Abstract
Caveolin-1 is a ubiquitously expressed integral membrane protein and essential for the formation of so-called Caveolae, small invaginations of the plasma membrane. Caveolae are involved in major physiological functions of the mammalian cell, including endocytosis and transcytosis processes, signal transduction and cholesterol homeostasis. During the last decade, it became evident that Caveolin-1 plays a key role in cancer progression and metastasis. As it has also been described as a tumor suppressor, the plethora of intracellular processes Caveolin-1 contributes to remains to be fully identified. Differences in pathophysiological protein function have been ascribed to cell-specific roles of Caveolin-1 and to cancer stage dependency. An important aspect of the protein in terms of cancer cure seems to be its relevance as a prognostic marker and for induction of metastasis. These diverse functions of Caveolin-1 were expanded by recent data showing its role in radio- and chemoresistance of tumor cells, a new aspect this review will concentrate on. Since resistance of tumor cells to conventional treatment regimes is still a major obstacle in cancer treatment, new targeting approaches in combination with conventional radio- and chemotherapy are highly desirable and of great interest to improve cancer patient cure.
Keywords: Caveolin-1, cancer, chemoresistance, ionizing radiation, metastasis, radioresistance
Introduction
Radioresistance of tumor cells is a multifactorial characteristic. One critical factor is the intrinsic cellular radiosensitivity, which depends on the repair capacity of radiation-induced DNA lesions [1]. Additional essential factors are hypoxia [2], differential gene expression [3], growth factor receptors, mutations in proto-oncogenes and tumor suppressor genes [4], expression of receptor tyrosine kinases [5] and adhesion of cells to extracellular matrix (ECM) molecules mediated by cell surface receptors named integrins [6-8]. Interactions between integrins and receptor tyrosine kinases are rendered possible by specific intracellular signaling and scaffolding proteins, of which the integral membrane protein named Caveolin is one [9].
Caveolins comprise a family of three proteins named Caveolin-1, -2 and -3. While Caveolin-1 and -2 are ubiquitously co-expressed with high expression levels especially in epithelial and endothelial cells, fibroblasts, smooth muscle cells, adipocytes and pneumocytes, is the expression of Caveolin-3 mainly restricted to striated, cardiac and smooth muscle cells [10] and has also been described in C6 glioma cells [11]. Caveolin oligomerizes at the plasma membrane and its main function is that of a structural and functional element of small glycolipid-/ cholesterol-rich invaginations of the plasma membrane named Caveolae. Approximately 100 to 200 Caveolin molecules are present per Caveola [12]. Caveolae are responsible for membrane trafficking, endocytosis and lipid homeostasis but also serve in signaling processes as a compartment where receptors and signaling proteins are concentrated [13-17]. Caveolin-1 in endothelial cells regulates angiogenesis, microvascular permeability and vascular remodeling [16, 18-20]. Caveolin-1 and -3 seem to be essential for Caveolae formation while the function of Caveolin-2 is less clear [21-23].
Apart from its signaling function in normal cells, Caveolin-1 takes part in pathophysiological processes such as inflammation [24], atherosclerosis and oncogenic transformation. Moreover, Caveolin-1 is thought to exert anti-apoptotic and tumor-promoting properties, stimulation of metastasis and to have prognostic value for patient survival and cancer recurrency. Contrariwise, Caveolin-1 also functions as tumor suppressor and pro-apoptotic protein [25-30]. Reasons for this Janus-like properties are postulated to be mainly due to cell-specific properties, physiological context, cellular transformation processes and cancer stage [31].
Since Caveolin-1 is the first identified and most widely explored family member, we focused in the following on the emerging role of Caveolin-1 as prognostic marker for cancer cure and on its impact on chemo- and radioresis-tance of cancer cells.
Caveolin structure and function
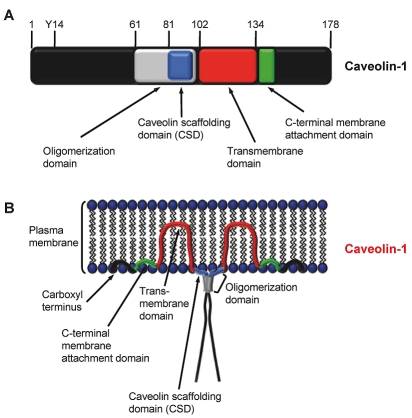
Caveolin protein family members Caveolin-1, -2 and -3 are integral membrane proteins with a molecular weight between 18 and 24 kDa [31, 32]. All three proteins are associated with membranes including the plasma membrane, endoplasmic reticulum and Golgi apparatus [31]. They possess a similar structure consisting of N- and C-terminal cytosolic domains, an oligomerization domain (residues 61-101 of Caveolin-1), including a Caveolin scaffolding domain (CSD; residues 82-101 of Caveolin-1) responsible for dimerization and interaction with signaling partners, a central transmembrane domain and a C-terminal membrane attachment domain [31-34]. Schematics of the Caveolin-1 domain structure and its localization in the plasma membrane are depicted in Figure 1A and Figure 1B. Moreover, Caveolin-1 is present in the cell in two isoforms, named α and β. Due to alternative splicing or initiation, Caveolin-1β is distinct from Caveolin-1α through a 31 amino acid residue deletion at the amino terminus [35-37].
Figure 1.
Structure of Caveolin-1. A, Important functional domains and phosphorylation site of Caveolin-1. Amino acid positions are depicted above the scheme. Y14, phosphorylation site at tyrosine 14. B, Localization of Caveolin-1 in the plasma membrane (modified from Williams and Lisanti [32]).
Caveolin-1 contains a tyrosine (Y) phosphorylation site at residue 14, which is apparently not present in Caveolin-1β. Phosphorylation at this amino acid residue leads to accumulation of the protein at focal adhesion sites and subsequent transmission of extracellular signals via intracellular pathways [38, 39]. Important to note is that activation of e.g. EGFR or Akt requires previous Src-mediated phosphorylation of Caveolin-1 at Y14 [40]. Also direct activation of the insulin receptor by Caveolin-1 and -3 has been observed [41]. As mentioned previously, Caveolin-1 has been shown to interact with β1 integrins and to promote Fyn-dependent Src homology containing (Shc) and mitogen-activated protein kinase (MAPK) phosphorylation [42-44]. How essential Caveolin-1 and its interaction with β1 integrin is can be seen from embryonic stem cell growth. Here, both proteins cooperate with p38 MAPK and the integrin signaling mediator Focal adhesion kinase (FAK) to provide optimal growth regulation [45]. Owing to its function as scaffold protein, Caveolin-1 seems also able to maintain a specific activity status of, for example, the important prosurvival protein Akt. Probably this action is facilitated by inhibitory binding of the Akt-regulating phosphatases protein phosphatase 1 and 2A to the CSD domain of Caveolin-1 [46]. This finding is supported by experiments in human pancreatic cancer cell lines, in which siRNA-mediated depletion of Caveolin-1 resulted in Akt dephosphorylation [47].
This short summary nicely exhibits the multi-functionality of Caveolin-1 and that most mechanisms this integral membrane protein serves in remain to be elucidated.
Caveolin-1 is associated with cancer progression and metastasis
First reports analyzing Caveolin-1 expression in different tumor entities such as breast cancer [48], colon cancer [49], ovarian carcinoma [50] and soft-tissue sarcomas [51] showed a down-regulation of Caveolin-1 expression as well as a tumor suppressor function of Caveolin-1. In addition, a more recent publication found that stromal loss of Caveolin-1 expression is associated with poor clinical outcome and survival of patients with basal-like and estrogen receptor/ progesterone receptor/HER2-negative breast cancers [52]. The most likely explanation of the discrepancies between Caveolin-1's role as tumor promoter versus tumor suppressor consists of a tumor stage dependency [31, 53, 54]. Similar hypotheses and evidence have been published for the cell-cell contact protein E-cadherin. A link between Caveolin-1 and E-cadherin is suggested by a recent study evaluating oncogenic transformation [31, 53]. In detail, Caveolin-1 seems to inhibit the expression of the anti-apoptotic protein Survivin only in the presence of E-cadherin via the β-catenin-Tcf/Lef pathway in human colon cancer and mouse melanoma cells [53] involving Cyclooxygenase-2 (COX-2) and prostaglandin E2 [54].
In contrast, later reports added further functional facets to Caveolin that indicate this protein to be overexpressed in different cancers, that it might serve as a prognostic factor for patient outcome and that it may contribute to metastatic spread (reviewed in: [31, 55-58]). Examples for increased expression of Caveolin-1 in tumor cells in comparison to normal tissues are prostate cancer (reviewed in [55]), ductal adenocarcinoma of the pancreas [47, 59], squamous cell carcinoma of the esophagus [60, 61], glioblastoma [62], renal cell carcinoma [27] and non-small cell lung carcinoma (NSCLC) [63] (summarized in Table 1).
Table 1.
Role of Caveolin in cancer progression and metastasis
| Authors | Year | Tumor entity | Main findings | Reference |
|---|---|---|---|---|
| Chen et al. | 2011 | lung | Increased Caveolin-1 expression indicated malignant progression and high invasion capability of NSCLC | [65] |
| Park et al. | 2010 | kidney | Caveolin-1 was upregulated in human renal cell carcinoma in comparison to adjacent tissue using cDNA microarray analysis | [27] |
| Li et al. | 2010 | lung | Caveolin-1 promoted lymph node metastasis in NSCLC | [26] |
| Rödel et al. | 2009 | rectum | Low Caveolin-1 expression in adenocarcinomas of the rectum was significantly related to better local control rates at 5 years and to an increased overall survival rate after preoperative chemoradiation | [64] |
| Hehlgans et al. | 2009 | pancreas | Caveolin-1 was overexpressed in pancreatic tumor cells compared to tumor stromal cells and pancreatic parenchymal cells | [47] |
| Tanase et al. | 2009 | pancreas | Caveolin-1 was overexpressed in ductal adenocarcinoma of the pancreas when compared to peritumoral tissue and associated with grade, stage and size of tumor | [59] |
| Ho et al. | 2008 | lung | Caveolin-1 expression was associated with a poor prognosis and with drug resistance in advanced NSCLC patients after Gemcitabine-based chemotherapy | [63] |
| Ando et al. | 2007 | esophagus | Caveolin-1 and -2 mRNA expression was significantly higher in esophageal squamous cell carcinoma than in corresponding normal esophageal mucosa | [61] |
| Barresi et al. | 2006 | brain (meningiomas) | Increased Caveolin-1 expression was correlated with worse patient survival and biological aggressiveness of meningiomas | [62] |
| Kato et al. | 2002 | esophagus | Caveolin-1 expression was correlated with a poor prognosis and lymph node metastasis in esophageal squamous cell carcinoma after surgery | [60] |
Concerning Caveolin-1 as prognostic marker, low Caveolin-1 expression levels in adenocarcinomas of the rectum were strongly associated with better local control rates at 5 years and increased overall survival after treatment [64]. In contrast, increased expression of Caveolin-1 in meningiomas and esophageal squamous cell carcinomas was associated with worse patient survival [60, 62]. As metastatic spread still severely hampers patient cure and survival and pursues to present a major obstacle in cancer treatment, studies have been performed to connect Caveolin-1 to this event. In NSCLC [26, 65], lung adenocarcinoma [25], esophageal squamous cell carcinoma [60] and renal cell carcinoma cells under doxorubicin treatment in a mouse model [27], Caveolin-1 expression showed a contribution to metastases formation. Studies to prove Caveolin-1 as essential determinant of migration and invasion of e.g. human head and neck squamous cell carcinoma (HNSCC) cells [66] and lung adenocarcinoma cells [25][67] supported this notion.
Functioning in such a great variety of physiological and pathophysiological cellular processes, researchers then asked the question if Caveolin -1 might also contribute to therapy resistance in cancer cells - a subject dealt with in the next section.
Role of Caveolin-1 in radio- and chemoresistance of human tumor cells
In recent years, the molecular mechanisms how Caveolin-1 is involved in cancer cell radio- and chemoresistance has been increasingly explored (summarized in Table 2). This interest arose from a study examining effects in whole body irradiated Caveolin-1 knockout mice [68]. The authors showed a significant decreased survival rate of Caveolin-1 deficient mice relative to Caveolin-1 wild-type mice. This decreased survival rate was ascribed to increased apoptosis and abnormal proliferation of intestinal crypt stem cells of the small intestine.
Table 2.
Role of Caveolin in radio- and chemoresistance
| Authors | Year | Tumor entity | Main findings | Reference |
|---|---|---|---|---|
| Park et al. | 2010 | kidney | Caveolin-1 knockdown sensitized human renal carcinoma cells to doxorubicin-induced apoptosis and reduced lung metastasis in a mouse model | [27] |
| Hehlgans et al. | 2009 | pancreas | Caveolin-1 knockdown sensitized pancreatic tumor cell lines grown in 3D lrECM to X-rays | [47] |
| Dittmann et al. | 2008 | lung, head & neck (SCC) | EGFR receptor internalization and transport into the nucleus upon irradiation depended on Src activation and Caveolin-1 | [70] |
| Ho et al. | 2008 | lung (NSCLC) | Caveolin-1 expression was associated with a poor prognosis and with drug resistance in advanced NSCLC patients after Gemcitabine-based chemotherapy | [63] |
| Cordes et al. | 2007 | pancreas | Knockdown of Caveolin-1 radiosensitized pancreatic cancer cell lines to ionizing radiation | [69] |
| Li et al. | 2005 | Enhanced radiosensitivity of Caveolin-1 deficient mice was associated with increased apoptosis and abnormal proliferation of intestinal crypt stem cells of the small intestine | [68] |
In 2007, our group reported radiosensitization and antiproliferative effects by siRNA-mediated knockdown of Caveolin-1 in pancreatic cancer cells MiaPaCa2, Panc1 and PATU8902, while overexpression of Caveolin-1 conferred radioresistance in these cell lines [69]. Mechanistically, Caveolin-1 knockdown was associated with changes in expression or phosphorylation of Akt, glycogen synthase kinase 3β, Paxillin, Src, c -Jun N-terminal kinase and mitogen-activated protein kinase. DNA microarray technology assisted us to detect Caveolin-1 overexpression in a subset of biopsies from human ductal adenocarcinoma of the pancreas as well as in human pancreatic tumor cell lines as compared to normal tissues. Interestingly, we found an interrelation between Caveolin-1 and β1 integrin and FAK. Following up on these observations, we continued in three-dimensional laminin-rich extracellular matrix (3D lrECM) based cell culture models using pancreatic tumor cells [47]. The radiosensitizing effects generated under 3D lrECM growth conditions corroborated our previous findings. Concerning the underlying mechanisms, we showed a connection of Caveolin-1 to DNA repair processes as silencing of Caveolin-1 resulted in increased levels of residual DNA double strand breaks in irradiated 3D cell cultures. Despite enhanced apoptosis in unirradiated and irradiated Caveolin-1 knockdown cell cultures, neither basal clonogenic cell survival nor radiosensitization were eventually affected by apoptotic processes [47]. Thus, how does Caveolin-1 participate in DNA repair? First findings suggested EGFR internalization into the cell nucleus to be Src-dependent and somewhat key to removal of DNA lesions by the DNA-Protein Kinase (DNA-PK) [70, 71]. Nevertheless, this issue is still under debate not only due to the fact that cell membrane to-cell nucleus translation of the EGFR seems not a general phenomenon but rather specific for selected cell lines in vitro.
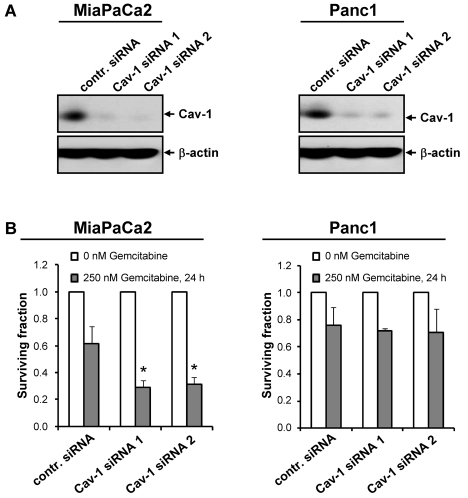
Evaluating the data generated in 3D lrECM cell culture more deeply, a decreased expression of integrin subunits was revealed upon Caveolin-1 depletion [47]. These results further supported the dependence of both, integrins and EGFR, on the integral membrane protein Caveolin-1, its putative role in resistance of tumor cells to ionizing radiation and chemotherapy and cellular signal transduction [6-8, 72, 73]. Histologically, several studies demonstrated co-localization of β1 integins and Caveolin-1 in human chondro-cytes [74], endothelial cells [75] and human pancreatic adenocarcinoma [47]. With regard to tumor cell multidrug resistance, most of the examinations have been performed in colon and breast cancer cell lines. Obviously, upregulation of Caveolin-1 expression in these cell lines mediated significant drug resistance [31, 49, 76]. In concordance with these findings, siRNA-mediated knockdown of Caveolin-1 decreased the incidence of lung metastasis from injected renal cell carcinoma cells under doxorubicin treatment in mouse experiments [27]. On the basis of the association of elevated Caveolin-1 expression with poor prognosis and Gemcitabine drug resistance of patients with advanced NSCLC [63], we investigated the chemosensitivity to Gemcitabine in Caveolin-1 knockdown pancreatic cancer cell cultures and found MiaPaCa2 to be chemosensitized to Gemcitabine, while the more resistant pancreatic cancer cell line Panc1 remained unaffected (Figure 2A and B).
Figure 2.
Cell line-dependent chemosensitization by Caveolin-1 knockdown of human pancreatic tumor cells to Gemcitabine. A, Confirmatory Western blot analysis of siRNA-mediated knockdown of Caveolin-1 in human pancreatic cancer cell lines MiaPaCa2 and Panc1 with two specific siRNAs directed against human Caveolin-1 (Cav-1 siRNA 1, 5'-GCUUCCUGAUUGAGAUUCAtt-3' [47]); Cav-1 siRNA 2, 5'-UGUGAUUGCAGAACCAGAAtt-3' [69]) (negative control siRNA #1 (Applied Biosystems, Darmstadt, Germany), contr. siRNA). B, For analysis of clonogenic survival, MiaPaCa2 or Panc1 cells were transfected with Caveolin-1 specific siRNA (Cav-1 siRNA 1/2) or control siRNA (contr. siRNA), plated as single cells on 6-well plates (BD, Heidelberg, Germany) and treated for 24 h with 250 nM Gemcitabine (Lilly Deutschland, Bad Homburg, Germany) 48 h after siRNA transfection. Colonies were counted microscopically after 9 days (MiaPaCa2) or 12 days (Panc1). Results are means ± s.d. (n = 3). Student's t-test compared Cav-1 siRNA transfected cells to siRNA controls. *P < 0.05.
Summary
Differential expression and/or function are prerequisites to be fulfilled by a specific target molecule to be designated as potential cancer target. Similarly important are these criteria for monotherapy application or combined application with conventional radio- and chemotherapy of a targeted drug against such a particular molecule. Consequently and in parallel, targeting efficacy is increased and normal tissue toxicity is reduced. As easily seen from this short review, Caveolin-1 is frequently overexpressed in a large range of tumor entities and the data point at a critical role of this integral membrane protein in carcinogenesis, tumor progression, metastatic spread and therapy resistance.
Whether a targeted anti-Caveolin-1 therapy might be feasible, reasonable and effective for optimization of cancer cure requires detailed clarification in future studies.
Acknowledgments
The research and authors were in part supported by a grant from the Bundesministerium für Bildung und Forschung (BMBF Contract 03ZIK041) and by a grant from the Medical Faculty Carl Gustav Carus, Dresden University of Technology, Germany (MedDrive program).
Glossary
Abbreviations
- COX-2
Cyclooxygenase-2
- CSD
Caveolin scaffolding domain
- DNA-DSB
DNA-double strand break
- DNA-PK
DNA-Protein Kinase
- ECM
extracellular matrix
- EGFR
epidermal growth factor receptor
- FAK
Focal adhesion kinase
- HNSCC
head and neck squamous cell carcinoma
- lrECM
laminin-rich extracellular matrix
- MAPK
mitogen-activated protein kinase
- NSCLC
non-small cell lung carcinoma
- Shc
Src homology containing
- Y
tyrosine
References
- 1.Jeggo P, Lobrich M. Radiation-induced DNA damage responses. Radiat Prot Dosimetry. 2006;122:124–127. doi: 10.1093/rpd/ncl495. [DOI] [PubMed] [Google Scholar]
- 2.Vaupel P. Tumor microenvironmental physiology and its implications for radiation oncology. Semin Radiat Oncol. 2004;14:198–206. doi: 10.1016/j.semradonc.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 3.Ogawa K, Murayama S, Mori M. Predicting the tumor response to radiotherapy using mi-croarray analysis (Review) Oncol Rep. 2007;18:1243–1248. [PubMed] [Google Scholar]
- 4.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 5.Rodemann HP, Dittmann K, Toulany M. Radiation-induced EGFR-signaling and control of DNA-damage repair. Int J Radiat Biol. 2007;83:781–791. doi: 10.1080/09553000701769970. [DOI] [PubMed] [Google Scholar]
- 6.Hehlgans S, Haase M, Cordes N. Signalling via integrins: implications for cell survival and anticancer strategies. Biochim Biophys Acta. 2007;1775:163–180. doi: 10.1016/j.bbcan.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 7.SandfortV, Koch U, Cordes N. Cell adhesion -mediated radioresistance revisited. Int J Radiat Biol. 2007;83:727–732. doi: 10.1080/09553000701694335. [DOI] [PubMed] [Google Scholar]
- 8.Park CC, Zhang HJ, Yao ES, Park CJ, Bissell MJ. Beta1 integrin inhibition dramatically enhances radiotherapy efficacy in human breast cancer xenografts. Cancer Res. 2008;68:4398–4405. doi: 10.1158/0008-5472.CAN-07-6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamada KM, Even-Ram S. Integrin regulation of growth factor receptors. Nat Cell Biol. 2002;4:E75–76. doi: 10.1038/ncb0402-e75. [DOI] [PubMed] [Google Scholar]
- 10.Song KS, Scherer PE, Tang Z, Okamoto T, Li S, Chafel M, Chu C, Kohtz DS, Lisanti MP. Expression of caveolin-3 in skeletal, cardiac, and smooth muscle cells. Caveolin-3 is a component of the sarcolemma and co-fractionates with dystrophin and dystrophin-associated gly-coproteins. J Biol Chem. 1996;271:15160–15165. doi: 10.1074/jbc.271.25.15160. [DOI] [PubMed] [Google Scholar]
- 11.Silva WI, Maldonado HM, Velazquez G, Rubio-Davila M, Miranda JD, Aquino E, Mayol N, Cruz-Torres A, Jardon J, Salgado-Villanueva IK. Caveolin isoform expression during differentiation of C6 glioma cells. Int J Dev Neurosci. 2005;23:599–612. doi: 10.1016/j.ijdevneu.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 12.Pelkmans L, Zerial M. Kinase-regulated quantal assemblies and kiss-and-run recycling of caveolae. Nature. 2005;436:128–133. doi: 10.1038/nature03866. [DOI] [PubMed] [Google Scholar]
- 13.Anderson RG. The caveolae membrane system. Annu Rev Biochem. 1998;67:199–225. doi: 10.1146/annurev.biochem.67.1.199. [DOI] [PubMed] [Google Scholar]
- 14.Fielding CJ, Fielding PE. Relationship between cholesterol trafficking and signaling in rafts and caveolae. Biochim Biophys Acta. 2003;1610:219–228. doi: 10.1016/s0005-2736(03)00020-8. [DOI] [PubMed] [Google Scholar]
- 15.Salanueva IJ, Cerezo A, Guadamillas MC, del Pozo MA. Integrin regulation of caveolin function. J Cell Mol Med. 2007;11:969–980. doi: 10.1111/j.1582-4934.2007.00109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chidlow JH, Jr, Sessa WC. Caveolae, caveo-lins, and cavins: complex control of cellular signalling and inflammation. Cardiovasc Res. 2010;86:219–225. doi: 10.1093/cvr/cvq075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 18.Bauer PM, Yu J, Chen Y, Hickey R, Bernatchez PN, Looft-Wilson R, Huang Y, Giordano F, Stan RV, Sessa WC. Endothelial-specific expression of caveolin-1 impairs microvascular permeability and angiogenesis. Proc Natl Acad Sci USA. 2005;102:204–209. doi: 10.1073/pnas.0406092102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu J, Bergaya S, Murata T, Alp IF, Bauer MP, Lin MI, Drab M, Kurzchalia TV, Stan RV, Sessa WC. Direct evidence for the role of caveolin-1 and caveolae in mechanotransduction and remodeling of blood vessels. J Clin Invest. 2006;116:1284–1291. doi: 10.1172/JCI27100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin MI, Yu J, Murata T, Sessa WC. Caveolin-1-deficient mice have increased tumor microvascular permeability, angiogenesis, and growth. Cancer Res. 2007;67:2849–2856. doi: 10.1158/0008-5472.CAN-06-4082. [DOI] [PubMed] [Google Scholar]
- 21.Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, Menne J, Lindschau C, Mende F, Luft FC, Schedl A, Haller H, Kurzchalia TV. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293:2449–2452. doi: 10.1126/science.1062688. [DOI] [PubMed] [Google Scholar]
- 22.Razani B, Wang XB, Engelman JA, Battista M, Lagaud G, Zhang XL, Kneitz B, Hou H, Jr, Christ GJ, Edelmann W, Lisanti MP. Caveolin-2-deficient mice show evidence of severe pulmonary dysfunction without disruption of caveolae. Mol Cell Biol. 2002;22:2329–2344. doi: 10.1128/MCB.22.7.2329-2344.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Razani B, Engelman JA, Wang XB, Schubert W, Zhang XL, Marks CB, Macaluso F, Russell RG, Li M, Pestell RG, Di Vizio D, Hou H, Jr, Kneitz B, Lagaud G, Christ GJ, Edelmann W, Lisanti MP. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J Biol Chem. 2001;276:38121–38138. doi: 10.1074/jbc.M105408200. [DOI] [PubMed] [Google Scholar]
- 24.Frank PG, Pavlides S, Cheung MW, Daumer K, Lisanti MP. Role of caveolin-1 in the regulation of lipoprotein metabolism. Am J Physiol Cell Physiol. 2008;295:C242–248. doi: 10.1152/ajpcell.00185.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ho CC, Huang PH, Huang HY, Chen YH, Yang PC, Hsu SM. Up-regulated caveolin-1 accentuates the metastasis capability of lung adeno-carcinoma by inducing filopodia formation. Am J Pathol. 2002;161:1647–1656. doi: 10.1016/S0002-9440(10)64442-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li M, Chen H, Diao L, Zhang Y, Xia C, Yang F. Caveolin-1 and VEGF-C promote lymph node metastasis in the absence of intratumoral lymphangiogenesis in non-small cell lung cancer. Tumori. 2010;96:734–743. doi: 10.1177/030089161009600516. [DOI] [PubMed] [Google Scholar]
- 27.Park J, Bae E, Lee C, Yoon SS, Chae YS, Ahn KS, Won NH. RNA interference-directed caveolin-1 knockdown sensitizes SN12CPM6 cells to doxorubicin-induced apoptosis and reduces lung metastasis. Tumour Biol. 2010;31:643–650. doi: 10.1007/s13277-010-0081-1. [DOI] [PubMed] [Google Scholar]
- 28.Carver LA, Schnitzer JE. Caveolae: mining little caves for new cancer targets. Nat Rev Cancer. 2003;3:571–581. doi: 10.1038/nrc1146. [DOI] [PubMed] [Google Scholar]
- 29.Galbiati F, Volonte D, Engelman JA, Watanabe G, Burk R, Pestell RG, Lisanti MP. Targeted downregulation of caveolin-1 is sufficient to drive cell transformation and hyperactivate the p42/44 MAP kinase cascade. Embo J. 1998;17:6633–6648. doi: 10.1093/emboj/17.22.6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams TM, Lisanti MP. Caveolin-1 in oncogenic transformation, cancer, and metastasis. Am J Physiol Cell Physiol. 2005;288:C494–506. doi: 10.1152/ajpcell.00458.2004. [DOI] [PubMed] [Google Scholar]
- 31.Quest AF, Gutierrez-Pajares JL, Torres VA. Caveolin-1: an ambiguous partner in cell signalling and cancer. J Cell Mol Med. 2008;12:1130–1150. doi: 10.1111/j.1582-4934.2008.00331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williams TM, Lisanti MP. The caveolin proteins. Genome Biol. 2004;5:214. doi: 10.1186/gb-2004-5-3-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Couet J, Sargiacomo M, Lisanti MP. Interaction of a receptor tyrosine kinase, EGF-R, with caveolins. Caveolin binding negatively regulates tyrosine and serine/threonine kinase activities. J Biol Chem. 1997;272:30429–30438. doi: 10.1074/jbc.272.48.30429. [DOI] [PubMed] [Google Scholar]
- 34.Li S, Couet J, Lisanti MP. Src tyrosine kinases, Galpha subunits, and H-Ras share a common membrane-anchored scaffolding protein, caveolin. Caveolin binding negatively regulates the auto-activation of Src tyrosine kinases. J Biol Chem. 1996;271:29182–29190. doi: 10.1074/jbc.271.46.29182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kogo H, Fujimoto T. Caveolin-1 isoforms are encoded by distinct mRNAs. Identification Of mouse caveolin-1 mRNA variants caused by alternative transcription initiation and splicing. FEBS Lett. 2000;465:119–123. doi: 10.1016/s0014-5793(99)01730-5. [DOI] [PubMed] [Google Scholar]
- 36.Scherer PE, Tang Z, Chun M, Sargiacomo M, Lodish HF, Lisanti MP. Caveolin isoforms differ in their N-terminal protein sequence and subcellular distribution. Identification and epi-tope mapping of an isoform-specific monoclonal antibody probe. J Biol Chem. 1995;270:16395–16401. doi: 10.1074/jbc.270.27.16395. [DOI] [PubMed] [Google Scholar]
- 37.Kogo H, Aiba T, Fujimoto T. Cell type-specific occurrence of caveolin-1alpha and -1beta in the lung caused by expression of distinct mRNAs. J Biol Chem. 2004;279:25574–25581. doi: 10.1074/jbc.M310807200. [DOI] [PubMed] [Google Scholar]
- 38.Lee H, Volonte D, Galbiati F, Iyengar P, Lublin DM, Bregman DB, Wilson MT, Campos-Gonzalez R, Bouzahzah B, Pestell RG, Scherer PE, Lisanti MP. Constitutive and growth factor-regulated phosphorylation of caveolin-1 occurs at the same site (Tyr-14) in vivo: identification of a c-Src/Cav-1/Grb7 signaling cassette. Mol Endocrinol. 2000;14:1750–1775. doi: 10.1210/mend.14.11.0553. [DOI] [PubMed] [Google Scholar]
- 39.Mettouchi A, Klein S, Guo W, Lopez-Lago M, Lemichez E, Westwick JK, Giancotti FG. Integrin-specific activation of Rac controls progression through the G(1) phase of the cell cycle. Mol Cell. 2001;8:115–127. doi: 10.1016/s1097-2765(01)00285-4. [DOI] [PubMed] [Google Scholar]
- 40.Zhang B, Peng F, Wu D, Ingram AJ, Gao B, Krepinsky JC. Caveolin-1 phosphorylation is required for stretch-induced EGFR and Akt activation in mesangial cells. Cell Signal. 2007;19:1690–1700. doi: 10.1016/j.cellsig.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 41.Yamamoto M, Toya Y, Schwencke C, Lisanti MP, Myers MG, Jr, Ishikawa Y. Caveolin is an activator of insulin receptor signaling. J Biol Chem. 1998;273:26962–26968. doi: 10.1074/jbc.273.41.26962. [DOI] [PubMed] [Google Scholar]
- 42.Wary KK, Mariotti A, Zurzolo C, Giancotti FG. A requirement for caveolin-1 and associated kinase Fyn in integrin signaling and anchorage-dependent cell growth. Cell. 1998;94:625–634. doi: 10.1016/s0092-8674(00)81604-9. [DOI] [PubMed] [Google Scholar]
- 43.Wei Y, Yang X, Liu Q, Wilkins JA, Chapman HA. A role for caveolin and the urokinase receptor in integrin-mediated adhesion and signaling. J Cell Biol. 1999;144:1285–1294. doi: 10.1083/jcb.144.6.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Del Pozo MA, Schwartz MA. Rac, membrane heterogeneity, caveolin and regulation of growth by integrins. Trends Cell Biol. 2007;17:246–250. doi: 10.1016/j.tcb.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 45.Lee SH, Lee YJ, Park SW, Kim HS, Han HJ. Caveolin-1 and integrin beta1 regulate embryonic stem cell proliferation via p38 MAPK and FAK in high glucose. J Cell Physiol. 2010 doi: 10.1002/jcp.22510. [DOI] [PubMed] [Google Scholar]
- 46.Li L, Ren CH, Tahir SA, Ren C, Thompson TC. Caveolin-1 maintains activated Akt in prostate cancer cells through scaffolding domain binding site interactions with and inhibition of serine/threonine protein phosphatases PP1 and PP2A. Mol Cell Biol. 2003;23:9389–9404. doi: 10.1128/MCB.23.24.9389-9404.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hehlgans S, Eke I, Storch K, Haase M, Baretton GB, Cordes N. Caveolin-1 mediated radiore-sistance of 3D grown pancreatic cancer cells. Radiother Oncol. 2009;92:362–370. doi: 10.1016/j.radonc.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 48.Lee SW, Reimer CL, Oh P, Campbell DB, Schnitzer JE. Tumor cell growth inhibition by caveolin re-expression in human breast cancer cells. Oncogene. 1998;16:1391–1397. doi: 10.1038/sj.onc.1201661. [DOI] [PubMed] [Google Scholar]
- 49.Bender FC, Reymond MA, Bron C, Quest AF. Caveolin-1 levels are down-regulated in human colon tumors, and ectopic expression of caveolin-1 in colon carcinoma cell lines reduces cell tumorigenicity. Cancer Res. 2000;60:5870–5878. [PubMed] [Google Scholar]
- 50.Wiechen K, Diatchenko L, Agoulnik A, Scharff KM, Schober H, Arlt K, Zhumabayeva B, Siebert PD, Dietel M, Schafer R, Sers C. Caveolin-1 is down-regulated in human ovarian carcinoma and acts as a candidate tumor suppressor gene. Am J Pathol. 2001;159:1635–1643. doi: 10.1016/S0002-9440(10)63010-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wiechen K, Sers C, Agoulnik A, Arlt K, Dietel M, Schlag PM, Schneider U. Down-regulation of caveolin-1, a candidate tumor suppressor gene, in sarcomas. Am J Pathol. 2001;158:833–839. doi: 10.1016/S0002-9440(10)64031-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Witkiewicz AK, Dasgupta A, Sammons S, Er O, Potoczek MB, Guiles F, Sotgia F, Brody JR, Mitchell EP, Lisanti MP. Loss of stromal caveolin-1 expression predicts poor clinical outcome in triple negative and basal-like breast cancers. Cancer Biol Ther. 2010;10:135–143. doi: 10.4161/cbt.10.2.11983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Torres VA, Tapia JC, Rodriguez DA, Lladser A, Arredondo C, Leyton L, Quest AF. E-cadherin is required for caveolin-1-mediated down-regulation of the inhibitor of apoptosis protein survivin via reduced beta-catenin-Tcf/Lef-dependent transcription. Mol Cell Biol. 2007;27:7703–7717. doi: 10.1128/MCB.01991-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rodriguez DA, Tapia JC, Fernandez JG, Torres VA, Munoz N, Galleguillos D, Leyton L, Quest AF. Caveolin-1-mediated suppression of cyclooxygenase-2 via a beta-catenin-Tcf/Lef-dependent transcriptional mechanism reduced prostaglandin E2 production and survivin expression. Mol Biol Cell. 2009;20:2297–2310. doi: 10.1091/mbc.E08-09-0939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thompson TC, Tahir SA, Li L, Watanabe M, Naruishi K, Yang G, Kadmon D, Logothetis CJ, Troncoso P, Ren C, Goltsov A, Park S. The role of caveolin-1 in prostate cancer: clinical implications. Prostate Cancer Prostatic Dis. 2010;13:6–11. doi: 10.1038/pcan.2009.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goetz JG, Lajoie P, Wiseman SM, Nabi IR. Caveolin-1 in tumor progression: the good, the bad and the ugly. Cancer Metastasis Rev. 2008;27:715–735. doi: 10.1007/s10555-008-9160-9. [DOI] [PubMed] [Google Scholar]
- 57.Shatz M, Liscovitch M. Caveolin-1: a tumor-promoting role in human cancer. Int J Radiat Biol. 2008;84:177–189. doi: 10.1080/09553000701745293. [DOI] [PubMed] [Google Scholar]
- 58.Burgermeister E, Liscovitch M, Rocken C, Schmid RM, Ebert MP. Caveats of caveolin-1 in cancer progression. Cancer Lett. 2008;268:187–201. doi: 10.1016/j.canlet.2008.03.055. [DOI] [PubMed] [Google Scholar]
- 59.Tanase CP, Dima S, Mihai M, Raducan E, Nicolescu MI, Albulescu L, Voiculescu B, Dumitrascu T, Cruceru LM, Leabu M, Popescu I, Hinescu ME. Caveolin-1 overexpression correlates with tumour progression markers in pancreatic ductal adenocarcinoma. J Mol Histol. 2009;40:23–29. doi: 10.1007/s10735-008-9209-7. [DOI] [PubMed] [Google Scholar]
- 60.Kato K, Hida Y, Miyamoto M, Hashida H, Shinohara T, Itoh T, Okushiba S, Kondo S, Katoh H. Overexpression of caveolin-1 in esophageal squamous cell carcinoma correlates with lymph node metastasis and pathologic stage. Cancer. 2002;94:929–933. [PubMed] [Google Scholar]
- 61.Ando T, Ishiguro H, Kimura M, Mitsui A, Mori Y, Sugito N, Tomoda K, Mori R, Harada K, Katada T, Ogawa R, Fujii Y, Kuwabara Y. The over-expression of caveolin-1 and caveolin-2 correlates with a poor prognosis and tumor progression in esophageal squamous cell carcinoma. Oncol Rep. 2007;18:601–609. [PubMed] [Google Scholar]
- 62.Barresi V, Cerasoli S, Paioli G, Vitarelli E, Giuffre G, Guiducci G, Tuccari G, Barresi G. Caveolin-1 in meningiomas: expression and clinico-pathological correlations. Acta Neuropathol. 2006;112:617–626. doi: 10.1007/s00401-006-0097-1. [DOI] [PubMed] [Google Scholar]
- 63.Ho CC, Kuo SH, Huang PH, Huang HY, Yang CH, Yang PC. Caveolin-1 expression is significantly associated with drug resistance and poor prognosis in advanced non-small cell lung cancer patients treated with gemcitabine-based chemotherapy. Lung Cancer. 2008;59:105–110. doi: 10.1016/j.lungcan.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 64.Rodel F, Capalbo G, Rodel C, Weiss C. Caveolin-1 as a prognostic marker for local control after preoperative chemoradiation therapy in rectal cancer. Int J Radiat Oncol Biol Phys. 2009;73:846–852. doi: 10.1016/j.ijrobp.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 65.Chen HL, Fan LF, Gao J, Ouyang JP, Zhang YX. Differential expression and function of the caveolin-1 gene in non-small cell lung carcinoma. Oncol Rep. 2011;25:359–366. doi: 10.3892/or.2010.1095. [DOI] [PubMed] [Google Scholar]
- 66.Nohata N, Hanazawa T, Kikkawa N, Mutallip M, Fujimura L, Yoshino H, Kawakami K, Chiyomaru T, Enokida H, Nakagawa M, Okamoto Y, Seki N. Caveolin-1 mediates tumor cell migration and invasion and its regulation by miR-133a in head and neck squamous cell carcinoma. Int J Oncol. 2011;38:209–217. [PubMed] [Google Scholar]
- 67.Luanpitpong S, Talbott SJ, Rojanasakul Y, Nimmannit U, Pongrakhananon V, Wang L, Chanvorachote P. Regulation of lung cancer cell migration and invasion by reactive oxygen species and caveolin-1. J Biol Chem. 2010;285:38832–38840. doi: 10.1074/jbc.M110.124958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li J, Hassan GS, Williams TM, Minetti C, Pestell RG, Tanowitz HB, Frank PG, Sotgia F, Lisanti MP. Loss of caveolin-1 causes the hyper-proliferation of intestinal crypt stem cells, with increased sensitivity to whole body gamma-radiation. Cell Cycle. 2005;4:1817–1825. doi: 10.4161/cc.4.12.2199. [DOI] [PubMed] [Google Scholar]
- 69.Cordes N, Frick S, Brunner TB, Pilarsky C, Grutzmann R, Sipos B, Kloppel G, McKenna WG, Bernhard EJ. Human pancreatic tumor cells are sensitized to ionizing radiation by knockdown of caveolin-1. Oncogene. 2007;26:6851–6862. doi: 10.1038/sj.onc.1210498. [DOI] [PubMed] [Google Scholar]
- 70.Dittmann K, Mayer C, Kehlbach R, Rodemann HP. Radiation-induced caveolin-1 associated EGFR internalization is linked with nuclear EGFR transport and activation of DNA-PK. Mol Cancer. 2008;7:69. doi: 10.1186/1476-4598-7-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dittmann K, Mayer C, Fehrenbacher B, Schaller M, Raju U, Milas L, Chen DJ, Kehlbach R, Rodemann HP. Radiation-induced epidermal growth factor receptor nuclear import is linked to activation of DNA-dependent protein kinase. J Biol Chem. 2005;280:31182–31189. doi: 10.1074/jbc.M506591200. [DOI] [PubMed] [Google Scholar]
- 72.Baumann M, Krause M, Hill R. Exploring the role of cancer stem cells in radioresistance. Nat Rev Cancer. 2008;8:545–554. doi: 10.1038/nrc2419. [DOI] [PubMed] [Google Scholar]
- 73.Krause M, Baumann M. Clinical biomarkers of kinase activity: examples from EGFR inhibition trials. Cancer Metastasis Rev. 2008;27:387–402. doi: 10.1007/s10555-008-9141-z. [DOI] [PubMed] [Google Scholar]
- 74.Schwab W, Kasper M, Gavlik JM, Schulze E, Funk RH, Shakibaei M. Characterization of caveolins from human knee joint cartilage: expression of caveolin-1, -2, and -3 in chondro-cytes and association with integrin beta1. Histochem Cell Biol. 2000;113:221–225. doi: 10.1007/s004180050441. [DOI] [PubMed] [Google Scholar]
- 75.Wickstrom SA, Alitalo K, Keski-Oja J. En-dostatin associates with integrin alpha5beta1 and caveolin-1, and activates Src via a tyrosyl phosphatase-dependent pathway in human endothelial cells. Cancer Res. 2002;62:5580–5589. [PubMed] [Google Scholar]
- 76.Lavie Y, Liscovitch M. Changes in lipid and protein constituents of rafts and caveolae in multidrug resistant cancer cells and their functional consequences. Glycoconj J. 2000;17:253–259. doi: 10.1023/a:1026553626537. [DOI] [PubMed] [Google Scholar]