Abstract
Guanine (G) rich G4T4G4 DNA and homologous PNA strands tend to form antiparallel dimeric quadruplexes, which can be hybridized to form a stable parallel DNA2PNA2 hybrid quadruplex. In contrast, the same DNA strands functionalised with large planar aromatic groups at the 5′-end lead to the formation of parallel DNA quadruplex, which cannot be perturbed by homologous PNA strands. Conformation and composition of the DNA quadruplexes can be programed by π-π-stacking interaction.
The guanine (G) rich telomeric DNA sequence of Oxytricha nova, d(G4T4G4) is known to form1 stable antiparallel hairpin dimeric quadruplexes through Hoogsteen hydrogen bond formation, in the presence of Na+ or K+ ion. Peptide nucleic acid (PNA) is a DNA analog2 that contains oligomeric N-(2-aminoethyl)gycine units bearing nucleobases at the glycine nitrogen through acetyl linkers. More recent effort has been directed to the hybridization2,3 of complementary as well as homologous PNA molecules with DNA and RNA for their importance in sequencing and sensing, antisense agents, and in gene technology. The hybrid DNA/PNA duplexes, triplexes, and quadruplexes display much higher thermal stability over the purely DNA and RNA superstructures.2 Enhanced stability of the DNA-PNA hybrid structures is attributed to the neutral peptidic backbones of the PNA strands, which in addition to diminishing the electrostatic repulsion between the phosphate backbones of the DNA strands, provide an optimal spacing between the nucleobases that matches the sugar-phosphate backbone of DNA and RNA. This structural advantage is reflected in the stability of duplexes formed by complementary DNA, PNA, and RNA strands (PNA-PNA > PNA-RNA > PNA-DNA > RNA-DNA > DNA-DNA).2b
Armitage et al.4 established that G4T4G4 DNA and PNA form a stable 2:2 hybrid quadruplex (Figure 1c), in which each of the two negatively charged DNA strands is flanked by two neutral PNA strands and the 5′-ends of the two DNAs are aligned with the N-termini of both PNA strands. As part of our interest in controlling the characteristics of quadruplexes,5 we set out to investigate whether or not the formation of homo and hybrid quadruplexes can be biased in either direction through chemical modification of the DNA and PNA strands. If the structural integrity of the functionalized DNA and PNA quadruplexes can be controlled, they can be employed to form microarrays of fluorescence biosensors, as well as energy and electron transfer materials.
Fig. 1.
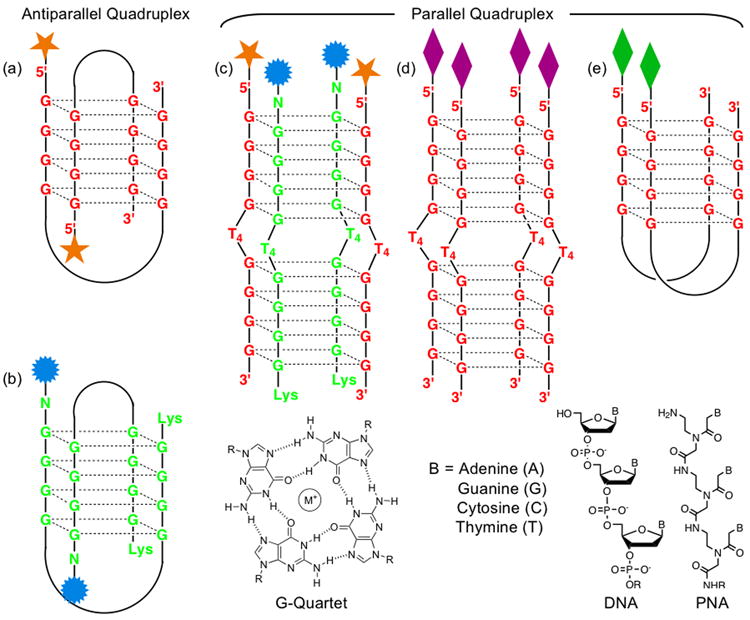
Graphical representation of G4T4G4 DNA and PNA quadruplexes, modified at the 5′- and N-terminals, respectively. (a) an antiparallel hairpin dimeric DNA quadruplex, (b) an antiparallel hairpin dimeric PNA quadruplex, (c) a parallel DNA2-PNA2 hybrid quadruplex, (d) a tetramolecular parallel DNA4 quadruplex, and (e) a dimeric parallel DNA2 quadruplex.
Herein we report that the stability, conformation, and composition of quadruplexes depend not only on the nucleobase sequence, but also on the properties of the head-groups that are attached to the 5′- and N-termini of the DNA and PNA strands, respectively (Figure 2). In order to investigate the formation of the hybrid quadruplexes by the G4T4G4 DNA and PNA strands in the presence of different head-groups, we functionalized the 5′- and N-termini of both strands with a number of aromatic units, ranging from energy donating and accepting small π-surfaces, like coumarin-2 (1) and coumarin-343 (2), to electron donating and accepting larger π-surfaces, such as porphyrin (3, 4) and perylene (5) units.
Fig. 2.
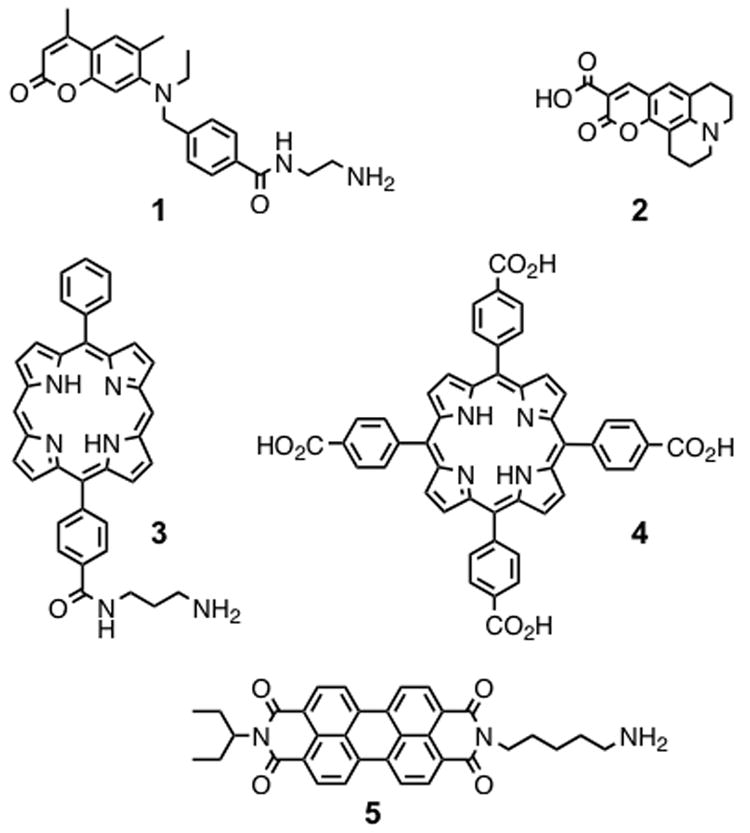
Coumarin-2 derivative (1), coumarin-343 (2), porphyrin derivatives (3 and 4), and perylene diimide (5) used as 5′- and N- head-groups for DNA and PNA, respectively.
5′-C10-carboxy-modified d(G4T4G4) oligonucleotide (ODN), 5′-C6-amino-modified d(G4T4G4) ODN, and homologous FmocNH-G4T4G4-LysNHBhoc PNA strands were used for 5′-end and N-end functionalization. The coumarin-2 derivative 1, porphyrin derivatives 3 and 4, as well as perylene diimide 5 were attached to the modified ODNs via solid-state amide coupling reactions,4 followed by cleavage of the resisn and HPLC purification to obtain three functionalized ODNs. Basic deprotection of the Fmoc group yielded an amine-terminated PNA strand, which allowed the peptide coupling with coumarin-343 (2). Cleavage of the resin and HPLC purification provided two functionalized PNA strands.4 All compounds were characterized by high resolution MALDI-TOF mass spectrometry (MS) and UV-Vis spectroscopy.
Formation of the DNA, PNA, and hybrid quadruplexes is a dynamic process, owing to hydrogen bonding, metal ion coordination, as well as π-π-stacking interactions between the nucleobases. All quadruplexes were formed4 by incubating single-stranded (SS) DNA and/or PNA at 20 μM concentrations in pH = 7.0 buffer, containing 10 mM sodium phosphate, 100 mM NaCl, and 0.1 mM EDTA at 90°C for 15 min, then cooling them slowly to 25°C and storing them at 4°C for 16 h. In the presence of Na+ ions, the circular dichroism (CD) spectrum of unmodified d(G4T4G4) ODN displays4 a maximum at 295 nm and a minimum at 265 nm, indicating the formation of an antiparallel dimeric hairpin DNA quadruplex (Supplementary Information). CD spectroscopy further confirmed that the dimeric antiparallel conformation was preserved (Figure 3) for the modified d(G4T4G4) ODNs functionalised at the 5′-end with smaller aromatic units, such as fluorescein4 and coumarin-2 (1) that cannot exert strong π-π-stacking interaction. The d(G4T4G4) ODNs modified with porphyrin 3 at the 5′-end, however, showed a CD peak at 265 nm and a trough at 245 nm, signals that are characteristic5 of a parallel DNA quadruplex. Two different quadruplex conformations can be explained by the difference in the strength of π-π-stacking interactions between the aromatic head-groups and their influence on the quadruplex formation. In the case of 1-DNA, the coumarin-2 unit is not large enough to provide any significant π-π-stacking interaction, allowing the formation of a dimeric antiparallel quadruplex (Figure 1a). In 3-DNA, the hydrophobic porphyrin head-group presents a large planar π-surface that templates the formation of a highly stable parallel quadruplex involving two or four DNA strands (Figure 1d, e), via π-π-stacking interactions in the aqueous environment. Porphyrin units are known5 to template G-quartet formation by preorganizing four guanine bases through π-stacking interaction. An almost identical CD spectrum — characteristic of a parallel DNA quadruplex (Figure 1d, e) — was recorded for 5-DNA, which carries a hydrophobic planar head-group, perylene diimide 5, at the 5′-end.
Fig. 3.
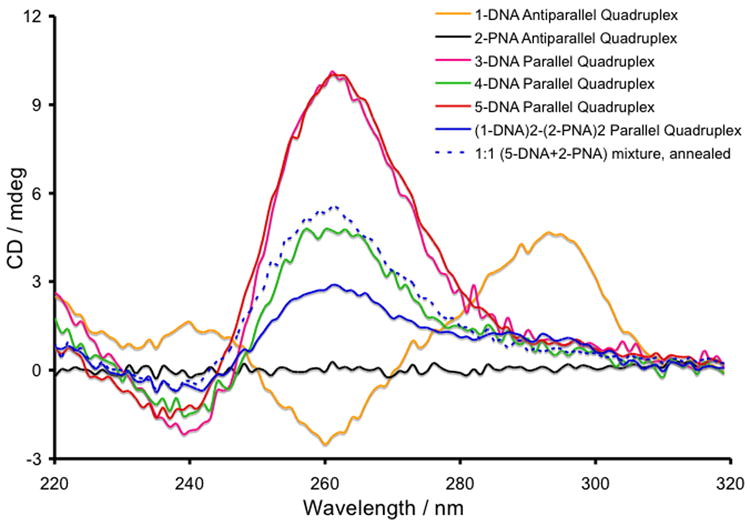
CD spectra demonstrating formation of DNA, PNA, and hybrid quadruplexes at 20 μM single-strand concentration of each species.
To destabilize the strong π-π-stacking interactions between the hydrophobic porphyrin units by electrostatic repulsion, we installed tetra-(4-carboxyphenyl) porphyrin 4 head-groups via amide bond formation on 5′-C6-amino-modified d(G4T4G4) ODN. The CD spectrum of the annealed sample showed a significant departure from that of 3-DNA. Although CD spectra of 3-DNA, 4-DNA and 5-DNA indicate the formation of respective parallel qudruplexes that show characteristic peaks at 265 nm and troughs at 245 nm, the 4-DNA quadruplex shows only 50% intensity of the 265 nm peak compared to that of the 3-DNA and 5-DNA quadruplexes (Figures 3) at the same SS concentrations (20 and 40 μM). Electrostatic repulsion between the negatively charged porphyrin residues in 4-DNA presumably destabilises the formation of quadruplexes leading to the formation of a parallel hairpin dimer (Figure 1e), allowing only two negatively charged prophyrins to come in close proximity. In contrast, the 3-DNA and 5-DNA strands predominantly form tetramolecular parallel quadruplexes (Figure 1d), by virtue of π-π-stacking interaction between neutral aromatic groups.
The quadruplex formed by comarin-343 (2) appended G4T4G4-Lys PNA strands under identical conditions did not show (Figure 3) any CD signal since the PNA backbone lacks a chiral center other than at the lysine residue. Armitage et al.5,6 demonstrated that akin to d(G4T4G4) ODN, H-G4T4G4-Lys PNA strand form a dimeric antiparallel quadruplex. We employed 2-PNA strand as a probe to examine the structural integrity (Figure 3) of the modified DNA quadruplexes carrying different 5′-residues. For this study, an equimolar amount (10 μM of each SS in the final solution mixture) of each of the preformed quadruplexes of 1-DNA and 5-DNA was treated separately with 2-PNA (10 μM SS in the final solution) under the same annealing conditions. In the presence of the 2-PNA probe, the CD spectrum of the dimeric antiparallel quadruplex of 1-DNA changed dramatically, showing complete disappearance of the 295 nm peak and 265 nm trough and emergence of a peak at 265 nm and a trough at 245 nm. These spectroscopic changes indicate that the dimeric antiparallel 1-DNA quadruplex is perturbed by two homologous PNA strands forming a tetramolecular hybrid parallel quadruplex (Figure 1c), which is a thermo-dynamically more stable assembly.4 The preformed parallel quadruplex of 5-DNA, however, did not show any change in the CD spectrum after treatment with an equimolar amount of the 2-PNA probe, apart from a reduction in the intensities of the 265 and 245 nm signals by 50%, presumably because of a two-fold dilution of the original solution. Had a (5-DNA)2(2-PNA)2 hybrid quadruplex formed, the resulting CD spectrum would have been similar to that recorded for the (1-DNA)2(2-PNA)2 quadruplex as a result of having the same helical pitch. These results suggest that the π-π-stacking interaction between the larger aromatic head groups, which template the formation of parallel homo-quadruplexes,5 are strong enough to resist a disruption by the PNA probe. Therefore, such π-π-stacking interaction or lack thereof can be exploited to bias the formation of either an all-DNA homo-quadruplex or a hybrid with a homlogous PNA.
To confirm the difference in compositions of the 3-DNA and 4-DNA parallel quadruplexes, we studied the CD response of quadruplex formed from 5′-TG8T3-3′ ODN at 20 and 40 μM SS concentrations in identical buffer conditions and compared them with those of 3-DNA at the same concentrations (Figure 4). The intensities of the 265 and 245 nm CD signals of both quadruplexes were virtually same at respective SS concentrations although the tetramolecular parallel quadruplex of TG8T3 presents eight contiguous planes of G-quartets, whereas the 3-DNA quadruplex possesses two sets of four contiguous G-quartet planes separated by a TTTT segment. Therefore, it is unlikely that the TTTT segment, separating two GGGG segments in 3-DNA, interferes with exciton-coupling among the G-quartet planes. This also confirms that while 3-DNA forms a tetramolecular quadruplex with eight G-quartets planes, 4-DNA forms a dimeric quadruplex with only four G-quartet planes, resulting in 50% reduction of the CD intensity for the latter at various concentrations (Figure 5).
Fig. 4.
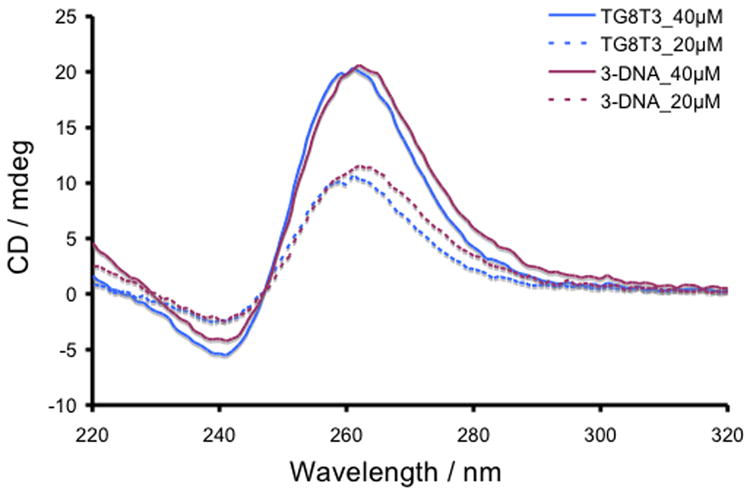
CD spectra demonstrating the formation of parallel quadruplexes of 5′-TG8T3-3′ ODN and 4-DNA at 20 and 40 μM single-strand concentration.
Fig. 5.
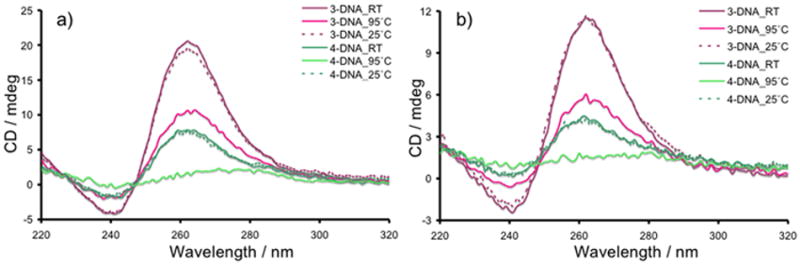
CD spectra demonstrating formation (RT), thermal dissociation (95 °C), and reformation (25 °C) of 3-DNA and 4-DNA quadruplexes at a) 40 μM and b) 20 μM SS concentrations of each species.
To understand the effects of 5′-residues on 3-DNA and 4-DNA quadruplexes, we investigated their thermal stabilities at two different concentrations (20 μM and 40 μM of SS) by CD spectroscopy. As expected, the intensity of the 265 nm peak and 245 trough at 25°C for both 3-DNA and 4-DNA quadruplexes were twice as high at 40 μM compared to those at 20 μM of initial SS concentrations (Figure 5). This suggests that 3-DNA and 4-DNA SS are completely consumed to form respective parallel quadruplexes at both concentrations. While the CD signals of 4-DNA quadruplex disappeared completely at 95°C as a result of thermal decomplexation, the intensity of 3-DNA quadruplex signals faded only partially, suggesting a higher thermal stability of the latter. Cooling of each quadruplex solution to 25°C reproduced the corresponding initial CD spectra recorded at room temperature, confirming the reversibility of the thermal decomplexation/reformation processes. We assert that a neutral porphyrin residue at the 5′-end of 3-DNA leads to the formation of a stable tetramolecular parallel quadruplex, which cannot be completely dissociated thermally in an aqueous buffer, containing Na+ ion. In contrast, to minimize the electrostatic repulsion between the charged porphyrin residues, 4-DNA forms a dimeric parallel quadruplex which can be thermally dissociated completely.
In summary, we have demonstrated that the perturbation of a DNA quadruplex by a homologous PNA strand can be programed by equipping the DNA strands with large planar aromatic head-groups. The nature of the 5′-modifications of d(G4T4G4) ODNs determines whether this telomeric sequence would form antiparallel or parallel quadruplexes.7 We could also control the stability, geometry, and molecularity of the parallel quadruplexes formed by the modified d(G4T4G4) ODNs by changing the nature of aromatic residues at their 5′-ends.
Supplementary Material
Acknowledgments
We thank the National Science Foundation for financial support of this reaserch.
Footnotes
Electronic Supplementary Information (ESI) available: [CD melting and control studies, as well as syntheses and characterizations of compounds 1 and 5 as well as those of functionalized DNA and PNA strands with compounds 1 through 5]. See DOI: 10.1039/b000000x/
References
- 1.Miyoshi D, Nakao A, Sugimoto N. Nucleic Acids Res. 2003;31:1156. doi: 10.1093/nar/gkg211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hyrup B, Nielsen PE. Bioorg Med Chem. 1996;4:5. doi: 10.1016/0968-0896(95)00171-9. [DOI] [PubMed] [Google Scholar]; Nielsen PE, Haaima G. Chem Soc Rev. 1997:73. [Google Scholar]; Uhlmann E, Peyman A, Breiphol G, Will DW. Angew Chem Int Ed. 1998;37:2797. doi: 10.1002/(SICI)1521-3773(19981102)37:20<2796::AID-ANIE2796>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 3.Marin VL, Armitage BA. Biochemistry. 2006;45:1745. doi: 10.1021/bi051831q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Datta B, Armitage BA. J Am Chem Soc. 2001;123:9612. doi: 10.1021/ja016204c. [DOI] [PubMed] [Google Scholar]; Datta B, Smitt C, Armitage BA. J Am Chem Soc. 2003;125:4111. doi: 10.1021/ja028323d. [DOI] [PubMed] [Google Scholar]; Roy S, Tanious FA, Wilson WD, Ly DH, Armitage BA. Biochemistry. 2007;46:10433. doi: 10.1021/bi700854r. [DOI] [PubMed] [Google Scholar]
- 5.Jayawickramarajah J, Tagore DM, Tsou LK, Hamilton AD. Angew Chem Int Ed. 2007;46:7583. doi: 10.1002/anie.200701883. [DOI] [PubMed] [Google Scholar]; Tagore DM, Sprinz KI, Fletcher S, Jayawickramarajah J, Hamilton AD. Angew Chem Int Ed. 2007;46:223. doi: 10.1002/anie.200603479. [DOI] [PubMed] [Google Scholar]
- 6.Krishnan-Ghosh Y, Stephens E, Balasubramanian S. J Am Chem Soc. 2004;126:5944. doi: 10.1021/ja031508f. [DOI] [PubMed] [Google Scholar]; Datta B, Bier ME, Roy S, Armitage BA. J Am Chem Soc. 2005;127:4199. doi: 10.1021/ja0446202. [DOI] [PubMed] [Google Scholar]
- 7.Benz A, Hartig JS. Chem Commun. 2008;37:4010. doi: 10.1039/b805227a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


