapies are being investigated, with many clinical trials currently underway [7-11]. The purpose of this paper is to review nanotechnology enhancements to energy-based cancer therapies and discuss results that may be applicable for combined therapies.
2. Energy-based therapies
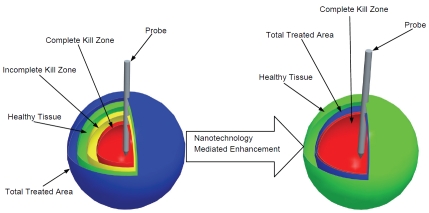
Some promising focus areas in energy-based therapy research are photodynamic, alternating magnetic field, microwave, radio frequency (RF), high intensity focused ultrasound (HIFU), and cryoablation therapies, each with their own advantages and disadvantages [8, 12-14]. An advantage of these methods over systemic treatments or surgical resection is a more localized destruction of diseased tissue while minimizing possible side effects such as systemic toxicity or infection. Also, these methods are considered minimally invasive and are primarily investigated as outpatient procedures. Energy-based therapies destroy tumor cells by causing a local temperature excursion within the designated treatment area. Commonly, this procedure is applied through a minimally invasive probe insertion technique or the focusing of external high energy sources. Although the individual implementation of these thermal ablation methodologies are different depending on the energy source, the fundamental therapeutic mechanisms for these therapies can be divided into two categories, damage from heating to hyper-thermic temperatures (usually > 43°C) or damage from cooling or freezing to cryothermic temperatures (usually < -20°C). The therapeutic benefit from both of these types of treatment are strongly temperature and time dependent with differing degrees of damage existing throughout a given treatment gradient, as shown in Figure 1 (left).
Figure 1.
Illustration of nanotechnology mediated enhancements that can improve energy-based cancer therapies. Classical energy-based treatments have limitations in treatment area visualization causing the total treated area to overlap with healthy tissue. Also, unquantified thermal distribution causes an uncertainty in the complete kill zone (left). By using nanotechnology mediated combined modality treatments, the total treatment area can be visualized to minimize healthy tissue overlap, and the complete kill zone can be expanded to the treated area edge.
In the complete kill zone, hyperthermic damage has been characterized by protein denaturation, cellular membrane damage, and vascular injury [12]. Alternatively, cryothermic damage has been characterized by mechanical damage from ice formation, cellular dehydration, ischemia from vascular damage, and post treatment immunological response [15]. Of the energy sources mentioned, all induce hyperthermic damage with the exception of cryoablation, which induces cryothermic damage. Although these methodologies have promising potential applications, they have problems that cannot be overlooked. Thermal ablation treatments are susceptible to uneven distribution of temperature profiles, and in the case of hyperthermic treatments, the treated area is not readily visible during the procedure and must be estimated from models or experimentation. Furthermore, the methods of implementation for the delivery of the thermal energy required for these treatments cause unintended damage to surrounding healthy tissue. In contrast, the iceball formed during cryothermic ablation treatment is visible through ultrasound or CT and easily tracked, but the determination of effectively treated area with temperature < -20 °C within the iceball is uncertain and must either be directly measured or estimated through models and experimentation [16-18]. The fluctuation in temperature gradient and uncertainty in treated area causes ablation treatments to be less specific than intended and in some cases possibly incomplete, as shown in Figure 1 (left) [7, 12, 19].
3. Adjuvants for energy-based therapy
To investigate enhancements to energy-based therapies, chemotherapeutic agents have been used as treatment adjuvants. It has been shown that both types of thermal ablation therapies, hyperthermic and cryothermic, have the potential to enhance the uptake of chemotherapeutic agents as well as induce a secondary immunological response that can enhance the extent of the removal of diseased tissue [15, 20-31]. Additionally, various salts, chemotherapeutic agents, and immunological factors have been tested for enhanced cryoablation treatment outcomes [15, 32-37].
While promising results and discoveries have been elucidated using adjuvants to enhance thermal ablation therapy, there are significant drawbacks associated with this treatment methodology that warrant further investigation. In particular, unquantified systemic toxicity, tumor specific targeting, and intratumoral drug distribution have left areas for improvements and research [38-55]. The focus of further research in this field has been to improve the treated area versus non-treated area by using nanotechnology as a resolution enhancing mechanism to expand the complete kill zone into the incomplete kill zone, sharply define treatment boundaries, and reduce the total treated area, as shown in Figure 1 (right).
4. Nanotechnology mediated enhancements to energy-based therapies
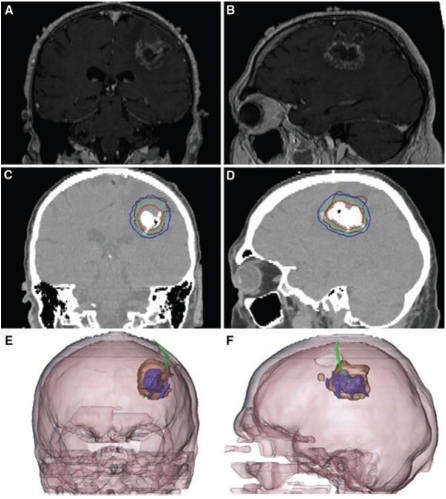
Over the past decade, nanotechnology has begun to be explored as a tool to increase the resolution of thermal ablation treatment area, tumor visualization, and improve treatment effectiveness [13, 14, 19]. The most direct method used for the enhancement of thermal therapy has been the systemic or local introduction of nanoparticles given concurrently with energy-based ablation treatments [56, 57]. For hyperthermic therapies, carbon nanotubes, gold nanoshells, and iron oxide nanoparticles have proven extremely useful for enhancing heating effects due to energy absorption by the nanoparticles during treatment [13, 19, 58]. Previous research has shown that the nanoparticles preferentially associate with tumors when given systemically or locally under the premise of the enhanced permeability and retention effect (EPR), which is often found in tumor vasculature [52, 53, 59-61]. Furthermore, the use of metallic or carbon nanoparticles as treatment adjuvants enables the treated region to be visualized through noninvasive means such as MRI and CT, as shown in Figure 2 [56].
Figure 2.
Imaging results from a preliminary study of locally delivered metallic nanoparticles in hyperthermic treatment of brain cancer: (A,B), pre-treatment brain MRI; (C,D), post nanoparticle delivery CT scan showing magnetic nanoparticle deposits as hyper-dense areas with the colored lines indicating calculated treatment temperatures between 40°C (blue) and 50°C (red) and the brown line representing the tumor area; and (E,F), 3-D reconstruction of fused MRI and CT showing the tumor (brown), magnetic fluid (blue) and thermometry catheter (green) [144].
To overcome problems with systemic toxicity and enable target specificity, nanocapsule carriers with targeting moieties have been investigated to preferentially deliver therapeutics to diseased tissue via cell surface receptors, as shown in Figure 3. Some cell surface receptors help transmit messages from the extracellular environment to the intracellular environment, and in many cancerous cells are overexpressed. Overexpression of these surface receptors and other similar hallmarks specific to cancer can serve as potential target areas due to their increased concentration in diseased tissue. Specifically, receptors to estrogen, folic acid, epidermal growth factor and others have been explored for potential treatment targets. Using the various cellular targeting moieties, preferential uptake of nanoparticles into target expressing cancer cells has been shown [23, 62-67].
Figure 3.
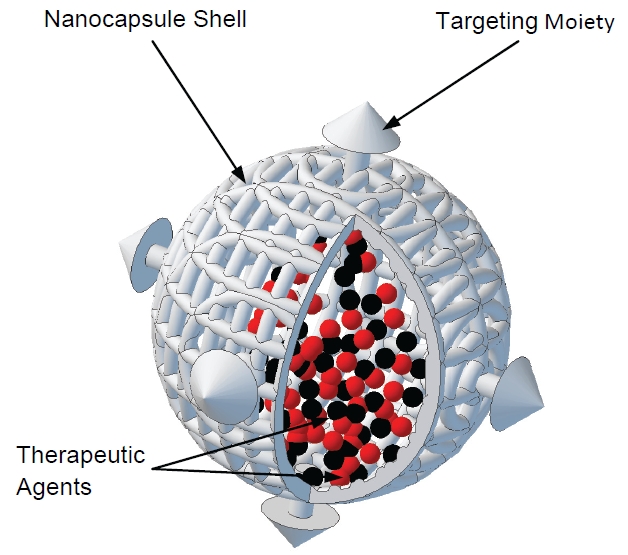
Illustration of nanocapsule containing therapeutic agents for targeted delivery to tumor cells. Nanoencapsulation technology has the potential to offer many combined modality approaches for treatment enhancements and personalized treatment targeting.
In addition to small molecular compound drugs, a Nobel-prize winning discovery of RNA interference (RNAi) has been extensively applied with the progress of delivery systems in several different experimental models and more recently in treatment of numerous diseases, including neurodegenerative disorders and cancer [68-72]. Small interfering RNAs (siRNAs) promote the cleavage of complementary mRNA to reduce protein production in mammalian cells and play a pivotal role in triggering RNAi [73, 74]. SiRNAs have short plasma half-life, fast degradation times in the physiological milieu, inefficient translocation into the cytoplasm, and lack of targeting ability. Therefore, successful siRNA-based gene targeting relies on the following conditions: improvement on stability and prevention of degradation by serum RNAses, efficient cellular uptake and subsequent intracellular release into the cytoplasm, as well as avoidance of intracellular immune responses, in vivo toxicity or rapid elimination in the liver or kidneys [73, 75-77].
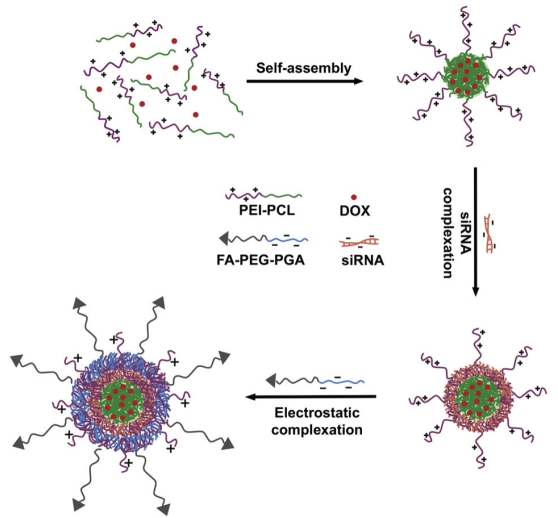
SiRNA, similar to DNA, carries a net negative charge on the sugar phosphate which prevents its contact and entrance to the lipid bilayer of the cell membrane, whose head groups are also negatively charged. In the early 1970s, Calcium phosphate (CaP) precipitates were used as transfection reagents of viral DNA as they are believed to be non-toxic [78]. CaP effectively protects the nucleic acids from enzymatic degradation and aided cellular delivery, but uncontrollable rapid growth of calcium phosphate crystals greatly reduced the transfection efficiency [79, 80]. To facilitate higher genocom-patibility and lower toxicity, non-viral delivery vectors became a good choice for gene-based therapies and in drug development. Non-viral delivery vectors include cationic lipids (e.g. DO-TAP and Oligofectamine), cationic polymers (e.g. PEI and DAB dendrimers) and non-ionic (uncharged) polymers (e.g. poly HPMA and PEG) [81-87]. Nanoparticles (NPs) such as the cationic polymer, polyethyleneimine (PEI), can act as envelopes to protect the siRNA from metabolism and excretion, but can also carry specific molecules designed to target the siRNA to specific tissue types. For example, hydrophobic DOX obtained by deprotonation accumulated in the PCL core of the cationic micelle assembled from PEI-PCL, as shown in Figure 4 [88]. More recently, gold nanoparticles were directly conjugated to siRNA, increasing the serum half-life more than six fold compared to free RNA duplexes [89]. Also, biodegradable nanoparticles have been developed and have shown good potential as carriers for anticancer drugs with a spherical structure [90].
Figure 4.
Formation of hierarchical nano-assemblies for combinatorial delivery of siRNA and anticancer drugs [88].
Within the past decade, the use of siRNA for RNAi has proven to be an effective nanomedicine for gene silencing therapy [91-96]. However, research into the delivery of siRNA via nanoparticles to target cells is still in its infancy. In cancer therapy, siRNA delivery via nanoparticles needs to satisfy two major concerns: to improve the therapeutic range by including more than one siRNA which acts on specific targets, but keep minimal toxicity and maximum patient safety; and to develop novel or modify established carrier systems to induce gene changes on siRNA mediated gene silencing, but avoid enhancing the off-target gene changes [97-99]. These emerging different new types of nanoparticles (biodegradable, gold, etc.) will facilitate the brilliance of RNAi and promote its application in clinical trials targeting specific tissues and diseases.
Recently, thermally responsive nanoencapsulation systems have been developed using temperature sensitive carriers designed to deliver chemotherapeutic agents preferentially to tumor sites. During the temperature change associated with energy-based treatment, a conformational or structural change in the delivery vehicle causes the release of chemotherapeutics from the carrier. Once released, therapeutic agents are free to diffuse away from their carrier and act on nearby targets with promising results [64, 100-114]. Additionally, as previously mentioned, the solubility of many chemotherapeutic substances in physiological conditions is very poor. Therefore, an added benefit of nanoencapsulation is the expansion of available chemotherapeutic agents that can be used for treatment. Moreover, an effect of energy-based treatments is enhanced uptake of chemotherapeutics possibly due to permeability changes. Utilizing the nanoparticle aided target delivery approach allows drugs released via a temperature controlled mechanism to be preferentially distributed at the tumor location with an increased uptake caused from the energy-based treatment. Use of nanoencapsulation technology also has the potential to reduce systemic toxicity because of localized delivery of agents to the treatment area for controlled release. Consequently, this combined treatment has the potential benefit of reducing the overall treatment area by allowing for an increase in the complete kill zone aided by chemotherapeutic agents [115, 116]. Preliminary results in animal studies for temperature sensitive carriers (liposomes) have prompted several currently ongoing clinical trials in various phases, I-III [117-121].
To provide additional improvements to the nanoparticle aided delivery methodology, facilitated drug release and treatment visualization, some experimental systems have co-encapsulated metallic nanoparticles alongside chemotherapeutic agents. This co-encapsulation paradigm allows metallic nanoparticles to act as agents for imaging and controlled release of chemotherapeutics through their energy absorbing properties [100, 101, 122-124]. Moreover, the delivery of metallic nanoparticles and chemotherapeutic agents simultaneously provide an approximated visualization of drug delivery localization and treatment area [125-129]. Therefore, this combined approach has the potential to reduce the total treatment area due to the energy absorbing properties of metallic nanoparticles, provide an increase to the complete kill zone from both targeted heating and chemotherapeutic agent delivery, and visual definition of treatment boundaries. However, more research into the development of this approach is necessary for clinical application to be realized, especially in the area of intratumoral nanoparticle distribution.
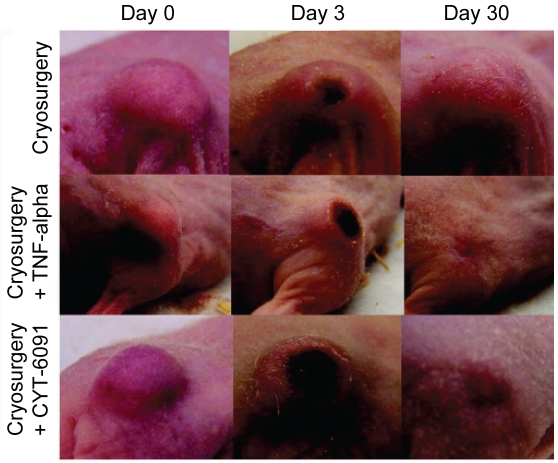
Exploration into immune response to enhance treatment has also been studied by conjugating TNF-α onto the surface of gold nanoparticles [130-135]. Results from animal model studies have shown a preferential biodistribution of gold -TNF-α within tumor locations with less systemic toxicity than free TNF-α. Furthermore, hyperthermic and cryothermic ablation treatment given after gold-TNF-α nanoparticle delivery increased the complete kill zone in animal models, as shown in Figure 5. From these initial studies, further tumor model applications and combinations with chemotherapeutic and co-encapsulation treatments are warranted [115].
Figure 5.
30 day observation of tumor size in mice treated with cryosurgery, TNF-α with cryosurgery, or gold-TNF-α nanoparticles (CYT-6091) with cryosurgery. CYT-6091 was found to have less systemic toxicity than free TNF-α and provided a similar benefit in tumor size reduction as the more toxic free TNF-α [135].
In addition to the nanoparticle mediated combined modality treatments, recent developments such as nanoscissor technology in conjunction with gene and gene product specific targeting and manipulation may bring about new areas of research focus for even more combined modality therapies with patient specific cancer targeting treatments [136-140]. Specifically, targeted DNA sequences have been manipulated through localized disruption by the utilization of the energy absorption properties of gold nanoparticles [136]. Furthermore, gold and polymeric nanoparticles have also been used for DNA/ oligonucleotide conjugation to regulate transcription and translation in cell models [137, 140]. Considering that this research has used energy absorption, metallic nanoparticles, and targeted delivery techniques similar to that used in previously mentioned research areas, it is not a far stretch to imagine that combined therapy applications with the correction or elimination of damaged DNA or initiation of apoptotic signaling through nanomanipulation techniques may be of future relevance. These techniques are still in their infancy and much more research and technical advancement is needed in order for this to become a practical and economic reality. However, the pace of advancement toward affordable and accessible gene research technology for potential treatment personalization applications is increasing rapidly [141].
While the majority of the advances made for nanotechnology derived delivery vehicles have been in the area of hyperthermic treatment, recent studies in our laboratories have focused on advancing cryoablation treatment using hypothermically responsive nanocapsules [142, 143]. The goal of this research has been to improve the effectiveness of cryoablation treatment by moving the complete kill zone closer to the edge of the ice ball (the total treated area) by releasing drugs from a nanocapsule carrier within the incomplete kill zone. If successful, the subsequent outcome of this treatment enhancement would yield a smaller ice ball needed to achieve a greater clinical response and thus less peripheral damage to adjacent tissues. As shown in Figure 6, our initial studies have shown that a thermally responsive nanocapsule system for delivery of chemotherapeutic agents during cryothermic ablation treatment is theoretically possible and further research in this area is warranted and ongoing.
Figure 6.

Cumulative release of ethidium bromide(EB) encapsulated in hypothermic temperature sensitive nanocapsules during dialysis. Before cold treatment, the nanocapsule solution was kept at 37°C to keep the encapsulated EB trapped within the capsule core to prevent release. After 3 hours, a cold treatment was given for 15 minutes to expand the nanocapsules and increase the wall permeability to allow the encapsulated EB to be liberated (released) out of the nanocapsule. As a result, a burst release of EB during dialysis is observable after the cold treatment [143].
The potential benefits offered by nanotechnology (target specificity, reduction of systemic toxicity for chemotherapeutics, and coencapsulation of adjuvants), bring nanoparticle mediated combined therapies to the forefront of potential enhancements to energy-based cancer therapies. Coupled with further understanding of host immune response and the possibility of patient specific treatments, nanoparticle mediated therapies can also provide the basis for many more interesting and novel treatment options previously not investigated. Further research into the nanoparticle mediated enhancements to energy-based therapies mentioned in this review should result in the final goal of expanding the complete kill zone while minimizing the total treatment area (or incomplete kill zone) and providing visualization of boundary zones needed to give energy-based therapies more clinical relevance and certainty, as shown in Figure 1 (right).
5. Summary and Conclusion
Through advanced understanding and application of functional nanomaterials, cellular targeting, and immune response, improvements in energy-based therapy have seen promising results. Furthermore, as gene sequencing technology advances to a much more affordable level, the personalization of nanotechnology derived delivery vehicles and therapeutics will make nanoparticle mediated combined therapies a much more focused and patient specific treatment option. Through further investigation and clinical trials, energy-based therapy assisted by nanotechnology may bring about a paradigm shift in primary cancer treatment in the not so distant future
Acknowledgments
This work was partially supported by a grant (to X.H.) from the Wendy Will Case Cancer Fund and a grant (to X.L.) from the American Cancer Society (119135-RSG-10-185-01-TBE).
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics, 2010. CA Cancer J Clin. 60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Schwartzberg LS. Chemotherapy-induced nausea and vomiting: Which antiemetic for which therapy? Oncology-New York. 2007;21:946–953. [PubMed] [Google Scholar]
- 3.American Cancer Society. Treatments and Side Effects. Accessed from: http://www.cancer.org/Treatment/TreatmentsandSideEffects/index on Jan. 27, 2011.
- 4.Chiappetta DA, Sosnik A. Poly(ethylene oxide)-poly(propylene oxide) block copolymer micelles as drug delivery agents: improved hydro-solubility, stability and bioavailability of drugs. Eur J Pharm Biopharm. 2007;66:303–317. doi: 10.1016/j.ejpb.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 5.McDonald MW, Fitzek MM. Proton Therapy. Current Problems in Cancer. 34:257–296. doi: 10.1016/j.currproblcancer.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 6.Halperin EC. Particle therapy and treatment of cancer. The Lancet Oncology. 2006;7:676–685. doi: 10.1016/S1470-2045(06)70795-1. [DOI] [PubMed] [Google Scholar]
- 7.Stauffer PR, Goldberg SN. Introduction: Thermal ablation therapy. International Journal of Hyperthermia. 2004;20:671–677. doi: 10.1080/02656730400007220. [DOI] [PubMed] [Google Scholar]
- 8.Timmerman RD, Bizekis CS, Pass HI, Fong Y, Dupuy DE, Dawson LA, Lu D. Local Surgical, Ablative, and Radiation Treatment of Metastases. CA Cancer J Clin. 2009;59:145–170. doi: 10.3322/caac.20013. [DOI] [PubMed] [Google Scholar]
- 9.Gannon WE. ClinicalTrials.gov [Internet] Bethesda (Md): National Library of Medicine (US); Combination Chemotherapy With or Without Microwave Thermotherapy Before Surgery in Treating Women With Locally Advanced Breast Cancer. 2000-[2011]. Available from http://clinicaltrials.gov/show/NCT00036985 NLM identifier: NCT00036985. [Google Scholar]
- 10.Gannon WE. ClinicalTrials.gov[Internet] Bethesda (Md): National Library of Medicine (US); Microwave Thermotherapy in Treating Women With Stage I or Stage II Breast Cancer. 2000-[2011]. Available from http://clinicaltrials.gov/show/NCT00036998 NLM identifier: NCT00036998. [Google Scholar]
- 11. ClinicalTrials.gov[Internet]. Bethesda (Md): National Library of Medicine (US). 2000-[2011]. Available from http://www.clinicaltrials.gov/ct2/results?term=ablation=cancer.
- 12.Shinohara K. Thermal ablation of prostate diseases: advantages and limitations. International Journal of Hyperthermia. 2004;20:679–697. doi: 10.1080/02656730412331286876. [DOI] [PubMed] [Google Scholar]
- 13.Manthe RL, Foy SP, Krishnamurthy N, Sharma B, Labhasetwar V. Tumor Ablation and Nanotechnology. Molecular Pharmaceutics. 2010;7:1880–1898. doi: 10.1021/mp1001944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moroz P, Jones SK, Gray BN. Magnetically mediated hyperthermia: current status and future directions. International Journal of Hyperthermia. 2002;18:267–284. doi: 10.1080/02656730110108785. [DOI] [PubMed] [Google Scholar]
- 15.Raghav G, Kyle A, Joel S, Franz S, Greg V, John B, John CB. Adjuvant Approaches to Enhance Cryosurgery. Journal of Biomechanical Engineering. 2009;131:074003. doi: 10.1115/1.3156804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Littrup PJ, Jallad B, Chandiwala-Mody P, D'Agostini M, Adam BA, Bouwman D. Cryotherapy for breast cancer: a feasibility study without excision. J Vasc Interv Radiol. 2009;20:1329–1341. doi: 10.1016/j.jvir.2009.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tatli S, Acar M, Tuncali K, Morrison PR, Silverman S. Percutaneous cryoablation techniques and clinical applications. Diagn Interv Radiol. 2010;16:90–95. doi: 10.4261/1305-3825.DIR.1922-08.0. [DOI] [PubMed] [Google Scholar]
- 18.Wang H, Littrup PJ, Duan Y, Zhang Y, Feng H, Nie Z. Thoracic masses treated with percutaneous cryotherapy: initial experience with more than 200 procedures. Radiology. 2005;235:289–298. doi: 10.1148/radiol.2351030747. [DOI] [PubMed] [Google Scholar]
- 19.Emily SD, Jennifer GM, Jennifer LW. Nanoparticles for Thermal Cancer Therapy. Journal of Biomechanical Engineering. 2009;131:074001. doi: 10.1115/1.3156800. [DOI] [PubMed] [Google Scholar]
- 20.Kong G, Braun RD, Dewhirst MW. Hyperthermia enables tumor-specific nanoparticle delivery: Effect of particle size. Cancer Research. 2000;60:4440–4445. [PubMed] [Google Scholar]
- 21.Issels RD. Hyperthermia adds to chemotherapy. European Journal of Cancer. 2008;44:2546–2554. doi: 10.1016/j.ejca.2008.07.038. [DOI] [PubMed] [Google Scholar]
- 22.Mir LM, Rubinsky B. Treatment of cancer with cryochemotherapy. British Journal of Cancer. 2002;86:1658–1660. doi: 10.1038/sj.bjc.6600306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahmed M, Goldberg SN. Combination ra-diofrequency thermal ablation and adjuvant IV liposomal doxorubicin increases tissue coagulation and intratumoural drug accumulation. International Journal of Hyperthermia. 2004;20:781–802. doi: 10.1080/02656730410001711655. [DOI] [PubMed] [Google Scholar]
- 24.Clarke DM, Baust JM, Van Buskirk RG, Baust JG. Addition of anticancer agents enhances freezing-induced prostate cancer cell death: implications of mitochondrial involvement. Cryobiology. 2004;49:45–61. doi: 10.1016/j.cryobiol.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 25.Yuan F, Zhou W, Zhang J, Zhang Z, Zou C, Huang L, Zhang Y, Dai Z. Anticancer drugs are synergistic with freezing in induction of apoptosis in HCC cells. Cryobiology. 2008;57:60–65. doi: 10.1016/j.cryobiol.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 26.Ikekawa S, Ishihara K, Tanaka S, Ikeda S. Basic studies of cryochemotherapy in a murine tumor system. Cryobiology. 1985;22:477–483. doi: 10.1016/0011-2240(85)90159-2. [DOI] [PubMed] [Google Scholar]
- 27.Forest V, Peoc'h M, Campos L, Guyotat D, Vergnon JM. Benefit of a combined treatment of cryotherapy and chemotherapy on tumour growth and late cryo-induced angiogenesis in a non-small-cell lung cancer model. Lung Cancer. 2006;54:79–86. doi: 10.1016/j.lungcan.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 28.Clarke DM, Baust JM, Van Buskirk RG, Baust JG. Chemo-cryo combination therapy: an adjunctive model for the treatment of prostate cancer. Cryobiology. 2001;42:274–285. doi: 10.1006/cryo.2001.2333. [DOI] [PubMed] [Google Scholar]
- 29.Forest V, Peoc'h M, Campos L, Guyotat D, Vergnon JM. Effects of cryotherapy or chemotherapy on apoptosis in a non-small-cell lung cancer xenografted into SCID mice. Cryobiology. 2005;50:29–37. doi: 10.1016/j.cryobiol.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 30.Ahmed M, Monsky WE, Girnun G, Lukyanov A, D’lppolito G, Kruskal JB, Stuart KE, Torchilin VP, Goldberg SN. Radiofrequency Thermal Ablation Sharply Increases Intratumoral Liposomal Doxorubicin Accumulation and Tumor Coagulation. Cancer Research. 2003;63:6327–6333. [PubMed] [Google Scholar]
- 31.Monsky WL, Kruskal JB, Lukyanov AN, Girnun GD, Ahmed M, Gazelle GS, Huertas JC, Stuart KE, Torchilin VP, Goldberg SN. Radio-frequency Ablation Increases Intratumoral Liposomal Doxorubicin Accumulation in a Rat Breast Tumor Model1. Radiology. 2002;224:823–829. doi: 10.1148/radiol.2243011421. [DOI] [PubMed] [Google Scholar]
- 32.Han B, Iftekhar A, Bischof JC. Improved cryosurgery by use of thermophysical and inflammatory adjuvants. Technol Cancer Res Treat. 2004;3:103–111. doi: 10.1177/153303460400300203. [DOI] [PubMed] [Google Scholar]
- 33.Kawaguchi Y, Sugiyama Y, Saji S. [Cryoimmunological therapy with local injection of OK-432 against advance or recurrent breast cancer] Gan To Kagaku Ryoho. 2003;30:1583–1586. [PubMed] [Google Scholar]
- 34.Han B, Swanlund DJ, Bischof JC. Cryoinjury of MCF-7 human breast cancer cells and inhibition of post-thaw recovery using TNF-alpha. Technol Cancer Res Treat. 2007;6:625–634. doi: 10.1177/153303460700600606. [DOI] [PubMed] [Google Scholar]
- 35.Chao BH, He X, Bischof JC. Pre-treatment inflammation induced by TNF-alpha augments cryosurgical injury on human prostate cancer. Cryobiology. 2004;49:10–27. doi: 10.1016/j.cryobiol.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 36.Sugiyama Y, Saji S, Miya K, Kunieda K, Takao H, Kawaguchi Y, Kimura A, Honda S, Matsui K. [Therapeutic effect of multimodal therapy, such as cryosurgery, locoregional immunotherapy and systemic chemotherapy against far advanced breast cancer] Gan To Kagaku Ryoho. 2001;28:1616–1619. [PubMed] [Google Scholar]
- 37.Jiang J, Goel R, Iftekhar MA, Visaria R, Belcher JD, Vercellotti GM, Bischof JC. Tumor necrosis factor-alpha-induced accentuation in cryoinjury: mechanisms in vitro and in vivo. Mol Cancer Ther. 2008;7:2547–2555. doi: 10.1158/1535-7163.MCT-07-2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salloum M, Ma R, Zhu L. Enhancement in treatment planning for magnetic nanoparticle hyperthermia: Optimization of the heat absorption pattern. International Journal of Hyperthermia. 2009;25:309–321. doi: 10.1080/02656730902803118. [DOI] [PubMed] [Google Scholar]
- 39.Ray PC, Yu HT, Fu PP. Toxicity and Environmental Risks of Nanomaterials: Challenges and Future Needs. Journal of Environmental Science and Health Part C-Environmental Carcino-genesis & Ecotoxicology Reviews. 2009;27:1–35. doi: 10.1080/10590500802708267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nat Rev Cancer. 2006;6:583–592. doi: 10.1038/nrc1893. [DOI] [PubMed] [Google Scholar]
- 41.Vasir JK, Labhasetwar V. Biodegradable nanoparticles for cytosolic delivery of therapeutics. Adv Drug Deliv Rev. 2007;59:718–728. doi: 10.1016/j.addr.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kumar MNVR. Nano and microparticles as controlled drug delivery devices. J Pharm Pharm Sci. 2000;3:234–258. [PubMed] [Google Scholar]
- 43.Vinogradov SV, Bronich TK, Kabanov AV. Nanosized cationic hydrogels for drug delivery: preparation, properties and interactions with cells. Adv Drug Deliv Rev. 2002;54:135–147. doi: 10.1016/s0169-409x(01)00245-9. [DOI] [PubMed] [Google Scholar]
- 44.Tanaka T, Decuzzi P, Cristofanilli M, Sakamoto JH, Tasciotti E, Robertson FM, Ferrari M. Nanotechnology for breast cancer therapy. Biomed Microdevices. 2009;11:49–63. doi: 10.1007/s10544-008-9209-0. [DOI] [PubMed] [Google Scholar]
- 45.Breunig M, Bauer S, Goepferich A. Polymers and nanoparticles: intelligent tools for intracel-lular targeting? Eur J Pharm Biopharm. 2008;68:112–128. doi: 10.1016/j.ejpb.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 46.Thurber GM, Schmidt MM, Wittrup KD. Antibody tumor penetration: transport opposed by systemic and antigen-mediated clearance. Adv Drug Deliv Rev. 2008;60:1421–1434. doi: 10.1016/j.addr.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kostarelos K, Emfietzoglou D, Papakostas A, Yang WH, Ballangrud A, Sgouros G. Binding and interstitial penetration of liposomes within avascular tumor spheroids. Int J Cancer. 2004;112:713–721. doi: 10.1002/ijc.20457. [DOI] [PubMed] [Google Scholar]
- 48.Zagaynova EV, Shirmanova MV, Kirillin MY, Khlebtsov BN, Orlova AG, Balalaeva IV, Sirotkina MA, Bugrova ML, Agrba PD, Kamensky VA. Contrasting properties of gold nanoparticles for optical coherence tomography: phantom, in vivo studies and Monte Carlo simulation. Phys Med Biol. 2008;53:4995–5009. doi: 10.1088/0031-9155/53/18/010. [DOI] [PubMed] [Google Scholar]
- 49.Nuttens VE, Wera AC, Bouchat V, Lucas S. Determination of biological vector characteristics and nanoparticle dimensions for radioim-munotherapy with radioactive nanoparticles. Appl Radiat Isot. 2008;66:168–172. doi: 10.1016/j.apradiso.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 50.Needham D, Dewhirst MW. The development and testing of a new temperature-sensitive drug delivery system for the treatment of solid tumors. Adv Drug Deliv Rev. 2001;53:285–305. doi: 10.1016/s0169-409x(01)00233-2. [DOI] [PubMed] [Google Scholar]
- 51.Kostarelos K, Emfietzoglou D, Papakostas A, Yang WH, Ballangrud AM, Sgouros G. Engineering lipid vesicles of enhanced intratumoral transport capabilities: correlating liposome characteristics with penetration into human prostate tumor spheroids. J Liposome Res. 2005;15:15–27. doi: 10.1081/lpr-64953. [DOI] [PubMed] [Google Scholar]
- 52.Wu NZ, Klitzman B, Rosner G, Needham D, Dewhirst MW. Measurement of material extravasation in microvascular networks using fluorescence video-microscopy. Microvasc Res. 1993;46:231–253. doi: 10.1006/mvre.1993.1049. [DOI] [PubMed] [Google Scholar]
- 53.Yuan F, Leunig M, Berk DA, Jain RK. Microvascular permeability of albumin, vascular surface area, and vascular volume measured in human adenocarcinoma LS174T using dorsal chamber in SCID mice. Microvasc Res. 1993;45:269–289. doi: 10.1006/mvre.1993.1024. [DOI] [PubMed] [Google Scholar]
- 54.Weinstein JN, Eger RR, Covell DG, Black CD, Mulshine J, Carrasquillo JA, Larson SM, Keenan AM. The pharmacology of monoclonal antibodies. Ann N Y Acad Sci. 1987;507:199–210. doi: 10.1111/j.1749-6632.1987.tb45802.x. [DOI] [PubMed] [Google Scholar]
- 55.Jung S, Patzelt A, Otberg N, Thiede G, Sterry W, Lademann J. Strategy of topical vaccination with nanoparticles. J Biomed Opt. 2009;14:021001. doi: 10.1117/1.3080714. [DOI] [PubMed] [Google Scholar]
- 56.Thiesen B, Jordan A. Clinical applications of magnetic nanoparticles for hyperthermia. International Journal of Hyperthermia. 2008;24:467–474. doi: 10.1080/02656730802104757. [DOI] [PubMed] [Google Scholar]
- 57.Jordan A. Jordan Andreas, et al. Vol. 9. International Journal of Hyperthermia; 1993. Hyperthermia classic commentary: ‘Inductive heating of ferrimagnetic particles and magnetic fluids: Physical evaluation of their potential for hyperthermia’; pp. 51–68. International Journal of Hyperthermia 2009; 25: 512-516. [DOI] [PubMed] [Google Scholar]
- 58.Kappiyoor R, Liangruksa M, Ganguly R, Puri IK. The effects of magnetic nanoparticle properties on magnetic fluid hyperthermia. Journal of Applied Physics. 2010:108. [Google Scholar]
- 59.Matsumura Y, Maeda H. A New Concept for Macromolecular Therapeutics in Cancer-Chemotherapy - Mechanism of Tumoritropic Accumulation of Proteins and the Antitumor Agent Smancs. Cancer Research. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 60.Wang X, Yang L, Chen Z, Shin DM. Application of Nanotechnology in Cancer Therapy and Imaging. CA Cancer J Clin. 2008;58:97–110. doi: 10.3322/CA.2007.0003. [DOI] [PubMed] [Google Scholar]
- 61.Dreher MR, Chilkoti A. Toward a Systems Engineering Approach to Cancer Drug Delivery. Journal of the National Cancer Institute. 2007;99:983–985. doi: 10.1093/jnci/djm042. [DOI] [PubMed] [Google Scholar]
- 62.Glazer ES, Curley SA. Radiofrequency Field-Induced Thermal Cytotoxicity in Cancer Cells Treated With Fluorescent Nanoparticles. Cancer. 2010;116:3285–3293. doi: 10.1002/cncr.25135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kikumori T, Kobayashi T, Sawaki M, Imai T. Anti-cancer effect of hyperthermia on breast cancer by magnetite nanoparticle-loaded anti-HER2 immunoliposomes. Breast Cancer Research and Treatment. 2009;113:435–441. doi: 10.1007/s10549-008-9948-x. [DOI] [PubMed] [Google Scholar]
- 64.Carpin LB, Bickford LR, Agollah G, Yu TK, Schiff R, Li Y, Drezek RA. Immunoconjugated gold nanoshell-mediated photothermal ablation of trastuzumab-resistant breast cancer cells. Breast Cancer Research and Treatment. 2011;125:27–34. doi: 10.1007/s10549-010-0811-5. [DOI] [PubMed] [Google Scholar]
- 65.Glazer ES, Zhu CH, Massey KL, Thompson CS, Kaluarachchi WD, Hamir AN, Curley SA. Noninvasive Radiofrequency Field Destruction of Pancreatic Adenocarcinoma Xenografts Treated with Targeted Gold Nanoparticles. Clinical Cancer Research. 2010;16:5712–5721. doi: 10.1158/1078-0432.CCR-10-2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Van Meir EG, Hadjipanayis CG, Norden AD, Shu H-K, Wen PY, Olson JJ. Exciting New Advances in Neuro-Oncology: The Avenue to a Cure for Malignant Glioma. CA Cancer J Clin. 60:166–193. doi: 10.3322/caac.20069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jensen SS, Andresen TL, Davidsen J, HÃyrup P, Shnyder SD, Bibby MC, Gill JH, JÃrgensen K. Secretory phospholipase A2 as a tumor-specific trigger for targeted delivery of a novel class of liposomal prodrug anticancer etherlipids. Molecular Cancer Therapeutics. 2004;3:1451–1458. [PubMed] [Google Scholar]
- 68.Cheng R, Feng F, Meng F, Deng C, Feijen J, Zhong Z. Glutathione-responsive nano-vehicles as a promising platform for targeted intracellu-lar drug and gene delivery. doi: 10.1016/j.jconrel.2011.01.030. Journal of Controlled Release In Press, Uncorrected Proof: [DOI] [PubMed] [Google Scholar]
- 69.Kenny GD, Kamaly N, Kalber TL, Brody LP, Sahuri M, Shamsaei E, Miller AD, Bell JD. Novel multifunctional nanoparticle mediates siRNA tumour delivery, visualisation and therapeutic tumour reduction in vivo. Journal of Controlled Release. 149:111–116. doi: 10.1016/j.jconrel.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 70.Collins RE, Cheng XD. Structural and bio-chemical advances in mammalian RNAi. Journal of Cellular Biochemistry. 2006;99:1251–1266. doi: 10.1002/jcb.21069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hannon GJ. RNA interference. Nature. 2002;418:244–251. doi: 10.1038/418244a. [DOI] [PubMed] [Google Scholar]
- 72.Bernards R. Exploring the uses of RNAi - Gene knockdown and the Nobel Prize. New England Journal of Medicine. 2006;355:2391–2393. doi: 10.1056/NEJMp068242. [DOI] [PubMed] [Google Scholar]
- 73.Bumcrot D, Manoharan M, Koteliansky V, Sah DWY. RNAi therapeutics: a potential new class of pharmaceutical drugs. Nature Chemical Biology. 2006;2:711–719. doi: 10.1038/nchembio839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fire A, Xu SQ, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 75.Gilmore IR, Fox SP, Hollins AJ, Akhtar S. Delivery Strategies for siRNA-mediated Gene Silencing. Current Drug Delivery. 2006;3:147–155. doi: 10.2174/156720106776359159. [DOI] [PubMed] [Google Scholar]
- 76.Li CX, Parker A, Menocal E, Xiang SL, Borodyansky L, Fruehauf JH. Delivery of RNA interference. Cell Cycle. 2006;5:2103–2109. doi: 10.4161/cc.5.18.3192. [DOI] [PubMed] [Google Scholar]
- 77.Gilmore IR, Fox SP, Hollins AJ, Muhammad SB, Akhtar S. The design and exogenous delivery of siRNA for post-transcriptional gene silencing. Journal of Drug Targeting. 2004;12:315–340. doi: 10.1080/10611860400006257. [DOI] [PubMed] [Google Scholar]
- 78.Graham FL, Vandereb AJ. New Technique for Assay of Infectivity of Human Adenovirus 5 DNA. Virology. 1973;52:456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- 79.Maitra A. Calcium phosphate nanoparticles: second-generation nonviral vectors in gene therapy. Expert Review of Molecular Diagnostics. 2005;5:893–905. doi: 10.1586/14737159.5.6.893. [DOI] [PubMed] [Google Scholar]
- 80.Jordan M, Schallhorn A, Wurm FM. Trans-fecting Mammalian Cells: Optimization of Critical Parameters Affecting Calcium-Phosphate Precipitate Formation. Nucleic Acids Research. 1996;24:596–601. doi: 10.1093/nar/24.4.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Minko T, Kopecková P, Kopecek J. Preliminary evaluation of caspases-dependent apop-tosis signaling pathways of free and HPMA co-polymer-bound doxorubicin in human ovarian carcinoma cells. Journal of Controlled Release. 2001;71:227–237. doi: 10.1016/s0168-3659(01)00220-6. [DOI] [PubMed] [Google Scholar]
- 82.Garbuzenko OB, Saad M, Betigeri S, Zhang M, Vetcher AA, Soldatenkov VA, Reimer DC, Pozharov VP, Minko T. Intratracheal Versus Intravenous Liposomal Delivery of siRNA, Antisense Oligonucleotides and Anticancer Drug. Pharmaceutical Research. 2009;26:382–394. doi: 10.1007/s11095-008-9755-4. [DOI] [PubMed] [Google Scholar]
- 83.Lee SH, Kim SH, Park TG. Intracellular siRNA delivery system using polyelectrolyte complex micelles prepared from VEGF siRNA-PEG conjugate and cationic fusogenic peptide. Biochemical and Biophysical Research Communications. 2007;357:511–516. doi: 10.1016/j.bbrc.2007.03.185. [DOI] [PubMed] [Google Scholar]
- 84.Hu-Lieskovan S, Heidel JD, Bartlett DW, Davis ME, Triche TJ. Sequence-specific knock-down of EWS-FLI1 by targeted, nonviral delivery of small interfering RNA inhibits tumor growth in a murine model of metastatic Ewing's sarcoma. Cancer Research. 2005;65:8984–8992. doi: 10.1158/0008-5472.CAN-05-0565. [DOI] [PubMed] [Google Scholar]
- 85.Urban-Klein B, Werth S, Abuharbeid S, Czubayko F, Aigner A. RNAi-mediated genetargeting through systemic application of polyethylenimine (PEI)-complexed siRNA in vivo. Gene Therapy. 2005;12:461–466. doi: 10.1038/sj.gt.3302425. [DOI] [PubMed] [Google Scholar]
- 86.Soutschek J, Akinc A, Bramlage B, Charisse K, Constien R, Donoghue M, Elbashir S, Geick A, Hadwiger P, Harborth J, John M, Kesavan V, Lavine G, Pandey RK, Racie T, Rajeev KG, Rohl I, Toudjarska I, Wang G, Wuschko S, Bumcrot D, Koteliansky V, Limmer S, Manoharan M, Vornlocher HP. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature. 2004;432:173–178. doi: 10.1038/nature03121. [DOI] [PubMed] [Google Scholar]
- 87.Sorensen DR, Leirdal M, Sioud M. Gene silencing by systemic delivery of synthetic siRNAs in adult mice. Journal of Molecular Biology. 2003;327:761–766. doi: 10.1016/s0022-2836(03)00181-5. [DOI] [PubMed] [Google Scholar]
- 88.Cao N, Cheng D, Zou S, Ai H, Gao J, Shuai X. The synergistic effect of hierarchical assemblies of siRNA and chemotherapeutic drugs co-delivered into hepatic cancer cells. Biomaterials. 32:2222–2232. doi: 10.1016/j.biomaterials.2010.11.061. [DOI] [PubMed] [Google Scholar]
- 89.Elbakry A, Zaky A, Liebkl R, Rachel R, Goepferich A, Breunig M. Layer-by-Layer Assembled Gold Nanoparticles for siRNA Delivery. Nano Letters. 2009;9:2059–2064. doi: 10.1021/nl9003865. [DOI] [PubMed] [Google Scholar]
- 90.Bhavsar MD, Amiji MM. Gastrointestinal distribution and in vivo gene transfection studies with nanoparticles-in-microsphere oral system (NiMOS) Journal of Controlled Release. 2007;119:339–348. doi: 10.1016/j.jconrel.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 91.Hood JD, Bednarski M, Frausto R, Guccione S, Reisfeld RA, Xiang R, Cheresh DA. Tumor regression by targeted gene delivery to the neovasculature. Science. 2002;296:2404–2407. doi: 10.1126/science.1070200. [DOI] [PubMed] [Google Scholar]
- 92.Pardridge WM. Intravenous, non-viral RNAi gene therapy of brain cancer. Expert Opinion on Biological Therapy. 2004;4:1103–1113. doi: 10.1517/14712598.4.7.1103. [DOI] [PubMed] [Google Scholar]
- 93.Kim B, Tang QQ, Biswas PS, Xu J, Schiffelers RM, Xie FY, Ansari AM, Scaria PV, Woodle MC, Lu P, Rouse BT. Inhibition of ocular angiogenesis by siRNA targeting vascular endothelial growth factor pathway genes - Therapeutic strategy for herpetic stromal keratitis. American Journal of Pathology. 2004;165:2177–2185. doi: 10.1016/S0002-9440(10)63267-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Toub N, Bertrand JR, Tamaddon A, Elhamess H, Hillaireau H, Maksimenko A, Maccario J, Malvy C, Fattal E, Couvreur P. Efficacy of siRNA nanocapsules targeted against the EWS-Fli1 oncogene in Ewing sarcoma. Pharmaceutical Research. 2006;23:892–900. doi: 10.1007/s11095-006-9901-9. [DOI] [PubMed] [Google Scholar]
- 95.Kortylewski M, Swiderski P, Herrmann A, Wang L, Kowolik C, Kujawski M, Lee H, Scuto A, Liu Y, Yang CM, Deng JH, Soifer HS, Raubitschek A, Forman S, Rossi JJ, Pardoll DM, Jove R, Yu H. In vivo delivery of siRNA to immune cells by conjugation to a TLR9 agonist enhances antitumor immune responses. Nature Biotechnology. 2009;27:925–U988. doi: 10.1038/nbt.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.MacDiarmid JA, Amaro-Mugridge NB, Madrid-Weiss J, Sedliarou I, Wetzel S, Kochar K, Brahmbhatt VN, Phillips L, Pattison ST, Petti C, Stillman B, Graham RM, Brahmbhatt H. Sequential treatment of drug-resistant tumors with targeted minicells containing siRNA or a cytotoxic drug. Nature Biotechnology. 2009;27:643–U697. doi: 10.1038/nbt.1547. [DOI] [PubMed] [Google Scholar]
- 97.Hollins AJ, Omidi Y, Benter IF, Akhtar S. Toxicogenomics of drug delivery systems: Exploiting delivery system-induced changes in target gene expression to enhance siRNA activity. Journal of Drug Targeting. 2007;15:83–88. doi: 10.1080/10611860601151860. [DOI] [PubMed] [Google Scholar]
- 98.Fedorov Y, Anderson EM, Birmingham A, Reynolds A, Karpilow J, Robinson K, Leake D, Marshall WS, Khvorova A. Off-target effects by siRNA can induce toxic phenotype. Rna-a Publication of the Rna Society. 2006;12:1188–1196. doi: 10.1261/rna.28106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jackson AL, Bartz SR, Schelter J, Kobayashi SV, Burchard J, Mao M, Li B, Cavet G, Linsley PS. Expression profiling reveals off-target gene regulation by RNAi. Nature Biotechnology. 2003;21:635–637. doi: 10.1038/nbt831. [DOI] [PubMed] [Google Scholar]
- 100.Liu TY, Hu SH, Liu KH, Shaiu RS, Liu DM, Chen SY. Instantaneous Drug Delivery of Magnetic/Thermally Sensitive Nanospheres by a High-Frequency Magnetic Field. Langmuir. 2008;24:13306–13311. doi: 10.1021/la801451v. [DOI] [PubMed] [Google Scholar]
- 101.Liu TY, Liu KH, Liu DM, Chen SY, Chen IW. Temperature-Sensitive Nanocapsules for Controlled Drug Release Caused by Magnetically Triggered Structural Disruption. Advanced Functional Materials. 2009;19:616–623. [Google Scholar]
- 102.Wells J, Sen A, Hui SW. Localized delivery to CT-26 tumors in mice using thermosensitive liposomes. International Journal of Pharmaceutics. 2003;261:105–114. doi: 10.1016/s0378-5173(03)00290-4. [DOI] [PubMed] [Google Scholar]
- 103.Lee SH, Choi SH, Kim SH, Park TG. Thermally sensitive cationic polymer nanocapsules for specific cytosolic delivery and efficient gene silencing of siRNA: Swelling induced physical disruption of endosome by cold shock. Journal of Controlled Release. 2008;125:25–32. doi: 10.1016/j.jconrel.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 104.Choi SH, Lee SH, Park TG. Temperaturesensitive pluronic/poly(ethylenimine) nanocapsules for thermally triggered disruption of intracellular endosomal compartment. Biomacromolecules. 2006;7:1864–1870. doi: 10.1021/bm060182a. [DOI] [PubMed] [Google Scholar]
- 105.Dromi S, Frenkel V, Luk A, Traughber B, Angstadt M, Bur M, Poff J, Xie JW, Libutti SK, Li KCP, Wood BJ. Pulsed-high intensity focused ultrasound and low temperature sensitive liposomes for enhanced targeted drug delivery and antitumor effect. Clinical Cancer Research. 2007;13:2722–2727. doi: 10.1158/1078-0432.CCR-06-2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Frenkel V, Etherington A, Greene M, Quijano J, Xie JW, Hunter F, Dromi S, Li KCP. Delivery of liposomal doxorubicin (Doxil) in a breast cancer tumor model: Investigation of potential enhancement by pulsed-high intensity focused ultrasound exposure. Academic Radiology. 2006;13:469–479. doi: 10.1016/j.acra.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 107.Ahmed M, Liu ZJ, Lukyanov AN, Signoretti S, Horkan C, Monsky WL, Torchilin VP, Goldberg SN. Combination radiofrequency ablation with intratumoral liposomal doxorubicEffect on drug accumulation and coagulation in multiple tissues and tumor types in animals. Radiology. 2005;235:469–477. doi: 10.1148/radiol.2352031856. [DOI] [PubMed] [Google Scholar]
- 108.Steinberg Y, Schroeder A, Talmon Y, Schmidt J, Khalfin RL, Cohen Y, Devoisselle JM, Begu S, Avnir D. Triggered release of aqueous content from liposome-derived solgel nanocapsules. Langmuir. 2007;23:12024–12031. doi: 10.1021/la702311f. [DOI] [PubMed] [Google Scholar]
- 109.Kong G, Anyarambhatla G, Petros WP, Braun RD, Colvin OM, Needham D, Dewhirst MW. Efficacy of Liposomes and Hyperthermia in a Human Tumor Xenograft Model: Importance of Triggered Drug Release. Cancer Research. 2000;60:6950–6957. [PubMed] [Google Scholar]
- 110.Needham D, Anyarambhatla G, Kong G, Dewhirst MW. A New Temperature-sensitive Liposome for Use with Mild Hyperthermia: Characterization and Testing in a Human Tumor Xenograft Model. Cancer Research. 2000;60:1197–1201. [PubMed] [Google Scholar]
- 111.Hauck ML, LaRue SM, Petros WP, Poulson JM, Yu D, Spasojevic I, Pruitt AF, Klein A, Case B, Thrall DE, Needham D, Dewhirst MW. Phase I Trial of Doxorubicin-Containing Low Temperature Sensitive Liposomes in Spontaneous Canine Tumors. Clinical Cancer Research. 2006;12:4004–4010. doi: 10.1158/1078-0432.CCR-06-0226. [DOI] [PubMed] [Google Scholar]
- 112.Ponce AM, Viglianti BL, Yu D, Yarmolenko PS, Michelich CR, Woo J, Bally MB, Dewhirst MW. Magnetic Resonance Imaging of Temperature-Sensitive Liposome Release: Drug Dose Painting and Antitumor Effects. Journal of the National Cancer Institute. 2007;99:53–63. doi: 10.1093/jnci/djk005. [DOI] [PubMed] [Google Scholar]
- 113.Yuh EL, Shulman SG, Mehta SA, Xie J, Chen L, Frenkel V, Bednarski MD, Li KCP. Delivery of Systemic Chemotherapeutic Agent to Tumors by Using Focused Ultrasound: Study in a Murine Modell. Radiology. 2005;234:431–437. doi: 10.1148/radiol.2342030889. [DOI] [PubMed] [Google Scholar]
- 114.Lindner LH, Eichhorn ME, Eibl H, Teichert N, Schmitt-Sody M, Issels RD, Dellian M. Novel Temperature-Sensitive Liposomes with Prolonged Circulation Time. Clinical Cancer Research. 2004;10:2168–2178. doi: 10.1158/1078-0432.ccr-03-0035. [DOI] [PubMed] [Google Scholar]
- 115.Park J-H, von Maltzahn G, Xu MJ, Fogal V, Kotamraju VR, Ruoslahti E, Bhatia SN, Sailor MJ. Cooperative nanomaterial system to sensitize, target, and treat tumors. Proceedings of the National Academy of Sciences. 107:981–986. doi: 10.1073/pnas.0909565107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chen Q, Tong S, Dewhirst MW, Yuan F. Targeting tumor microvessels using doxorubicin encapsulated in a novel thermosensitive liposome. Molecular Cancer Therapeutics. 2004;3:1311–1317. [PubMed] [Google Scholar]
- 117.Wood B. ClinicalTrials.gov[Internet] Bethesda (Md): National Library of Medicine (US); Heat Activated Liposomal Doxorubicin and Radiofrequency Ablation in Treating Patients With Primary or Metastatic Liver Tumors. 2000-[2011]. Available from http://clinicaltrials.gov/show/NCT00093444 NLM identifier: NCT00093444. [Google Scholar]
- 118.Van Doren C. ClinicalTrials.gov[Internet] Bethesda (Md): National Library of Medicine (US); Liposomal Doxorubicin and thermal therapy in treating patients with prostate cancer. 2000-[2011]. Available from http://clinicaltrials.gov/show/NCT00061867 NLM identifier: NCT00061867. [Google Scholar]
- 119.Poon RT, Lencioni R. ClinicalTrials.gov[Internet] Bethesda (Md): National Library of Medicine (US); Phase 3 Study of ThermoDox With Radiofrequency Ablation (RFA) in Treatment of Hepatocellular Carcinoma (HCC) 2000-[2011]. Available from http://clinicaltrials.gov/show/NCT00617981 NLM identifier: NCT00617981. [Google Scholar]
- 120.Borys N. ClinicalTrials.gov[Internet] Bethesda (Md): National Library of Medicine (US); A Study of ThermoDox™ in Combination With Radiofrequency Ablation (RFA) in Primary and Metastatic Tumors of the Liver. 2000-[2011]. Available from http://clinicaltrials.gov/show/NCT00441376 NLM identifier: NCT00441376. [Google Scholar]
- 121.Borys N. ClinicalTrials.gov[Internet] Bethesda (Md): National Library of Medicine (US); Phase 1/2 Study of ThermoDox With Approved Hyperthermia in Treatment of Breast Cancer Recurrence at the Chest Wall (DIGNITY) 2000-[2011]. Available from http://clinicaltrials.gov/show/NCT00826085 NLM identifier: NCT00826085. [Google Scholar]
- 122.Yoshida M, Watanabe Y, Sato M, Maehara T, Aono H, Naohara T, Hirazawa H, Horiuchi A, Yukumi S, Sato K, Nakagawa H, Yamamoto Y, Sugishita H, Kawachi K. Feasibility of chemohyperthermia with docetaxel-embedded magnetoliposomes as minimally invasive local treatment for cancer. International Journal of Cancer. 2010;126:1955–1965. doi: 10.1002/ijc.24864. [DOI] [PubMed] [Google Scholar]
- 123.Li FR, Yan WH, Guo YH, Qi H, Zhou HX. Preparation of carboplatin-Fe@C-loaded chitosan nanoparticles and study on hyperthermia combined with pharmacotherapy for liver cancer. International Journal of Hyperthermia. 2009;25:383–391. doi: 10.1080/02656730902834949. [DOI] [PubMed] [Google Scholar]
- 124.Tai LA, Tsai PJ, Wang YC, Wang YJ, Lo LW, Yang CS. Thermosensitive liposomes entrapping iron oxide nanoparticles for controllable drug release. Nanotechnology. 2009:20. doi: 10.1088/0957-4484/20/13/135101. [DOI] [PubMed] [Google Scholar]
- 125.Jain TK, Reddy MK, Morales MA, Leslie-Pelecky DL, Labhasetwar V. Biodistribution, clearance, and biocompatibility of iron oxide magnetic nanoparticles in rats. Molecular Pharmaceutics. 2008;5:316–327. doi: 10.1021/mp7001285. [DOI] [PubMed] [Google Scholar]
- 126.Jain TK, Richey J, Strand M, Leslie-Pelecky DL, Flask CA, Labhasetwar V. Magnetic nanoparticles with dual functional properties: Drug delivery and magnetic resonance imaging. Biomaterials. 2008;29:4012–4021. doi: 10.1016/j.biomaterials.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jain TK, Morales MA, Sahoo SK, Leslie-Pelecky DL, Labhasetwar V. Iron oxide nanoparticles for sustained delivery of anticancer agents. Molecular Pharmaceutics. 2005;2:194–205. doi: 10.1021/mp0500014. [DOI] [PubMed] [Google Scholar]
- 128.Jain TK, Foy SP, Erokwu B, Dimitrijevic S, Flask CA, Labhasetwar V. Magnetic resonance imaging of multifunctional pluronic stabilized iron-oxide nanoparticles in tumor-bearing mice. Biomaterials. 2009;30:6748–6756. doi: 10.1016/j.biomaterials.2009.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zou P, Yu Y, Wang YA, Zhong Y, Welton A, Galbal n C, Wang S, Sun D. Superparamagnetic Iron Oxide Nanotheranostics for Targeted Cancer Cell Imaging and pH-Dependent Intracellular Drug Release. Molecular Pharmaceutics. 7:1974–1984. doi: 10.1021/mp100273t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pedro RN, Thekke-Adiyat T, Goel R, Shenoi M, Slaton J, Schmechel S, Bischof J, Anderson JK. Use of Tumor Necrosis Factor-alphacoated Gold Nanoparticles to Enhance Radiofrequency Ablation in a Translational Model of Renal Tumors. Urology. 2010;76:494–498. doi: 10.1016/j.urology.2010.01.085. [DOI] [PubMed] [Google Scholar]
- 131.Goel R, Shah N, Visaria R, Paciotti GF, Bischof JC. Biodistribution of TNF-alphacoated gold nanoparticles in an in vivo model system. Nanomedicine. 2009;4:401–410. doi: 10.2217/nnm.09.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Visaria R, Bischof JC, Loren M, Williams B, Ebbini E, Paciotti G, Griffin R. Nanotherapeutics for enhancing thermal therapy of cancer. International Journal of Hyperthermia. 2007;23:501–511. doi: 10.1080/02656730701611241. [DOI] [PubMed] [Google Scholar]
- 133.Ito A, Honda H, Kobayashi T. Cancer immunotherapy based on intracellular hyperthermia using magnetite nanoparticles: a novel concept of “heat-controlled necrosis” with heat shock protein expression. Cancer Immunology Immunotherapy. 2006;55:320–328. doi: 10.1007/s00262-005-0049-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Goel R, Swanlund D, Coed J, Paciotti GF, Bischof JC. Article on TNF-alpha-based accentuation in cryoinjury (vol 6, pg 2039, 2007) Molecular Cancer Therapeutics. 2007;6:2383–2383. doi: 10.1158/1535-7163.MCT-06-0676. [DOI] [PubMed] [Google Scholar]
- 135.Goel R, Swanlund D, Coad J, Paciotti GF, Bischof JC. TNF-alpha-based accentuation in cryoinjury - dose, delivery, and response. Molecular Cancer Therapeutics. 2007;6:2039–2047. doi: 10.1158/1535-7163.MCT-06-0676. [DOI] [PubMed] [Google Scholar]
- 136.Tsai TL, Shieh DB, Yeh CS, Tzeng Y, Htet K, Chuang KS, Hwu JR, Su WC. The down regulation of target genes by photo activated DNA nanoscissors. Biomaterials. 2010;31:6545–6554. doi: 10.1016/j.biomaterials.2010.04.058. [DOI] [PubMed] [Google Scholar]
- 137.Conde J, de la Fuente JM, Baptista PV. In vitro transcription and translation inhibition via DNA functionalized gold nanoparticles. Nanotechnology. 2010:21. doi: 10.1088/0957-4484/21/50/505101. [DOI] [PubMed] [Google Scholar]
- 138.Csaki A, Garwe F, Steinbruck A, Maubach G, Festag G, Weise A, Riemann I, Konig K, Fritzsche W. A parallel approach for subwavelength molecular surgery using gene-specific positioned metal nanoparticles as laser light antennas. Nano Letters. 2007;7:247–253. doi: 10.1021/nl061966x. [DOI] [PubMed] [Google Scholar]
- 139.Hamad-Schifferli K, Schwartz JJ, Santos AT, Zhang SG, Jacobson JM. Remote electronic control of DNA hybridization through inductive coupling to an attached metal nanocrystal antenna. Nature. 2002;415:152–155. doi: 10.1038/415152a. [DOI] [PubMed] [Google Scholar]
- 140.Chisholm EJ, Vassaux G, Martin-Duque P, Chevre R, Lambert O, Pitard B, Merron A, Weeks M, Burnet J, Peerlinck I, Dai M-S, Alusi G, Mather SJ, Bolton K, Uchegbu IF, Schatzlein AG, Baril P. Cancer-Specific Transgene Expression Mediated by Systemic Injection of Nanoparticles. Cancer Research. 2009;69:2655–2662. doi: 10.1158/0008-5472.CAN-08-2657. [DOI] [PubMed] [Google Scholar]
- 141.Pettersson E, Lundeberg J, Ahmadian A. Generations of sequencing technologies. Genomics. 2009;93:105–111. doi: 10.1016/j.ygeno.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 142.Zhang WJ, Rong JH, Wang Q, He XM. The encapsulation and intracellular delivery of trehalose using a thermally responsive nanocapsule. Nanotechnology. 2009:20. doi: 10.1088/0957-4484/20/27/275101. [DOI] [PubMed] [Google Scholar]
- 143.Zhang WJ, Gilstrap K, Wu LY, Bahadur KCR, Moss MA, Wang QA, Lu XB, He XM. Synthesis and Characterization of Thermally Responsive Pluronic F127-Chitosan Nanocapsules for Controlled Release and Intracellular Delivery of Small Molecules. Acs Nano. 2010;4:6747–6759. doi: 10.1021/nn101617n. [DOI] [PubMed] [Google Scholar]
- 144.Maier-Hauff K, Ulrich F, Nestler D, Niehoff H, Wust P, Thiesen B, Orawa H, Budach V, Jordan A. Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. Journal of Neuro-Oncology. :1–8. doi: 10.1007/s11060-010-0389-0. [DOI] [PMC free article] [PubMed] [Google Scholar]