Abstract
Prostate cancer (PCa) is initially regulated by androgens, such as testosterone and dihydrotestosterone, which regulates cell proliferation and survival by activating the androgen receptor (AR), but later progresses to an aggressive, metastatic, androgen-independent stage for which, currently, there is no cure. Here, we argue that prevention of PCa progression is a better strategy compared to trying to cure the disease once it has already progressed. Statins inhibit the mevalonate pathway, thus preventing the synthesis of cholesterol, geranylgeranyl pyrophosphate and farnesyl pyrophosphate. Multiple clinical studies have shown an inverse relationship between statin use and PCa risk, especially the risk for developing advanced metastatic cancer. Biochemical investigations have largely corroborated the positive effect of statins on PCa risk, showing that statins inhibited cell proliferation, induced apoptosis, and decreased cell migration and invasion in PCa cells in vitro. However, investigations of the biochemical mechanism of statin action in preventing advanced/high risk PCa remains inconclusive, as statins can act through cholesterol, geranylgeranyl, or farnesyl mediated signals. This review discusses the current clinical and biochemical findings on the use of statins in preventing PCa. Evidence of statin action through cholesterol as well as geranylgeranylation and farnesylation has been discussed. As cholesterol is a precursor of androgen production, it can reduce PCa risk by decreasing the levels of circulating testosterone, which in turn reduces the levels of interprostatic dihydrotestosterone, a strong ligand for the AR. Cholesterol was also shown to accumulate in lipid rafts and regulate the activation of the phosphatidylinositol 3-kinase/Akt pathway. However, clinical evidence from multiple studies also point to the existence of cholesterol-independent pathways mediating statin action in PCa patients. In particular, ligand-activated AR activation is seen in early stage PCa and activation of the cholesterol pathway did not indicate an effect on metastasis. Cell migration and invasion, on the other hand, is regulated strongly by members of the Ras superfamily of small GTPases, especially the Rho family, which is geranylgeranylated. This review, therefore, also compares the effects of statins on both cholesterol and geranylgeranylated and farnesylated small GTPases regulating tumor progression and metastasis in biochemical and clinical studies.
Keywords: Mevalonate pathway, cholesterol, geranylgeranyl pyrophosphate, farnesyl pyrophosphate, Akt, androgen receptor, metastasis, Ras, Rac, Rho
Introduction
Prostate Cancer (PCa) is the most common malignancy, and the second most common cause of cancer-related deaths among men in the United States [1]. Most patients in the United States undergo radical prostatectomy or radiation therapy for treatment of localized PCa [2], and a majority of patients with prostate confined tumors show decreased levels of serum prostate specific antigen (PSA), a surrogate marker for treatment failure, once the prostate gland and tumor are removed. However, evidence of increasing PSA values following primary treatment occurs in approximately 15-30% of patients and relates to tumor recurrence [3, 4]. Recurrent PCa is treated with androgen withdrawal therapy (AWD), which causes hormonal suppression of androgen production and may include treatment with androgen receptor (AR) antagonists (including flutamide or bicalu-tamide) [5, 6]. Patients usually respond initially to such treatment but eventually relapse indicating the development of castration resistant PCa (CRPC) [7]. Despite extensive research to find a cure for CRPC, the only treatment options currently available to these patients are do-cetaxel (Taxotere) [8, 9] and the cancer vaccine Sipuleucel-T (Provenge; Dendreon) [10], which extends survival by 3 and 4 months, respectively. Hence, it is imperative to investigate possible therapeutic agents which decrease the risk of recurrence and progression of PCa.
3-Hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors, commonly known as statins, are cholesterol-lowering drugs that are the second most prescribed therapeutic drug class in the United States, after painkillers such as acetaminophen [11]. As of 2010, approximately 24 million Americans use these medications [12]. Statin therapy is highly recommended and used for the prevention of cardiovascular disease in men forty years or older, who incidentally also have the highest risk of developing PCa [13]. Investigations revealed that, in addition to cholesterol-lowering effects, statins also have proapoptotic and antimetastatic effects in cancer cells [14]. As a result, several studies have investigated the possible therapeutic value of statins in PCa treatment. Many investigators assume that the effect of statins on PCa stems from their effects on cholesterol and point to the accumulation of cholesterol in solid tumors, and studies linking the increased risk of aggressive PCa to elevated cholesterol levels [15]. However, in vitro studies show that statins may also inhibit PCa by preventing the activation of small GTP-binding proteins of the Ras superfamily [16-19] which play important roles in the development and progression of PCa. In this review, we will compare literature investigating the effects of cholesterol vs Ras GTPases on PCa and seek to determine which of these mechanisms mediate the effect of statins on PCa risk reduction.
Downstream targets of statins in the mevalonate pathway
There are a number of statins that have been produced to date: atorvastatin, (Lipitor, the best selling pharmaceutical in history), lovastatin, simvastatin, pravastatin, pitavastatin, rosuvastatin, mevastatin, cerivastatin and fluvastatin. It has been suggested that lipophilic (hydrophobic) statins (e.g. atorvastatin, simvastatin, lovastatin, fluvastatin) may be able to affect cancer more than the hydrophillic statins (e.g. pravastatin and rosuvastatin), since hydrophobic statins have greater intracellular access and are able to cross biological membranes [11]. Hydrophobic statins inhibit proliferation of various PCa cell lines by inducing G1 cell cycle arrest. Lovastatin achieved this arrest at 0.5 mmol/L, a concentration easily achieved in the serum following oral administration [20]. The hydrophilic pravastatin, however, was less effective at inhibiting HMG-CoA reductase in PC-3 cells and had to be present at 200 times higher concentrations to effect a cell cycle arrest, since it's uptake into cells is not as efficient as the other statins [20]. In addition, the bioavailability of the HMG-CoA reductase inhibitors is limited by extensive first-pass metabolism [21]. The differences in metabolism among the various statins lead to different distributions of the drugs in the liver (via enterohepatic circulation) or peripheral tissues (via systemic circulation) at equivalent doses. Table 1 summarizes the various biological properties of statins [13, 21-24].
Table 1.
Pharmacological properties of Statins
| Statin Name | Brand Name | Lipophilicity | Bioavailability % | Hepatic Extraction (%absorbed) | Observed Mechanism in PCa cells in vitro |
|---|---|---|---|---|---|
| Atorvastatin | Lipitor | Lipophilic (+4.1) | 12 | 20-30 | Growth inhibition, induction of apoptosis and autophagy |
| Lovastatin | Mevacor | Lipophilic (+4.3) | <5 | 40-70 | Cell cycle arrest, induction of apoptosis, suppression of RhoA activity |
| Simvastatin | Zocor | Lipophilic (+4.7) | <5 | 50-80 | Induction of apoptosis through depletion of intracellular cholesterol |
| Pravastatin | Pravacol | Hydrophilic (-0.2) | 17 | 50-70 | Cell cycle arrest at high concentrations |
| Pitavastatin | Livalo | Lipophilic | 80 | ||
| Rosuvastatin | Crestor | Hydrophilic (-0.3) | 20 | Growth inhibition, induction of apoptosis, inhibition of angiogenesis | |
| Fluvastatin | Lescol | Lipophilic (+3.2) | 10-35 | 40-70 | Cell cycle arrest |
| Mevastatin* | Compactin | Lipophilic | |||
| Cerivastatin* | Baycol | Lipophilic | 60 | 50-60 |
Not FDA approved as of 2010.
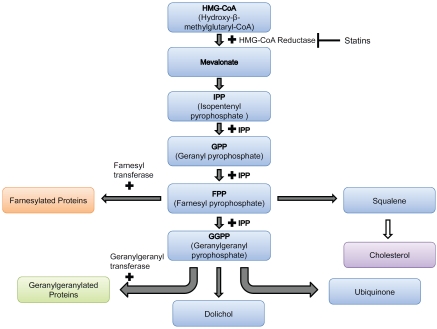
As shown in Figure 1, statins inhibit the synthesis of HMG-CoA reductase, which catalyzes a rate-limiting step in the mevalonate pathway. These inhibitors decrease hepatic cholesterol production, consequently decreasing hepatic Low Density Lipoprotein (LDL)-cholesterol uptake and ultimately cause a 20-60% decrease in plasma LDL-cholesterol level [21]. In PCa, feedback regulation of cholesterol absorption and synthesis is lost [25], resulting in the upregulation of the mevalonate pathway and increased LDL receptor expression. This can affect risk of PCa in two ways - by decreased cholesterol metabolism as well as by inhibition of other products of the mevalonate pathway. Intracellular depletion of cholesterol can induce apoptosis through inhibition of the phosphatidylinositol-3-kinase (PI3K)/Akt pathway, which plays a key role in mediating signals for cell growth, cell survival, and cell cycle progression [26-28]. Cholesterol, a critical component in biological membranes, accumulates in “lipid rafts”, which were shown to be important in mediating signal transduction pathways, including the PI3K-Akt pathway [15]. Further details of this pathway will be provided later in this review.
Figure 1.
HMG-CoA is reduced to mevalonate, which is converted into 5-carbon units (C5), called isopentenyl pyrophosphate (IPP). Two C5 units combine to form a C10 compound, geranyl pyrophosphate, which in turn combines with IPP to form the C15 compound farnesol pyrophosphate (FPP). FPP is a common intermediate for the biosynthesis of cholesterol, dolichol and ubiquinone as well as protein isoprenylation. FPP combines with IPP to form the C20 all-trans-geranylgeranyl pyrophosphate (GGPP). The isoprenoids FPP and GGPP are transferred by farnesyl and geranylgeranyl protein transferases respectively to various proteins, such as small GTP-binding proteins, for targeting and activation. Statins inhibit the reduction of HMG-CoA to mevalonate by preventing its binding to HMG-CoA reduc-tase, and hence statin treatment precludes all these events.
In addition, since statins function at an early step in the synthesis of cholesterol, they also inhibit the production of isoprenoids such as geranylgeranyl pyrophosphate (GGPP) and farnesyl pyrophosphate (FPP) [29] (Figure 1). These isoprenoids are involved in cellular signal transduction by activating farnesylated proteins (for example, Ras proteins) and geranylger-anylated proteins (for example, RhoA and Rac1). Geranylgeranyl transferase and farnesyl transferase use GPP and FPP for post-translational modifications of small GTP-binding proteins of Ras and Rho families [21], which must first undergo prenylation (farnesylation or geranylgeranylation) to associate with the plasma membrane and be activated [30]. The association is accomplished by the addition of the prenyl group to the COOH terminus of these proteins [21, 30]. Current studies indicate that geranylgeranylation is a crucial step in the apoptotic, angiogenic and inflammatory effects of statins [31], in addition to cell growth and proliferation [16]. Thus, PCa may be affected by cholesterol or the downstream targets of GPP and FPP.
Effect of statins on prostate cancer
Statins and overall prostate cancer risk
Multiple clinical studies have investigated the association of statin use with PCa incidence or development. Most studies were initially designed to investigate the effects of statins on cardiovascular disease, with cancer usually as a secondary end point or an adverse effect finding [31]. However, several recent studies have examined the direct relationship between statins and PCa (reviewed in [13]). Most of these studies are observational or retrospective in nature; however, the results do show promising trends, allowing for some associations to be made based on a relatively large human data set. Corresponding biochemical studies performed in vitro largely support an inverse relationship between statin use and PCa cell death or cell cycle arrest. Despite this, some studies have reported no correlation or a positive correlation, as described below:
Many clinical studies show an inverse relationship between statin use and PCa risk. Haukka et al. detected a linear decreasing trend relating exposure to statins with PCa in 5,871 cases [32]. A large study of 83,373 men from western Washington State by Boudreau et al. found no overall association between statin use and PCa; however, they found reduced risk of PCa with hydrophobic statin use, and indications of a reduced risk among ever users of statins [33]. Another study on 37,248 patients at the VA New England Healthcare System found a statistically significant lower risk for total cancer in statin users compared to non-users [34]. A dose-response relationship likely exists between statins and cancer incidence because the risk of cancer incidence appeared to decrease with an increase in equivalent statin dose [34, 35].
In contrast, several other studies report largely inconclusive or negative results when correlating statin use and cancer risk [11]. Specifically, several large population studies have found no link between statin use and PCa. For instance, Soto et al reported that a study of 968 patients treated with radiation therapy (RT); where 23% of the patients had been taking statins during RT, showed no effect of statin use on PCa outcomes [36]. In addition, a few clinical studies have reported an increased incidence of cancer with statin use, but largely with inconclusive results. A study on 361,859 Kaiser Permanente patients in Northern California, found an overall increased rate of cancer in male statin users, as well as an increased risk in stage I PCa but a decreased risk in stage II PCa [37]. However, the same study notes that another Kaiser study (California Men's Health Study) found a 28% reduction in risk of PCa in statin users [37]. Possible mechanisms which could result in such a risk included increased mitotic abnormalities and/or immunosuppression caused by alterations in leukocyte function (discussed in [11]). Increased mitotic abnormalities can interfere with the proper function of centromeres, thus increasing the risk for mutations. Statins also may inhibit promoter function and cause binding to leukocyte function antigen, which could lead to immunosuppression. However, statin concentrations used in such findings largely are based on animal models, and are higher than those used in humans for clinical treatment [11].
Statin use was also associated with a lower proportion of prostatectomy patients with positive surgical margins and lower tumor volume [38]. In a case control study, no overall association was found between statin use and PCa risk [39]. Despite this, risk related to statin use was modified by body mass index, and obese men who used statins had an increased risk relative to obese nonusers [39].
Taken together, it appears that there is a discrepancy between the studies described above, where some show a positive, others negative and yet others, no correlation between statin use and PCa risk. While there appears to be a correlation between hydrophobic statin use and a reduced risk of PCa incidence, there is no overall consensus based on the studies described so far, as to the overall effect of statins on the development of PCa. Therefore, further studies were required to delineate this relationship, as described below.
Statins and reduced risk of advanced/aggressive prostate cancer
The discrepancy between the effects of statins on PCa risk from various studies can be explained by the fact that while statins may or may not have an overall benefit in preventing PCa, they specifically prevent advanced or aggressive PCa. As mentioned earlier, the study on Kaiser Permanente patients in Northern California found an increased risk in stage I PCa but a decreased risk in stage II PCa in male statin users [37]. Several studies found that statins reduced advanced PCa risk but not overall PCa risk [14, 40, 41]. Platz et al. conducted a study on 34,438 health professionals, and found no overall reduction in PCa risk with statin use. However, an analysis of the extent of the disease showed a significant (46%) reduction in advanced PCa risk (compared with non-drug users), and the risk decreased with increasing duration of use [14]. The risk reduction was even stronger for metastatic and fatal disease [42, 43]. Metaanalysis of 6 randomized clinical trials and 13 observational studies revealed a statistically significant inverse relationship between statin use and advanced disease, but not overall risk of developing PCa [44].
As described in the Introduction, 15-30% of patients experience biochemical or clinical recurrence within 10 years after undergoing radical prostatectomy (RP). Biochemical recurrence is marked by an increase in postoperative PSA levels to 0.4 ng/mL followed by another increase [45]. A study by Hamilton et al. found that 1319 men who underwent RP and took statins at the time of surgery, had a 30% lower risk for biochemical recurrence (p=0.03) than men not taking statins [46]. The reduced risk was dose dependent, with no reduction in men who took the simvastatin dose-equivalent of 20 mg or less and a 46% reduction in men who took the dose-equivalent of 20 mg or more. Time to recurrence after RP is associated with the risk of PCa progression and death; hence, these studies suggest a potential therapeutic value in statin use [46]. Overall, these studies indicate an effect of statin use on PCa progression, which usually involves increased cell migration or metastasis - rather than on the development of the primary tumor.
Statins and radiosensitization
Inhibition of the HMG-CoA reductase pathway by statins has been associated with in vitro radiosensitization. In a study on 512 patients treated with permanent brachytherapy (internal RT), statins (especially atorvastatin, which has one of the greatest bioavailabilities) improved the clinical presentation of PCa in an 8 year biochemical progression free survival [47]. Additionally, a 2010 study by Gutt el al. found that statin use improved freedom from biochemical failure (FFBF) and relapse-free survival (RFS) in PCa patients treated with radiotherapy (RT) [12]. This effect was seen across all RT dose ranges and was independent of AWD use. The authors of this study suggest that lowered LDL levels through statin may modify intra-prostatic hormonal levels, resulting in a differential effect of RT. Surprisingly, they also note that patients undergoing more aggressive radiation treatments derive less benefit from statin use [12]. This may explain the inconclusive RT results in an aforementioned study by Soto et al., which used higher radiation doses and saw no benefits in patients taking statins [36].
The beneficial effect of statins on RT is easy to understand. As RT kills cells by causing DNA damage, and resistance to RT is primarily caused by the ability of cells to achieve DNA repair following this damage, it is likely that statins cause radiosensitization by preventing DNA repair following radiation induced DNA damage. As a result, cells located in different phases of the cell cycle have a wide variation in sensitivity to ionizing radiation. For instance, cells located in the G1 and G2-M phases of the cell cycle are most sensitive to ionizing radiation-induced cell death. Therefore, statins may sensitize these cells to radiation through G1 cell cycle arrest [21]. It has been shown that Ras overexpression, which promotes cell cycle progression, may confer radiation resistance whereas that Ras-associated increase in radiation resistance can be reversed by lovastatin in osteosarcoma cells [21]. The negative effect of higher doses of radiations is harder to explain. It is likely that high radiation doses kill cells regardless of drug use and therefore, the effect of statins are not immediately obvious at these RT doses.
Possible mechanisms of statin effect on prostate cancer cells
A large number of in vitro and preclinical studies have been conducted to explain the effects of statins on PCa risk. These studies demonstrated that statins induce G1 arrest in PCa cell lines [20, 21]. This effect of statins was demonstrated not only in epithelial but also in endothelial cells. As a result, statins also may inhibit angiogenesis, and reduce tumor growth through this mechanism. Rosuvastatin, a relatively new statin drug, was shown to inhibit PCa cell growth and decrease tumor size in mice xenograft models. Moreover, rosuvastatin inhibited angiogenesis, by reducing the number of blood vessels within tumors of statin-treated mice [48]. A direct effect of statins on angiogenesis via cholesterol inhibition vs. isoprenoid inhibition was studied in mice and showed that whereas low grade cerivastatin or atorvastain consumption (0.005 to 0.01 μmol/L) lowered cholesterol levels and promoted endothelial cell proliferation and angiogenesis, in keeping with its cardioprotective functions, at high statin concentrations (0.05 to 1 μmol/L), despite lowered cholesterol, angiogenesis was inhibited, supporting cholesterol independent effects of statins in tumor suppression [49]. The anti-angiogenic effect of statins was reversed by GGPP, clearly demonstrating a role for geranylgeranylated proteins in this effect [49]. Thus, decreased PCa risk can be attributed to statin-induced suppression of tumor growth, induction of apoptosis, and/or inhibition of angiogenesis [11]. The deleterious effect of statins on angiogenesis in tumors, of course, is at odds with its effects on promoting angiogenesis in the cardiovasculature and will be discussed later.
Statins also have anti-inflammatory properties, and inflammation is an important process affecting PCa growth. Thus, statins may reduce PCa progression, in part by inhibiting inflammation. In a study on 236 men, statin intake before surgery was significantly associated with reduced risk of inflammation of surrounding malignant glands within RP specimens. The significant risk reduction for tumor inflammation among statin users was independent of demographic, clinical, and pathological characteristics [50].
Another possible mechanism of statins' action is the suppression of androgen production. Cholesterol is a required intermediate in androgen synthesis in the testis and adrenal glands. Thus, it is possible that reducing the available substrate for testosterone and dihydrotestosterone (DHT) production may suppress circulating androgen levels, thereby reducing androgen-dependent PCa tumor growth. The presence of cholesterol may promote castrate resistant PCa by conferring the ability to produce androgens despite castration. Locke et al. reported the ability of castration-resistant PCa cells to synthesize androgens from cholesterol precursors [51]. This effect of cholesterol has significant consequences in the detection of PCa as well. Androgens activate the AR which in turn transcriptionally regulates PSA production. Therefore, lowering of cholesterol by statin use directly reduces serum PSA levels. A study by Loeb et al. of 504 statin users at the Johns Hopkins School of Medicine showed a decline in PSA levels with statin use [38]. The study by Gutt et al mentioned above also correlated statin use with lower PSA and clinical stage at diagnosis [12]. Even among men who were free of PCa, statin use lowered PSA levels in proportion to the decrease in LDL levels [35, 46].
However, Hall et al. [52] failed to demonstrate that statin use decreases circulating hormones in patients, such as free testosterone, dehy-droepiandrosterone sulfate, or luteinizing hormone, suggesting that they may act by inhibiting ligand-independent rather than dependent AR transcriptional activity. An explanation for this observation may be found in the results from another study, showing that statins may lower intraprostatic androgen levels, despite not lowering serum androgen levels in patients [53]. Consequently, changes in intraprostatic androgen synthesis can change factors necessary for prostate growth and decrease PSA levels in men [53]. Because PSA is widely used as a screening test for PCa, the effect of statins or cholesterol on PSA levels independent of any biological influence on PCa can create a detection bias [13]. In support of this hypothesis, no correlation was observed between statins and PCa incidence in a small cohort of Korean patients who nonetheless, showed that mean PSA and PSA density were significantly lower in patients on statins [54]. Fortunately, the majority of studies reported here, take this bias into account - however, it still has to be kept in mind for older studies when this effect of cholesterol was not known.
Do statins prevent prostate cancer risk by inhibiting cholesterol production?
Because the primary site of cholesterol synthesis is the liver, statins that are currently available have been selected for their capacity to target the liver and decrease cholesterol biosynthesis [55], whereas they accumulate poorly in other tissues [55, 56]. This decreases their bioavailability in other tissues such as the prostate. It was estimated that less than 5% of an oral dose of simvastatin reaches the general circulation as an active inhibitor [56]. This is because simvastatin and lovastatin are consumed as prodrugs and require esterase-dependent conversion to the active form [57]. Humans lack high levels of serum esterases, and thus these statins remain in their pro-drug form and accumulate mainly in the liver [56]. On the other hand, statins such as pravastatin and atorvastatin do not require this enzymatic conversion; however, due to the hydrophobic nature of pravastatin, it's bioavailability in the liver is about 20% [58], while atorvastatin (Lipitor) remains the most bioavailable statin currently in the market - however, it too has low bioavailability in peripheral tissues. Based on these results, Solomon and Freeman have argued that any anti-tumor effects within peripheral tissues are likely to arise indirectly from potent LDL lowering effect, and not from the inhibition of iso-prenoid synthesis within tumor cells [56]. However, in vitro studies showed that lovastatin achieved G1 arrest arrest at 0.5 mmol/L, a concentration easily achieved in the serum following oral administration [20]. It may be argued that this could be due to the production of GGPP and FPP in the liver which is then systemically delivered to their sites of action, such as the prostate.
Indeed, a number of studies report a positive correlation between cholesterol levels and PCa risk. Mondul et al. examined the association between plasma cholesterol concentration and PCa in a large prospective cohort study conducted in Washington County, MD [59]. There was no overall association between cholesterol concentration and incidence of total, advanced, or organ-confined PCa. However, compared to men with high cholesterol (240 mg/dl), men with desirable (200 mg/dl) or borderline (200 to 240 mg/dl) levels were less likely to develop high-grade PCa [59]. Similar conclusions were drawn from a study in a cohort including 5112 PCa patients, where the authors investigated associations among triglycerides (TG), total cholesterol (TC), and PCa while taking into account glucose [60]. The results of the study showed that glucose and lipid metabolism influence PCa risk [60]. Another study showed that men with low cholesterol had a lower risk of Gleason 8 to 10 PCa than men with high cholesterol, although no association was present for PCa overall [43]. However, these correlations could be coincidental and given the effect of the mevalonate pathway and its increased activity in PCa -upregulation of cholesterol would be accompanied by an upregulation of isoprenyl groups, which may increase PCa risk by a mechanism independent of cholesterol metabolism.
The discriminating factor, of course, would be the effect of lowering cholesterol specifically, rather than reducing all of the downstream effectors of the mevalonate pathway. A 2007 Finnish study of 49,446 men reported that statin users had a 25% lower risk of advanced PCa when compared with men who took other cholesterol-lowering drugs or no cholesterol medication (described in [41]). The effect was dose-dependent, with increased statin use linked to increased positive outcomes [41]. This suggests that statins have a reduced risk of developing advanced or high grade PCa but not necessarily all cholesterol lowering drugs. This and similar studies, therefore, point to a mechanism of action of statins other than the lowering of cholesterols per se.
In vitro mechanisms of cholesterol action in prostate cancer cells
Direct correlation between cholesterol metabolism and PCa was provided in the form of in vitro studies that confirmed that upregulation of cholesterol increases the risk of PCa. In a series of experiments, Michael Freeman and co-investigators have shown that cholesterol depletion in lipid rafts on biological membranes may influence and downregulate oncogenic signals. They showed that LNCaP human PCa cells contain cholesterol-rich lipid rafts that mediate epidermal growth factor (EGF)-induced and constitutive signaling through the PI3K/Akt pathway [61]. Disruption of these lipid rafts inhibited, while their reconstitution restored, EGF receptor/PI3K/Akt signaling which regulated apoptosis. Treatment with 20 μM simvastatin caused cholesterol depletion of lipid rafts, followed by downregulation of the PI3K-Akt pathway and apoptosis [28, 61]. Elevation of circulating cholesterol in SCID mice promoted tumor growth, increased Akt phosphorylation, and reduced apoptosis in the xenografts [28]. The phosphorylation of Akt, which is an important step in intracellular oncogenic signaling, is regulated by caveolin-1, a cholesterol-regulated protein. Caveolin-1 is the main structural protein of caveolae, commonly found in lipid rafts. Studies have also shown that raft-dependent signaling events can be inhibited by altering cholesterol content in the raft sites [62].
AR was found to localize to a lipid raft compartment in androgen-sensitive LNCaP prostate adenocarcinoma cells. AR can send rapid, non-genomic signals, activating various pathways in response to androgen stimulation. Studies also demonstrated that the treatment of AR with androgen rapidly activated Akt1 in a non-genomic manner, through the activation of upstream PI3K. Endogenous AR interacted with endogenous Akt1 preferentially in raft areas, and androgen addition increased this interaction [63]. This study also showed that a complex forms between AR and Akt in the lipid raft membrane sections; while treatment of LNCaP cells with the AR antagonist bicalutamide prevented the complex formation, whereas LY294002 (PI3K inhibitor) and Src inhibitor PP2 only had marginal effects. Thus, the binding of AR to Akt may bypass the PI3K and Src pathways. Additionally, the major effects of androgen on Akt phosphorylation activity occurred in raft fractions. These findings indicate that the AR complex may activate Akt directly in raft sections of the cell membrane [63]. Taken together, these studies hypothesize that cholesterols may regulate PCa risk by upregulating an interaction between the AR and Akt, which in turn promoted tumor cell growth. These studies, however, do not account for the greater effect of cholesterol (and statins) in high grade and advanced PCa.
Cholesterol-independent mechanisms of statin action in prostate cancer cells
Despite the strong argument for cholesterol deregulation as a mediator of statin action in PCa cells, several lines of evidence point to a cholesterol independent mechanism in statin induced reduction in PCa risk. A study on 1,812 men by Hall et al. measured whether levels of circulating androgens and their carrier protein, sex hormone-binding globulin (SHBG), varied by statin use. The study found no relationship between statin use and free testosterone, dehy-droepiandrosterone sulfate, or luteinizing hormone [52]. The study concluded that it is unlikely that statins affect circulating androgens and PCa risk through a hormonal mechanism. In a longitudinal study of 1214 PCa-free men who were prescribed statins but who had undergone routine PSA testing before and after statin intake, serum PSA decline after statins initiation was significantly associated with statin dose and the decline in LDL-cholesterol levels [35]; however, even after adjusting for LDL changes, statin use still remained associated with the PSA decline, suggesting that there may be both cholesterol and non-cholesterol-mediated mechanisms by which statin treatment influences PSA [35].
A study by Mondul et al. on 2,574 men showed that statin users experienced a greater decline in PSA levels compared to non-statin users, although the difference was not statistically significant [64]. Detection bias was accounted for, but did not explain the inverse association between statin use and lowered PSA levels. The observed inverse relationship between statin use and PSA levels could indirectly suggest that statins may influence PSA concentration through cholesterol-lowering properties. However, other mechanisms are also possible, since PSA levels decreased even after controlling for cholesterol levels [64].
In contrast to in vitro and animal studies, a 1989 study in the Lancet showed that total cholesterol levels were reduced in patients with evidence of metastasis (n = 30) compared with those without metastasis (n = 73) [65]. LDL was cleared faster in patients with metastatic disease compared to patients without metastasis and in healthy controls [65]. A study in 127 Chinese patients with prostate cancer with bone metastasis undergoing hormone treatment showed that patients who showed good response to the treatment (n=54) had higher cholesterol levels compared to those who showed poor response (n=73) [66]. These studies contradict a recent metabolomics study where the mean cholesterol level in PCa bone metastases was 127.30 mg/g (n=20) as compared to 81.06 and 35.85 mg/g in bone metastases of different origin (n=7) and normal bone (n=14), respectively (p=0.0002 and 0.001) [67]. The smaller number of specimen used in the latter study may therefore indicate a subset of patients who contrast the former two. Overall, these studies suggest that cholesterol lowering is not the only mechanism by which statins decrease the risk of PCa, and that other mechanisms may exist as well.
Statin action through isoprenylation
Geranygeranylated and farnesylated small GTP-binding proteins
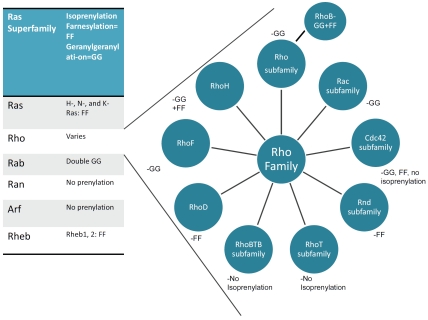
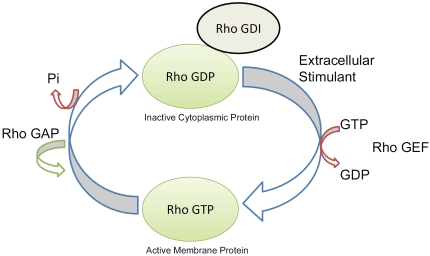
Small GTP-binding proteins of the Ras super-family are involved in such diverse cellular functions as cytokinesis, cell motility, cell adhesion and cell proliferation [16, 31]. This superfamily consists of 5 families with a total of more than 150 members: Ras, Rho, Rab, Ran and Arf (reviewed in [68, 69]) (Figure 2). All members of this family contain an N-terminal GTPase domain and switch between an active GTP-bound and an inactive GDP-bound state modulated by a family of regulators. RhoA, for example, forms a complex with the GDP-dissociation inhibitor (Rho-GDI) in the cytoplasm and remains GDP-bound unless activated by a stimulant and by guanine nucleotide exchange factors (GEF), which exchange GTP for GDP. Upon release of binding proteins and Rho-GDI, activated RhoA in its GTP-bound form can be translocated to the membrane, where it can bind its target protein and regulate downstream effectors. GTPase activating proteins (GAPs) enhance GTPase activity, increasing GTP hydrolysis, which reconverts activated Rho to its inactivated Rho-GDI/GDP bound form, which is then sequestered in the cytoplasm [70] (Figure 3). Most of the 150 members of the Ras superfamily are similarly regulated, but they utilize different regulatory factors.
Figure 2.
Table reflecting the various families included in the Ras superfamily of small GTP-binding proteins, and the isoprenyl group by which they are tethered to the membrane. All members of the Ras and Rheb families are far-nesylated, Rab family members are double geranylgeranylated while Ran and Arf GTPases are not prenylated. (right) In contrast, members of the Rho family are further divided into subfamilies which have varied prenylation properties. Rho and Rac GTPases are geranylgeranylated, whereas Rnd GTPases are farnesylated, while others like members of the RhoBTB and RhoT families are not prenylated. The exception is the cdc42 family, which has members that are either geranylgeranylated, farnesylated or neither. RhoH, RhoF, and RhoD are not categorized within the Rho subfamilies but are part of the Rho family at large. RhoH can be both geranylgeranylated and farnesylated, RhoD is farnesylated, and RhoF is predicted to be geranylgeranylated [114]. In addition, RhoB is exceptional because it is both geranylgeranylated and farnesylated.
Figure 3.
Rho GTPases switch between an active GTP-bound and an inactive GDP-bound state modulated by a family of regulators. Inactive Rho-GDP forms a complex with the GDP-dissociation inhibitor (Rho-GDI) in the cytoplasm and remains GDP-bound unless activated by a stimulant and by the guanine nucleotide exchange factors (RhoGEF) which exchange GTP for GTP. Rho in its GTP-bound form is then translocated to the membrane, where it acts on its target protein as a GTPase. Then, GTPase activating proteins (RhoGAP) enhance GTPase activity and reconvert activated Rho to its inactivated GDP bound form, after which, it is translocated to the cytoplasm by Rho-GDI.
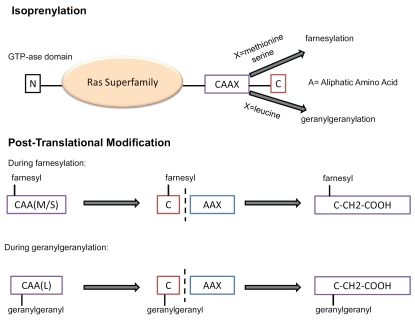
Members of the Ras superfamily that are regulated by isoprenylation, include H-, N- and K-Ras, as well as Rheb-1 and -2, which are farnesylated, whereas Rab GTPases require double geranylgeranylation [71]. Ran GTPases are, on the other hand, primarily nuclear localized and do not require prenylation for its activation [72]. The Rho family is unusual, since it has members that are geranylgeranylated, farnesylated, both or neither (Figure 2). As shown in Figure 4, all prenylated members of the Ras superfamily are characterized by a CAAX motif at their C-terminal, where A is any aliphatic amino acid and X is a COOH-terminal amino acid that specifies which prenyltransferase will act. Although recent experiments suggest more complex relationships, it is generally believed that CAAX farnesyltransferase preferentially recognizes methionine or serine at the X position, whereas CAAX geranylgeranyl transferase prefers leucine at the X position [73]. During post-translational modification, a farnesyl or geranylgeranyl group is first attached to the cysteine group, then the AAX sequence is cleaved and the isoprenylated cysteine is carboxymethylated [74].
Figure 4.
Mechanism of prenylation of the Ras superfamily GTPases by farnesyl pyrophosphate and geranylgeranyl pyrophosphate. (upper) Prenylated members of the Ras superfamily are characterized by a CAAX motif at their C-terminal, where A is any aliphatic amino acid and X is a COOH-terminal amino acid that specifies which prenyltrans-ferase will act. Although recent experiments suggest more complex relationships, it is generally believed that CAAX farnesyltransferase preferentially recognizes methionine or serine at the X position and CAAX geranylgeranyl trans-ferase prefers leucine at the X position. For farnesylation, X=methionine or serine whereas for geranylgeranylation, X = leucine. (lower) During post-translational modification, a farnesyl or geranylgeranyl group is first attached to the cysteine group, then the AAX sequence is cleaved and the isoprenylated cysteine is carboxymethylated.
Rho proteins are known to regulate both cell growth and motility, and consists of over 40 members which have been subclassified into Rho, Rac, cdc42, Rnd, RhoT and RhoBTB sub-familes (Figure 2) (for detailed reviews see [75, 76]). In addition, other members such as RhoD, RhoF and RhoH are included in the Rho family but not necessarily in one of the subfamilies. Unlike the RhoBTB and RhoT/Miro subfamilies, most members of the other Rho subfamilies are isoprenylated - but with significant exceptions. The Rho and Rac subfamilies are mostly all geranylgeranylated, but in addition, RhoB is both geranylgeranylated and farnesylated, while the Rnd subfamily is only farnesylated. Within the cdc42 subfamily, cdc42 itself and Wrch-1 (RhoU) are geranylgeranylated, most other members of the cdc42 family are farnesylated, except Wrch-2 (also called RhoV), which is not prenylated. A number of these proteins, in addition, are also palmitoylated, including RhoB. Both prenylation and palmitoylation helps tether the active form of the protein to the plasma membrane, its site of activity, and this localization is severely affected by statin treatment. RhoA, for example, is localized in the cytoplasm when treated with lovastatin, whereas in untreated, healthy cells, it is located in the plasma membrane envelope, not only in PCa, but also in other cell types and organ sites [77].
Role of isoprenylated small GTPases in cell invasion, migration and metastasis in prostate cancer
Many of the Rho family GTPases are involved in metastasis. The effects of RhoA are mostly mediated by Rho kinase, a serine-threonine kinase that induces the formation of actin stress fibers and focal adhesions by phosphorylating myosin light chain (MLC), which promotes actin-binding to myosin II [78]. The Rho-kinase inhibitor, Y-27632, inhibited chemotactic migration in vitro and metastatic growth of PC3 cells in immune-compromised mice, reduced MLC phosphorylation and markedly altered cell morphology [79], indicating that invasiveness of human prostate cancer is facilitated by the Rho/Rho-kinase pathway. RhoC GTPase is also expressed and activated in PC-3 cells, whereas RhoC inhibition promotes IGF-I stimulated random motility but decreases in vitro invasion and metastases, and results in drastic morphologic changes and alterations in the expression and distribution of focal adhesion-related proteins [80]. Studies indicated that RhoC was required for directed migration and invasion, whereas Rac1 was required for tumor cells to degrade the basement membrane, allowing them to escape the blood vessel (diapedesis) [81]. RhoC was increased in highly metastatic sublines of LNCaP cells, compared to the parental line, whereas treatment with a small hairpin RNA (shRNA) specific for RhoC suppressed collagen-mediated invasion [82]. Significantly, RhoC activation was selectively blocked with antibodies to α2β1 indicating that RhoC was activated downstream of integrin activation. In contrast, RhoC had no effect on cell proliferation in vitro or on tumor growth in mice [83]. Immunohistochemical analyses on tumor specimens from 63 PCa patients showed that RhoC expression had no significant correlation with Gleason grade, but had significant positive correlation with both lymph node and distant metastasis, and was inversely correlated with patient survival [83].
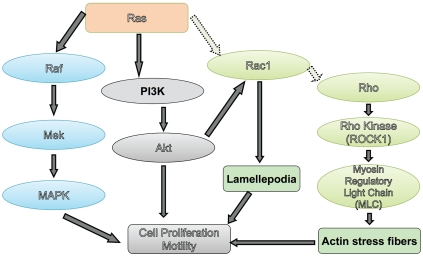
Ras overexpression has also been associated with increased metastasis. Early studies showed that H-ras overexpression increased the metastatic ability of AT2 cells [84], although the incidence of Ras mutations in PCa patients are rare [85]. Oncogenic Ras promoted metastasis to multiple organs, including bone and brain -however, detailed analysis indicated that activation of the Raf/ERK pathway downstream of Ras stimulated metastasis to the brain, while activation of the RalGEF pathway led to bone metastases, but did not affect subcutaneous tumor growth [86]. Ras has multiple effectors -including Rac and Rho and can signal to various pathways, including proliferation (through ERK/MAPK) and motility, and is one of the most common oncogenes displaying aberrant activation in human cancers (Figure 5). It can also interact with the PI3K/Akt pathway, either by PI3K interaction or through Rac1, which in turn activates p21-activated kinase (PAK), an Akt interacting protein (Figure 5). However, since Ras activation through mutagenesis is rare in human PCa, these proteins are likely to play a more important role in metastasis. Taken together, these studies indicate an important role for isoprenylated small GTPases in advanced PCa rather than in early stage PCa.
Figure 5.
Signaling pathways downstream of Ras, a farnesylated small GTPase. Ras directly activates the mitogen activated protein kinase (MAPK) cascade, through phosphorylation of Raf (or other MAPK kinase kinase, MAPKKK) which in turn phosphorylates MEK (or other MAPK kinases), which then phosphorylates MAPK. On the other hand, Ras also activates Rac GTPases, which in turn activates p21-activated kinase (PAK), an Akt interacting protein. Rac also can activate Rho GTPases, which regulates actin stress fiber and focal adhesion formation by activating Rho kinase, which in turn regulates the activity of myosin light chain which controls actin polymerization.
Effect of statins on isoprenylated small GTPases
Multiple in vitro studies have demonstrated that statins inhibit isoprenylation of small GTPases in PCa cell lines. Most of these studies utilized lovastatin, the first statin to come on the market in 1987 [87], although mevastatin had been the first statin drug to be produced [88]. In vitro studies utilized lovastatin at 50 μM, which completely inhibited the production of GGPP and FPP in PCa cells [89]. Lovastatin was shown to inhibit PCa cell growth, alone or in combination with other drugs [90]. In 1999, we first demonstrated that PCa cell growth was regulated by geranylgeranylation and not by farnesylation [16]. When treated with lovastatin, PCa cell lines derived from transgenic mice with adenocarcinoma of the prostate (TRAMP) showed in-activation of the small GTPase RhoA. The geranylgeranylated Rho family, including RhoA, Rac1, and Cdc42Hs, are involved in cell shape regulation and actin filament assembly. Inactivating these proteins with lovastatin caused cell rounding and actin filament disassembly. Our results showed that activation of Rho proteins was necessary for cell proliferation [16]. Exogenous addition of geranylgeraniol (GGOL), but not farnesol (FOL), rescued TRAMP cells from the anti-proliferative effects of lovastatin and also translocated RhoA from the cytosol to the membrane where RhoA was active. Active RhoA was also found to be necessary for TRAMP cell proliferation.
Our results were upheld by a 2008 study by Hoque et al., showing that lovastatin and simvastatin decreased cell survival in three PCa cell lines (PC3, DU145, and LNCaP) by inducing apoptosis and cell growth arrest at G1 phase and by suppressing RhoA activation [17]. This study also showed that HMG-CoA induced apoptosis may be mainly mediated through the depletion of geranylgeranylated proteins. A geranylgeranyl transferase inhibitor (GGTI) mimicked the effects of lovastatin in causing apoptosis; however, a farnesyl transferase inhibitor (FTI) was much less effective in triggering apoptosis. Furthermore, application of lovastatin and simvastatin was shown to suppress RhoA activation [17]. Overall, the underlying molecular mechanism of statins' action is thought to be mediated through the inactivation of Rho proteins, which in turn induces enzymatic activity and/or G1 cell cycle [17]. The effect of geranylgeranylated proteins like RhoA on cell cycle progression has been demonstrated in not only PCa but also in other types of cells [91].
Other evidence has also been presented showing effectiveness of geranylgeranylated proteins in apoptosis-independent PCa cell death. Atorvastatin treatment of AR negative PC3 cells caused rapid cell death, arresting cell cycle at the G1 phase [92], and showed increased expression of LC3-II (a marker of autophagy [93, 94]) by more than ten-fold in a dose-dependent manner [92]; however, autophagy was reversed by the addition of geranylgeraniol (GGOL) but not farnesol (FOL) [92]. Addition of other mevalonate pathway products (ubiquinone, squalene) did not affect LC3-II expression, indicating a role for geranylgeranylated proteins in this respect. Normal prostate RWPE1 and AR positive LNCaP cells were not affected by atorvastatin, suggesting a protective action of androgens against atovastain-induced autophagy. Taken together, these results indicate that atorvastatin acts by inhibiting GGPP synthesis [92].
Some of the effects of statins attributed to cholesterol depletion in lipid rafts may also be due to inhibition of RhoA activation. We previously showed that RhoA activation resulted in the activation of a PI3K-dependent pathway, which activated both Akt and p70S6 kinase (a downstream target of the PI3K/mTOR pathway) [95], and is inhibited by statin use. The involvement of p70S6 kinase in the mediation of RhoA's effects likely explains the role of statins in autophagy induction, described above [92], as the mTOR pathway is known to prevent autophagy [96]. Overall, these studies indicate an important role for isoprenylated small GTPases in metastasis and suggest that the greater effect of statins in advanced PCa may be, at least partly, attributed to the inhibition of the same GTPases by these drugs.
Expression of geranylgeranylated or farnesylated proteins in clinical samples from prostate cancer patients
Although farnesylated Ras proteins may not affect PCa cell growth, they play an important role in PCa metastasis. Unlike other solid cancers such as pancreatic or lung cancers which express ras mutations, such activation of Ha-, Ki - or N-Ras are not found in PCa in American or French patients [85, 97], although some studies reported such mutations in Japanese PCa patients [98], but not Chinese patients [99]. Despite this, high grade PCa tissues were repeatedly found to express high Ras expression [100, 101], especially at sites of bone metastasis [102], and H-ras expression has been suggested as a biomarker of advanced PCa [103]. In support of this, N-Ras expression was associated with the development of hormone refractory PCa, and a shorter time to relapse [104]. Other studies, however, disputed such findings and found no difference between Ras expression in benign vs. malignant prostate tissues [105, 106]. Increased expression of Rheb and Rho GTPases has also been reported in prostate tumor tissue compared to normal prostate [107, 108]. In particular, Rac proteins were highly expressed in PIN and carcinoma compared to benign prostate tissue [109]. On the other hand RhoE was underexpressed in prostate tumor tissue compared to benign prostate [110], which underscores its role in suppressing cell cycle progression. The Rac-specific guanine nucleotide exchange factor, Tiam1, plays a major role in oncogenicity, tumour invasion and metastasis. Tiam1 was significantly overexpressed in both PIN (p<0.001) and PCa (p<0.001) when compared to benign secretory epithelium, and was statistically significantly associated with disease recurrence (p=0.016), the presence of lymph vessel invasion (p=0.031), high Gleason scores (p=0.044) and decreased disease-free survival (p=0.03) [111]. These studies point to the diversity of the Ras superfamily of small GTPases and the variety of functions these proteins conduct.
Do statins prevent prostate cancer risk by inhibiting isoprenoid production in prostate cancer patients?
Despite strong evidence in vitro, there are arguments that statins may not be able to inhibit isoprenylated small GTPases in the prostate. Solomon and Freeman [56] point out that since statins have been designed to accumulate in the liver, very little of these drugs actually are released into the circulation, and may not reach the prostate in sufficient quantities to affect tumor growth. They argue that the concentrations of the drugs released in the plasma, (lovastatin from a single 40 mg dose is estimated 9.5-15 ng/ml in the plasma; for a 40 mg dose of simvastatin it is approx. 10 ng/ml; and for a single 40 mg dose of pravastatin it is approx. 50 ng/ml) are far below those required to demonstrate biological effects on cells in culture [56]. Yet it can also be argued that luminal epithelial cells in culture may not express the enzymes required to break up the prodrugs used: whereas the prostate expresses a number of other cells, including mainly the mesenchymal stromal cells, where drug metabolism may be more efficient. Further, GGPP and FPP are also produced in the liver and can be released into the circulation where it acts on various organs. Hence, statins may affect their production directly at the site of synthesis.
The effect of statins in peripheral tissue is obvious from the most common side-effect of statins, skeletal muscle myopathy [112], which may be traced to a geranylgeranylation defect induced by statin use in muscle cells [113]. Hence it is likely that lower concentrations of statins may be required to affect the prostatic stroma in situ compared to pure cultures of prostate epithelial cells in vitro, which in turn will make the drug widely available to the neighboring epithelial cells. As of now, there is little clinical evidence that distinguishes the effects of statins on cholesterolemia vs. those on isoprenyl formation. Further studies are needed to go beyond the obvious in vitro and pre-clinical studies to determine whether statins affect PCa through inhibiting cholesterol formation or by affecting the function of geranylgeranylated or farnesylated small GTP-binding proteins of the Ras, Rho or Rab families. Atorvastatin, which has a much higher bioavailability, displayed biochemical effects at much lower concentrations (1 μM compared to 50 μM for lovastatin), and induced cell death and cell cycle arrest at doses as low as 5 μM [92]. This effect was reversed by addition of geranylgeraniol, indicating an effect mediated by isoprenylation [92]. These studies indicate the possibility of statin action being mediated by iso-prenylated small GTPases preferentially over, or in addition to, cholesterol metabolism.
Conclusion
Based on this and similar results in animal models, we conclude that statins, especially statins with high bioavailability, inhibit cholesterol formation and exert cardioprotective effects at low concentrations, whereas at high concentrations would also be capable of tumor suppression by inhibition of small GTPases involved in cell proliferation, survival, inflammation, angiogenesis and metastasis. It is likely that higher statin levels, therefore, will be more effective in preventing PCa risk compared to those required for cardioprotection; however, further studies are required to determine the effects of higher statin concentrations on common side effects such as skeletal muscle myopathy, and increased liver enzymes.
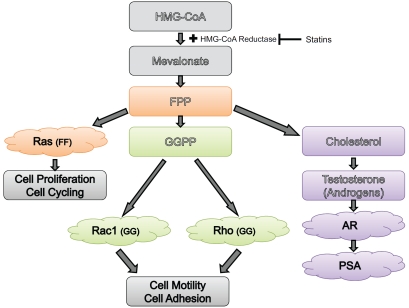
The mechanism of a potential preventive effect of statins on PCa needs to be clarified further by experimental studies. A study should consider the three main end products of the mevalonate pathway (cholesterol, GPP, and FPP), and how PCa cell proliferation can be mediated with statin use. A summary of the three possible pathways is shown in Figure 6. Current research suggests that statins most likely use either/both cholesterol and GPP mediated pathways (more so than FPP mediated pathways) for inducing cell death and/or cell cycle arrest. Moreover, the geranylgeranylated protein RhoA was shown to be necessary for cell proliferation, and GGOL was able to rescue cells from statin induced cell death. While the cholesterol pathway, through the action of lipid rafts is also probable, it remains unclear whether statins' effects on oncogenic signals are solely through the oncogenic Akt/AR complexes formed on lipid rafts. Overall, statin use was also correlated with PSA decline, which seems to indicate that more than cholesterol lowering effects may be at play. Also, a significant reduction in cholesterol has been required to observe induced apoptosis: levels which may not be attainable solely through statin use in vivo. Thus, a study on statins' effect on GGPP mediated apoptosis and cell cycle arrest could enhance understanding the processes involved in prostate carcinogenesis and potentially identify new therapeutic targets for PCa.
Figure 6.
Inhibition of prostate cancer cell proliferation through statin use, and its impact on the HMG-CoA pathway. Three possible mechanisms are indicated: cholesterol mediated pathways, farnesylated protein mediated pathways and, geranylgeranylated protein mediated pathways. Cholesterol is presumed to act through its assembly in lipid rafts, thereby activating PI3K/Akt, however, it also directly affects prostate cancer by mediating the production of testosterone, which in the prostate is converted to dihydrotestos-terone, a strong ligand for the androgen receptor (AR). At the same time, farnesyl pyrophosphosphates can regulate PCa progression by activating Ras GTPases, which need farnesylation for membrane binding and activation, whereas they also activate Rac and Rho GTPases, which require geranylgeranylation, and regulate cell motility, invasion and metastasis. Statins may reduce risk of advanced/aggressive PCa by inhibiting one or more of these pathways.
References
- 1.Williams H, Powell IJ. Epidemiology, pathology, and genetics of prostate cancer among African Americans compared with other ethnicities. Methods Mol Biol. 2009;472:439–453. doi: 10.1007/978-1-60327-492-0_21. [DOI] [PubMed] [Google Scholar]
- 2.Burkhardt JH, Litwin MS, Rose CM, Correa RJ, Sunshine JH, Hogan C, Hayman JA. Comparing the costs of radiation therapy and radical prostatectomy for the initial treatment of early-stage prostate cancer. J Clin Oncol. 2002;20:2869–2875. doi: 10.1200/JCO.2002.11.136. [DOI] [PubMed] [Google Scholar]
- 3.Roehl KA, Han M, Ramos CG, Antenor JA, Catalona WJ. Cancer progression and survival rates following anatomical radical retropubic prostatectomy in 3,478 consecutive patients: long-term results. J Urol. 2004;172:910–914. doi: 10.1097/01.ju.0000134888.22332.bb. [DOI] [PubMed] [Google Scholar]
- 4.Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–1597. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 5.Bruchovsky N, Klotz L, Crook J, Goldenberg SL. Locally advanced prostate cancer-biochemical results from a prospective phase II study of intermittent androgen suppression for men with evidence of prostate-specific antigen recurrence after radiotherapy. Cancer. 2007;109:858–867. doi: 10.1002/cncr.22464. [DOI] [PubMed] [Google Scholar]
- 6.Schmid HP, Keuler FU, Altwein JE. Rising prostate-specific antigen after primary treatment of prostate cancer: sequential hormone manipulation. Urol Int. 2007;79:95–104. doi: 10.1159/000106320. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Kreisberg JI, Ghosh PM. Crosstalk between the androgen receptor and the phosphatidylinositol 3-kinase/Akt pathway in prostate cancer. Curr Cancer Drug Targets. 2007;7:591–604. doi: 10.2174/156800907781662248. [DOI] [PubMed] [Google Scholar]
- 8.Petrylak D. Therapeutic options in androgen-independent prostate cancer: building on do-cetaxel. BJU Int. 2005;96(Suppl 2):41–46. doi: 10.1111/j.1464-410X.2005.05946.x. [DOI] [PubMed] [Google Scholar]
- 9.Boehmer A, Anastasiadis AG, Feyerabend S, Nagele U, Kuczyk M, Schilling D, Corvin S, Merseburger AS, Stenzl A. Docetaxel, es-tramustine and prednisone for hormone-refractory prostate cancer: a single-center experience. Anticancer Res. 2005;25:4481–4486. [PubMed] [Google Scholar]
- 10.Small EJ, Schellhammer PF, Higano CS, Redfern CH, Nemunaitis JJ, Valone FH, Verjee SS, Jones LA, Hershberg RM. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol. 2006;24:3089–3094. doi: 10.1200/JCO.2005.04.5252. [DOI] [PubMed] [Google Scholar]
- 11.Gonyeau MJ, Yuen DW. A clinical review of statins and cancer: helpful or harmful? Pharmacotherapy. 2010;30:177–194. doi: 10.1592/phco.30.2.177. [DOI] [PubMed] [Google Scholar]
- 12.Gutt R, Tonlaar N, Kunnavakkam R, Karrison T, Weichselbaum RR, Liauw SL. Statin use and risk of prostate cancer recurrence in men treated with radiation therapy. J Clin Oncol. 2010;28:2653–2659. doi: 10.1200/JCO.2009.27.3003. [DOI] [PubMed] [Google Scholar]
- 13.Murtola TJ, Visakorpi T, Lahtela J, Syvala H, Tammela T. Statins and prostate cancer prevention: where we are now, and future directions. Nat Clin Pract Urol. 2008;5:376–387. doi: 10.1038/ncpuro1146. [DOI] [PubMed] [Google Scholar]
- 14.Platz EA, Leitzmann MF, Visvanathan K, Rimm EB, Stampfer MJ, Willett WC, Giovannucci E. Statin drugs and risk of advanced prostate cancer. J Natl Cancer Inst. 2006;98:1819–1825. doi: 10.1093/jnci/djj499. [DOI] [PubMed] [Google Scholar]
- 15.Freeman MR, Solomon KR. Cholesterol and prostate cancer. J Cell Biochem. 2004;91:54–69. doi: 10.1002/jcb.10724. [DOI] [PubMed] [Google Scholar]
- 16.Ghosh PM, Ghosh-Choudhury N, Moyer ML, Mott GE, Thomas CA, Foster BA, Greenberg NM, Kreisberg JI. Role of RhoA activation in the growth and morphology of a murine prostate tumor cell line. Oncogene. 1999;18:4120–4130. doi: 10.1038/sj.onc.1202792. [DOI] [PubMed] [Google Scholar]
- 17.Hoque A, Chen H, Xu XC. Statin induces apoptosis and cell growth arrest in prostate cancer cells. Cancer Epidemiol Biomarkers Prev. 2008;17:88–94. doi: 10.1158/1055-9965.EPI-07-0531. [DOI] [PubMed] [Google Scholar]
- 18.Lee J, Lee I, Park C, Kang WK. Lovastatin-induced RhoA modulation and its effect on senescence in prostate cancer cells. Biochem Biophys Res Commun. 2006;339:748–754. doi: 10.1016/j.bbrc.2005.11.075. [DOI] [PubMed] [Google Scholar]
- 19.Shack S, Gorospe M, Fawcett TW, Hudgins WR, Holbrook NJ. Activation of the cholesterol pathway and Ras maturation in response to stress. Oncogene. 1999;18:6021–6028. doi: 10.1038/sj.onc.1203002. [DOI] [PubMed] [Google Scholar]
- 20.Sivaprasad U, Abbas T, Dutta A. Differential efficacy of 3-hydroxy-3-methylglutaryl CoA reductase inhibitors on the cell cycle of prostate cancer cells. Mol Cancer Ther. 2006;5:2310–2316. doi: 10.1158/1535-7163.MCT-06-0175. [DOI] [PubMed] [Google Scholar]
- 21.Chan KK, Oza AM, Siu LL. The statins as anticancer agents. Clin Cancer Res. 2003;9:10–19. [PubMed] [Google Scholar]
- 22.Igel M, Sudhop T, von Bergmann K. Pharmacology of 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors (statins), including rosuvastatin and pitavastatin. J Clin Pharmacol. 2002;42:835–845. doi: 10.1177/009127002401102731. [DOI] [PubMed] [Google Scholar]
- 23.Shitara Y, Sugiyama Y. Pharmacokinetic and pharmacodynamic alterations of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors: drug-drug interactions and interindividual differences in transporter and metabolic enzyme functions. Pharmacol Ther. 2006;112:71–105. doi: 10.1016/j.pharmthera.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 24.Williams D, Feely J. Pharmacokineticpharmacodynamic drug interactions with HMG -CoA reductase inhibitors. Clin Pharmacokinet. 2002;41:343–370. doi: 10.2165/00003088-200241050-00003. [DOI] [PubMed] [Google Scholar]
- 25.Chen Y, Hughes-Fulford M. Human prostate cancer cells lack feedback regulation of low-density lipoprotein receptor and its regulator, SREBP2. Int J Cancer. 2001;91:41–45. doi: 10.1002/1097-0215(20010101)91:1<41::aid-ijc1009>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 26.Li YC, Park MJ, Ye SK, Kim CW, Kim YN. Elevated levels of cholesterol-rich lipid rafts in cancer cells are correlated with apoptosis sensitivity induced by cholesterol-depleting agents. Am J Pathol. 2006;168:1107–1118. doi: 10.2353/ajpath.2006.050959. quiz 1404-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oh HY, Lee EJ, Yoon S, Chung BH, Cho KS, Hong SJ. Cholesterol level of lipid raft microdomains regulates apoptotic cell death in prostate cancer cells through EGFR-mediated Akt and ERK signal transduction. Prostate. 2007;67:1061–1069. doi: 10.1002/pros.20593. [DOI] [PubMed] [Google Scholar]
- 28.Zhuang L, Kim J, Adam RM, Solomon KR, Freeman MR. Cholesterol targeting alters lipid raft composition and cell survival in prostate cancer cells and xenografts. J Clin Invest. 2005;115:959–968. doi: 10.1172/JCI200519935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grunler J, Ericsson J, Dallner G. Branchpoint reactions in the biosynthesis of cholesterol, dolichol, ubiquinone and prenylated proteins. Biochim Biophys Acta. 1994;1212:259–277. doi: 10.1016/0005-2760(94)90200-3. [DOI] [PubMed] [Google Scholar]
- 30.Sinensky M, Lutz RJ. The prenylation of proteins. Bioessays. 1992;14:25–31. doi: 10.1002/bies.950140106. [DOI] [PubMed] [Google Scholar]
- 31.Demierre MF, Higgins PD, Gruber SB, Hawk E, Lippman SM. Statins and cancer prevention. Nat Rev Cancer. 2005;5:930–942. doi: 10.1038/nrc1751. [DOI] [PubMed] [Google Scholar]
- 32.Haukka J, Sankila R, Klaukka T, Lonnqvist J, Niskanen L, Tanskanen A, Wahlbeck K, Tiihonen J. Incidence of cancer and statin usage–record linkage study. Int J Cancer. 2010;126:279–284. doi: 10.1002/ijc.24536. [DOI] [PubMed] [Google Scholar]
- 33.Boudreau DM, Yu O, Buist DS, Miglioretti DL. Statin use and prostate cancer risk in a large population-based setting. Cancer Causes Control. 2008;19:767–774. doi: 10.1007/s10552-008-9139-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farwell WR, Scranton RE, Lawler EV, Lew RA, Brophy MT, Fiore LD, Gaziano JM. The association between statins and cancer incidence in a veterans population. J Natl Cancer Inst. 2008;100:134–139. doi: 10.1093/jnci/djm286. [DOI] [PubMed] [Google Scholar]
- 35.Hamilton RJ, Goldberg KC, Platz EA, Freedl, SJ The influence of statin medications on prostate-specific antigen levels. J Natl Cancer Inst. 2008;100:1511–1518. doi: 10.1093/jnci/djn362. [DOI] [PubMed] [Google Scholar]
- 36.Soto DE, Daignault S, Sandler HM, Ray ME. No effect of statins on biochemical outcomes after radiotherapy for localized prostate cancer. Urology. 2009;73:158–162. doi: 10.1016/j.urology.2008.02.055. [DOI] [PubMed] [Google Scholar]
- 37.Friedman GD, Flick ED, Udaltsova N, Chan J, Quesenberry CP, Jr, Habel LA. Screening statins for possible carcinogenic risk: up to 9 years of follow-up of 361,859 recipients. Pharmacoepidemiol Drug Saf. 2008;17:27–36. doi: 10.1002/pds.1507. [DOI] [PubMed] [Google Scholar]
- 38.Loeb S, Kan D, Helfand BT, Nadler RB, Catalona WJ. Is statin use associated with prostate cancer aggressiveness? BJU Int. 2010;105:1222–1225. doi: 10.1111/j.1464-410X.2009.09007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Agalliu I, Salinas CA, Hansten PD, Ostrander EA, Stanford JL. Statin use and risk of prostate cancer: results from a populationbased epidemiologic study. Am J Epidemiol. 2008;168:250–260. doi: 10.1093/aje/kwn141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jacobs EJ, Rodriguez C, Bain EB, Wang Y, Thun MJ, Calle EE. Cholesterol-lowering drugs and advanced prostate cancer incidence in a large U.S. cohort. Cancer Epidemiol Biomarkers Prev. 2007;16:2213–2217. doi: 10.1158/1055-9965.EPI-07-0448. [DOI] [PubMed] [Google Scholar]
- 41.Murtola TJ, Tammela TL, Lahtela J, Auvinen A. Cholesterol-lowering drugs and prostate cancer risk: a population-based casecontrol study. Cancer Epidemiol Biomarkers Prev. 2007;16:2226–2232. doi: 10.1158/1055-9965.EPI-07-0599. [DOI] [PubMed] [Google Scholar]
- 42.Platz EA, Clinton SK, Giovannucci E. Association between plasma cholesterol and prostate cancer in the PSA era. Int J Cancer. 2008;123:1693–1698. doi: 10.1002/ijc.23715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Platz EA, Till C, Goodman PJ, Parnes HL, Figg WD, Albanes D, Neuhouser ML, Klein EA, Thompson IM, Jr, Kristal AR. Men with low serum cholesterol have a lower risk of highgrade prostate cancer in the placebo arm of the prostate cancer prevention trial. Cancer Epidemiol Biomarkers Prev. 2009;18:2807–2813. doi: 10.1158/1055-9965.EPI-09-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bonovas S, Filioussi K, Sitaras NM. Statin use and the risk of prostate cancer: A metaanalysis of 6 randomized clinical trials and 13 observational studies. International Journal of Cancer. 2008;123:899–904. doi: 10.1002/ijc.23550. [DOI] [PubMed] [Google Scholar]
- 45.Stephenson AJ, Kattan MW, Eastham JA, Dotan ZA, Bianco FJ, Jr, Lilja H, Scardino PT. Defining biochemical recurrence of prostate cancer after radical prostatectomy: a proposal for a standardized definition. J Clin Oncol. 2006;24:3973–3978. doi: 10.1200/JCO.2005.04.0756. [DOI] [PubMed] [Google Scholar]
- 46.Hamilton RJ, Banez LL, Aronson WJ, Terris MK, Platz EA, Kane CJ, Presti JC, Jr, Amling CL, Freedland SJ. Statin medication use and the risk of biochemical recurrence after radical prostatectomy: results from the Shared Equal Access Regional Cancer Hospital (SEARCH) Database. Cancer. 2010;116:3389–3398. doi: 10.1002/cncr.25308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moyad MA, Merrick GS, Butler WM, Wallner KE, Galbreath RW, Kurko B, Adamovich E. Statins, especially atorvastatin, may favorably influence clinical presentation and biochemical progression-free survival after brachytherapy for clinically localized prostate cancer. Urology. 2005;66:1150–1154. doi: 10.1016/j.urology.2005.08.053. [DOI] [PubMed] [Google Scholar]
- 48.Wang C, Tao W, Wang Y, Bikow J, Lu B, Keating A, Verma S, Parker TG, Han R, Wen XY. Rosuvastatin, identified from a zebrafish chemical genetic screen for antiangiogenic compounds, suppresses the growth of prostate cancer. Eur Urol. 2010;58:418–426. doi: 10.1016/j.eururo.2010.05.024. [DOI] [PubMed] [Google Scholar]
- 49.Weis M, Heeschen C, Glassford AJ, Cooke JP. Statins have biphasic effects on angiogenesis. Circulation. 2002;105:739–745. doi: 10.1161/hc0602.103393. [DOI] [PubMed] [Google Scholar]
- 50.Banez LL, Klink JC, Jayachandran J, Lark AL, Gerber L, Hamilton RJ, Masko EM, Vollmer RT, Freedland SJ. Association between statins and prostate tumor inflammatory infiltrate in men undergoing radical prostatectomy. Cancer Epidemiol Biomarkers Prev. 2010;19:722–728. doi: 10.1158/1055-9965.EPI-09-1074. [DOI] [PubMed] [Google Scholar]
- 51.Locke JA, Guns ES, Lubik AA, Adomat HH, Hendy SC, Wood CA, Ettinger SL, Gleave ME, Nelson CC. Androgen levels increase by intratumoral de novo steroidogenesis during progression of castration-resistant prostate cancer. Cancer Res. 2008;68:6407–6415. doi: 10.1158/0008-5472.CAN-07-5997. [DOI] [PubMed] [Google Scholar]
- 52.Hall SA, Page ST, Travison TG, Montgomery RB, Link CL, McKinlay JB. Do statins affect androgen levels in men? Results from the Boston area community health survey. Cancer Epidemiol Biomarkers Prev. 2007;16:1587–1594. doi: 10.1158/1055-9965.EPI-07-0306. [DOI] [PubMed] [Google Scholar]
- 53.Mener DJ. Prostate specific antigen reduction following statin therapy: Mechanism of action and review of the literature. IUBMB Life. 2010;62:584–590. doi: 10.1002/iub.355. [DOI] [PubMed] [Google Scholar]
- 54.Ku JH, Jeong CW, Park YH, Cho MC, Kwak C, Kim HH. Relationship of statins to clinical presentation and biochemical outcomes after radical prostatectomy in Korean patients. Prostate Cancer Prostatic Dis. 2010 doi: 10.1038/pcan.2010.39. [DOI] [PubMed] [Google Scholar]
- 55.Garrett IR, Mundy GR. The role of statins as potential targets for bone formation. Arthritis Res. 2002;4:237–240. doi: 10.1186/ar413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Solomon KR, Freeman MR. Do the cholesterol-lowering properties of statins affect cancer risk? Trends Endocrinol Metab. 2008;19:113–121. doi: 10.1016/j.tem.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 57.Rao S, Porter DC, Chen X, Herliczek T, Lowe M, Keyomarsi K. Lovastatin-mediated G1 arrest is through inhibition of the proteasome, independent of hydroxymethyl glutaryl-CoA reductase. Proc Natl Acad Sci U S A. 1999;96:7797–7802. doi: 10.1073/pnas.96.14.7797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koitabashi Y, Kumai T, Matsumoto N, Watanabe M, Sekine S, Yanagida Y, Kobayashi S. Orange juice increased the bioavailability of pravastatin, 3-hydroxy-3-methylglutaryl CoA reductase inhibitor, in rats and healthy human subjects. Life Sci. 2006;78:2852–2859. doi: 10.1016/j.lfs.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 59.Mondul AM, Clipp SL, Helzlsouer KJ, Platz EA. Association between plasma total cholesterol concentration and incident prostate cancer in the CLUE II cohort. Cancer Causes Control. 2010;21:61–68. doi: 10.1007/s10552-009-9434-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Van Hemelrijck M, Garmo H, Holmberg L, Walldius G, Jungner I, Hammar N, Lambe M. Prostate cancer risk in the Swedish AMORIS study: the interplay among triglycerides, total cholesterol, and glucose. Cancer. 2010 doi: 10.1002/cncr.25758. [DOI] [PubMed] [Google Scholar]
- 61.Zhuang L, Lin J, Lu ML, Solomon KR, Freeman MR. Cholesterol-rich lipid rafts mediate akt-regulated survival in prostate cancer cells. Cancer Res. 2002;62:2227–2231. [PubMed] [Google Scholar]
- 62.Di Vizio D, Adam RM, Kim J, Kim R, Sotgia F, Williams T, Demichelis F, Solomon KR, Loda M, Rubin MA, Lisanti MP, Freeman MR. Caveolin-1 interacts with a lipid raftassociated population of fatty acid synthase. Cell Cycle. 2008;7:2257–2267. doi: 10.4161/cc.7.14.6475. [DOI] [PubMed] [Google Scholar]
- 63.Cinar B, Mukhopadhyay NK, Meng G, Freeman MR. Phosphoinositide 3-kinaseindependent non-genomic signals transit from the androgen receptor to Akt1 in membrane raft microdomains. J Biol Chem. 2007;282:29584–29593. doi: 10.1074/jbc.M703310200. [DOI] [PubMed] [Google Scholar]
- 64.Mondul AM, Selvin E, De Marzo AM, Freedland SJ, Platz EA. Statin drugs, serum cholesterol, and prostate-specific antigen in the National Health and Nutrition Examination Survey 2001-2004. Cancer Causes Control. 2010;21:671–678. doi: 10.1007/s10552-009-9494-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Henriksson P, Eriksson M, Ericsson S, Rudling M, Stege R, Berglund L, Angelin B. Hypocholesterolaemia and increased elimination of low-density lipoproteins in metastatic cancer of the prostate. Lancet. 1989;2:1178–1180. doi: 10.1016/s0140-6736(89)91790-x. [DOI] [PubMed] [Google Scholar]
- 66.Chen SS, Chen KK, Lin AT, Chang YH, Wu HH, Chang LS. Correlation between pretreatment serum biochemical markers and treatment outcome for prostatic cancer with bony metastasis. J Chin Med Assoc. 2009;72:301–306. doi: 10.1016/S1726-4901(09)70376-4. [DOI] [PubMed] [Google Scholar]
- 67.Thysell E, Surowiec I, Hornberg E, Crnalic S, Widmark A, Johansson AI, Stattin P, Bergh A, Moritz T, Antti H, Wikstrom P. Metabolomic characterization of human prostate cancer bone metastases reveals increased levels of cholesterol. PLoS One. 2010;5:e14175. doi: 10.1371/journal.pone.0014175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wennerberg K, Rossman KL, Der CJ. The Ras superfamily at a glance. J Cell Sci. 2005;118:843–846. doi: 10.1242/jcs.01660. [DOI] [PubMed] [Google Scholar]
- 69.Colicelli J. Human RAS superfamily proteins and related GTPases. Sci STKE. 2004;2004:RE13. doi: 10.1126/stke.2502004re13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schmidt A, Hall A. Guanine nucleotide exchange factors for Rho GTPases: turning on the switch. Genes Dev. 2002;16:1587–1609. doi: 10.1101/gad.1003302. [DOI] [PubMed] [Google Scholar]
- 71.Calero M, Chen CZ, Zhu W, Winand N, Havas KA, Gilbert PM, Burd CG, Collins RN. Dual prenylation is required for Rab protein localization and function. Mol Biol Cell. 2003;14:1852–1867. doi: 10.1091/mbc.E02-11-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Richards SA, Lounsbury KM, Macara IG. The C terminus of the nuclear RAN/TC4 GTPase stabilizes the GDP-bound state and mediates interactions with RCC1, RAN-GAP, and HTF9A/RANBP1. J Biol Chem. 1995;270:14405–14411. doi: 10.1074/jbc.270.24.14405. [DOI] [PubMed] [Google Scholar]
- 73.Seabra MC, Reiss Y, Casey PJ, Brown MS, Goldstein JL. Protein farnesyltransferase and geranylgeranyltransferase share a common alpha subunit. Cell. 1991;65:429–434. doi: 10.1016/0092-8674(91)90460-g. [DOI] [PubMed] [Google Scholar]
- 74.Adamson P, Marshall CJ, Hall A, Tilbrook PA. Post-translational modifications of p21rho proteins. J Biol Chem. 1992;267:20033–20038. [PubMed] [Google Scholar]
- 75.Wennerberg K, Der CJ. Rho-family GTPases: it's not only Rac and Rho (and I like it) J Cell Sci. 2004;117:1301–1312. doi: 10.1242/jcs.01118. [DOI] [PubMed] [Google Scholar]
- 76.Bustelo XR, Sauzeau V, Berenjeno IM. GTP-binding proteins of the Rho/Rac family: regulation, effectors and functions in vivo. Bioessays. 2007;29:356–370. doi: 10.1002/bies.20558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ghosh PM, Mott GE, Ghosh-Choudhury N, Radnik RA, Stapleton ML, Ghidoni JJ, Kreisberg JI. Lovastatin induces apoptosis by inhibiting mitotic and post-mitotic events in cultured mesangial cells. Biochim Biophys Acta. 1997;1359:13–24. doi: 10.1016/s0167-4889(97)00091-8. [DOI] [PubMed] [Google Scholar]
- 78.Yao L, Romero MJ, Toque HA, Yang G, Caldwell RB, Caldwell RW. The role of RhoA/Rho kinase pathway in endothelial dysfunction. J Cardiovasc Dis Res. 2010;1:165–170. doi: 10.4103/0975-3583.74258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Somlyo AV, Bradshaw D, Ramos S, Murphy C, Myers CE, Somlyo AP. Rho-kinase inhibitor retards migration and in vivo dissemination of human prostate cancer cells. Biochem Biophys Res Commun. 2000;269:652–659. doi: 10.1006/bbrc.2000.2343. [DOI] [PubMed] [Google Scholar]
- 80.Yao H, Dashner EJ, van Golen CM, van Golen KL. RhoC GTPase is required for PC-3 prostate cancer cell invasion but not motility. Oncogene. 2006;25:2285–2296. doi: 10.1038/sj.onc.1209260. [DOI] [PubMed] [Google Scholar]
- 81.Sequeira L, Dubyk CW, Riesenberger TA, Cooper CR, van Golen KL. Rho GTPases in PC-3 prostate cancer cell morphology, invasion and tumor cell diapedesis. Clin Exp Metastasis. 2008;25:569–579. doi: 10.1007/s10585-008-9173-3. [DOI] [PubMed] [Google Scholar]
- 82.Hall CL, Dubyk CW, Riesenberger TA, Shein D, Keller ET, van Golen KL. Type I collagen receptor (alpha2beta1) signaling promotes prostate cancer invasion through RhoC GTPase. Neoplasia. 2008;10:797–803. doi: 10.1593/neo.08380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Iiizumi M, Bandyopadhyay S, Pai SK, Watabe M, Hirota S, Hosobe S, Tsukada T, Miura K, Saito K, Furuta E, Liu W, Xing F, Okuda H, Kobayashi A, Watabe K. RhoC promotes metastasis via activation of the Pyk2 pathway in prostate cancer. Cancer Res. 2008;68:7613–7620. doi: 10.1158/0008-5472.CAN-07-6700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ichikawa T, Schalken JA, Ichikawa Y, Steinberg GD, Isaacs JT. H-ras expression, genetic instability, and acquisition of metastatic ability by rat prostatic cancer cells following v-H-ras oncogene transfection. Prostate. 1991;18:163–172. doi: 10.1002/pros.2990180209. [DOI] [PubMed] [Google Scholar]
- 85.Gumerlock PH, Poonamallee UR, Meyers FJ, deVere White RW. Activated ras alleles in human carcinoma of the prostate are rare. Cancer Res. 1991;51:1632–1637. [PubMed] [Google Scholar]
- 86.Yin J, Pollock C, Tracy K, Chock M, Martin P, Oberst M, Kelly K. Activation of the Ral-GEF/Ral pathway promotes prostate cancer metastasis to bone. Mol Cell Biol. 2007;27:7538–7550. doi: 10.1128/MCB.00955-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Krukemyer JJ, Talbert RL. Lovastata new cholesterol-lowering agent. Pharmacotherapy. 1987;7:198–210. doi: 10.1002/j.1875-9114.1987.tb03524.x. [DOI] [PubMed] [Google Scholar]
- 88.Endo A, Kuroda M, Tanzawa K. Competitive inhibition of 3-hydroxy-3-methylglutaryl coenzyme A reductase by ML-236A and ML-236B fungal metabolites, having hypocholesterolemic activity. FEBS Lett. 1976;72:323–326. doi: 10.1016/0014-5793(76)80996-9. [DOI] [PubMed] [Google Scholar]
- 89.Danesi R, McLellan CA, Myers CE. Specific labeling of isoprenylated proteins: application to study inhibitors of the post-translational farnesylation and geranylgeranylation. Biochem Biophys Res Commun. 1995;206:637–643. doi: 10.1006/bbrc.1995.1090. [DOI] [PubMed] [Google Scholar]
- 90.de Souza PL, Castillo M, Myers CE. Enhancement of paclitaxel activity against hormone-refractory prostate cancer cells in vitro and in vivo by quinacrine. Br J Cancer. 1997;75:1593–1600. doi: 10.1038/bjc.1997.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ghosh PM, Moyer ML, Mott GE, Kreisberg JI. Effect of cyclin E overexpression on lovastatin-induced G1 arrest and RhoA inactivation in NIH3T3 cells. J Cell Biochem. 1999;74:532–543. doi: 10.1002/(sici)1097-4644(19990915)74:4<532::aid-jcb3>3.3.co;2-5. [DOI] [PubMed] [Google Scholar]
- 92.Parikh A, Childress C, Deitrick K, Lin Q, Rukstalis D, Yang W. Statin-induced autophagy by inhibition of geranylgeranyl biosynthesis in prostate cancer PC3 cells. Prostate. 2010;70:971–981. doi: 10.1002/pros.21131. [DOI] [PubMed] [Google Scholar]
- 93.Kim RH, Bold RJ, Kung HJ. ADI, autophagy and apoptosis: metabolic stress as a therapeutic option for prostate cancer. Autophagy. 2009;5:567–568. doi: 10.4161/auto.5.4.8252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kim RH, Coates JM, Bowles TL, McNerney GP, Sutcliffe J, Jung JU, Gandour-Edwards R, Chuang FY, Bold RJ, Kung HJ. Arginine deiminase as a novel therapy for prostate cancer induces autophagy and caspaseindependent apoptosis. Cancer Res. 2009;69:700–708. doi: 10.1158/0008-5472.CAN-08-3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ghosh PM, Bedolla R, Mikhailova M, Kreisberg JI. RhoA-dependent murine prostate cancer cell proliferation and apoptosis: role of protein kinase Czeta. Cancer Res. 2002;62:2630–2636. [PubMed] [Google Scholar]
- 96.Wu Z, Chang PC, Yang JC, Chu CY, Wang LY, Chen NT, Ma AH, Desai SJ, Lo SH, Evans CP, Lam KS, Kung HJ. Autophagy Blockade Sensitizes Prostate Cancer Cells towards Src Family Kinase Inhibitors. Genes Cancer. 2010;1:40–49. doi: 10.1177/1947601909358324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Moyret-Lalle C, Marcais C, Jacquemier J, Moles JP, Daver A, Soret JY, Jeanteur P, Ozturk M, Theillet C. ras, p53 and HPV status in benign and malignant prostate tumors. Int J Cancer. 1995;64:124–129. doi: 10.1002/ijc.2910640209. [DOI] [PubMed] [Google Scholar]
- 98.Konishi N, Hiasa Y, Matsuda H, Tao M, Tsuzuki T, Hayashi I, Kitahori Y, Shiraishi T, Yatani R, Shimazaki J, et al. Intratumor cellular heterogeneity and alterations in ras oncogene and p53 tumor suppressor gene in human prostate carcinoma. Am J Pathol. 1995;147:1112–1122. [PMC free article] [PubMed] [Google Scholar]
- 99.Chia SJ, Tang WY, Elnatan J, Yap WM, Goh HS, Smith DR. Prostate tumours from an Asian population: examination of bax, bcl-2, p53 and ras and identification of bax as a prognostic marker. Br J Cancer. 2000;83:761–768. doi: 10.1054/bjoc.2000.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Viola MV, Fromowitz F, Oravez S, Deb S, Finkel G, Lundy J, Hand P, Thor A, Schlom J. Expression of ras oncogene p21 in prostate cancer. N Engl J Med. 1986;314:133–137. doi: 10.1056/NEJM198601163140301. [DOI] [PubMed] [Google Scholar]
- 101.Agnantis NJ, Constantinidou AE, Papaevagelou M, Apostolikas N. Comparative immunohistochemical study of ras-p21 oncoprotein in adenomatous hyperplasia and adenocarcinoma of the prostate gland. Anticancer Res. 1994;14:2135–2140. [PubMed] [Google Scholar]
- 102.Fan K. Heterogeneous subpopulations of human prostatic adenocarcinoma cells: potential usefulness of P21 protein as a predictor for bone metastasis. J Urol. 1988;139:318–322. doi: 10.1016/s0022-5347(17)42397-4. [DOI] [PubMed] [Google Scholar]
- 103.Idikio HA, Manickavel V. Correlation of blood group antigen expression and oncogene-related proteins in malignant prostatic tissues. Pathol Res Pract. 1991;187:189–197. doi: 10.1016/S0344-0338(11)80770-3. [DOI] [PubMed] [Google Scholar]
- 104.Traynor P, McGlynn LM, Mukhergee R, Grimsley SJ, Bartlett JM, Edwards J. An increase in N-Ras expression is associated with development of hormone refractory prostate cancer in a subset of patients. Dis Markers. 2008;24:157–165. doi: 10.1155/2008/918087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Varma VA, Austin GE, O'Connell AC. Antibodies to ras oncogene p21 proteins lack immunohistochemical specificity for neoplastic epithelium in human prostate tissue. Arch Pathol Lab Med. 1989;113:16–19. [PubMed] [Google Scholar]
- 106.Bushman EC, Nayak RN, Bushman W. Immunohistochemical staining of ras p21: staining in benign and malignant prostate tissue. J Urol. 1995;153:233–237. doi: 10.1097/00005392-199501000-00083. [DOI] [PubMed] [Google Scholar]
- 107.Muller JM, Metzger E, Greschik H, Bosserhoff AK, Mercep L, Buettner R, Schule R. The transcriptional coactivator FHL2 transmits Rho signals from the cell membrane into the nucleus. EMBO J. 2002;21:736–748. doi: 10.1093/emboj/21.4.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kobayashi T, Shimizu Y, Terada N, Yamasaki T, Nakamura E, Toda Y, Nishiyama H, Kamoto T, Ogawa O, Inoue T. Regulation of androgen receptor transactivity and mTOR-S6 kinase pathway by Rheb in prostate cancer cell proliferation. Prostate. 2010;70:866–874. doi: 10.1002/pros.21120. [DOI] [PubMed] [Google Scholar]
- 109.Engers R, Ziegler S, Mueller M, Walter A, Willers R, Gabbert HE. Prognostic relevance of increased Rac GTPase expression in prostate carcinomas. Endocr Relat Cancer. 2007;14:245–256. doi: 10.1677/ERC-06-0036. [DOI] [PubMed] [Google Scholar]
- 110.Bektic J, Pfeil K, Berger AP, Ramoner R, Pelzer A, Schafer G, Kofler K, Bartsch G, Klocker H. Small G-protein RhoE is underexpressed in prostate cancer and induces cell cycle arrest and apoptosis. Prostate. 2005;64:332–340. doi: 10.1002/pros.20243. [DOI] [PubMed] [Google Scholar]
- 111.Engers R, Mueller M, Walter A, Collard JG, Willers R, Gabbert HE. Prognostic relevance of Tiam1 protein expression in prostate carcinomas. Br J Cancer. 2006;95:1081–1086. doi: 10.1038/sj.bjc.6603385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Meador BM, Huey KA. Statin-associated myopathy and its exacerbation with exercise. Muscle Nerve. 2010;42:469–479. doi: 10.1002/mus.21817. [DOI] [PubMed] [Google Scholar]
- 113.Cao P, Hanai J, Tanksale P, Imamura S, Sukhatme VP, Lecker SH. Statin-induced muscle damage and atrogin-1 induction is the result of a geranylgeranylation defect. FASEB J. 2009;23:2844–2854. doi: 10.1096/fj.08-128843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Roberts PJ, Mitin N, Keller PJ, Chenette EJ, Madigan JP, Currin RO, Cox AD, Wilson O, Kirschmeier P, Der CJ. Rho Family GTPase modification and dependence on CAAX motifsignaled posttranslational modification. J Biol Chem. 2008;283:25150–25163. doi: 10.1074/jbc.M800882200. [DOI] [PMC free article] [PubMed] [Google Scholar]