Abstract
Objective
The goal of this study was to compare internal carotid artery (ICA) intima-media thickness (IMT) with common carotid artery (CCA) IMT as global markers of cardiovascular disease (CVD).
Methods
Cross-sectional measurements of the mean CCA IMT and maximum ICA IMT were made on ultrasound images acquired from the Framingham Offspring cohort (n = 3316; mean age, 58 years; 52.7% women). Linear regression models were used to study the associations of the Framingham risk factors with CCA and ICA IMT. Multivariate logistic regression models and receiver operating characteristic (ROC) curve analysis were used to compare the associations of prevalent CVD with CCA and ICA IMT and determine sensitivity and specificity.
Results
The association between age and the mean CCA IMT corresponded to an increase of 0.007 mm/y; the increase was 0.037 mm/y for the ICA IMT. Framingham risk factors accounted for 28.6% and 27.5% of the variability in the CCA and ICA IMT, respectively. Age and gender contributed 23.5% to the variability of the CCA IMT and 22.5% to that of the ICA IMT, with the next most important factor being systolic blood pressure (1.9%) for the CCA IMT and smoking (1.6%) for the ICA IMT. The CCA IMT and ICA IMT were statistically significant predictors of prevalent CVD, with the ICA IMT having a larger area under the ROC curve (0.756 versus 0.695).
Conclusions
Associations of risk factors with CCA and ICA IMT are slightly different, and both are independently associated with prevalent CVD. Their value for predicting incident cardiovascular events needs to be compared in outcome studies.
Keywords: atherosclerosis, carotid artery, disease prevalence, intima-media thickness, risk factors
Carotid artery intima-media thickness (IMT) is associated with cardiovascular risk factors and prevalent cardiovascular disease (CVD) and is predictive of cardiovascular events.1–8
Research protocols on which these results are based measure IMT differently: as the mean common carotid artery (CCA) IMT,9–12 the mean of the mean CCA and internal carotid artery (ICA) IMT,13 the mean of the maximum CCA and ICA IMT,6,14 and the mean CCA IMT and maximum ICA IMT.15 Although consensus panels have recommended using the mean CCA IMT and maximum ICA IMT11,16 for cardiovascular risk assessment, associations with prevalent disease and risk factors have not been made in a large patient cohort with well-defined end points of prevalent disease.
We hypothesized that the maximum ICA IMT and mean CCA IMT are equivalent in their associations with risk factors and prevalent CVD. We studied these two parameters in the context of global cardiovascular risk assessment.
Materials and Methods
This study was performed on members of the Framingham Offspring cohort undergoing examination cycle 6 from March 1995 to September 1998. The total number of individuals seen during cycle 6 clinic visits was 3532, of which 1657 were men and 1875 were women. The carotid ultrasound data presented in this study were acquired on 1593 men and 1784 women. The age range of the study participants was 26 to 83 years. Details of the study design have been published elsewhere.17 All participants provided informed consent, and the study protocol was approved by the Institutional Review Board of the Boston Medical Center.
During the clinic visit, a medical history was obtained, and a physical examination was performed. The risk factors relevant to this study were systolic blood pressure (BP), cigarette smoking status, use of hypertension medications, diabetes, total cholesterol level, and high-density lipoprotein (HDL) cholesterol level. These risk factors form the Framingham risk score and are used, in combination, for global assessment of cardiovascular risk.18 Use of lipid-lowering medications was also determined.
Systolic BP was determined from the average of 2 resting readings taken by a physician using a 14-cm BP cuff on the right arm. Smoking status was based on a self-reported history of cigarette smoking, and the level of exposure was estimated in terms of pack-years. The presence of diabetes was based on a history of diabetes, either a current or previous fasting glucose level of 126 mg/dL or higher, or current or previous use of antihyperglycemic medication. All lipid analyses were performed at the Framingham Heart Study Laboratory according to the Standardization Program of the Centers for Disease Control and Prevention and the National Heart Lung and Blood Institute Lipid Research Clinics.19,20
Ultrasound Studies
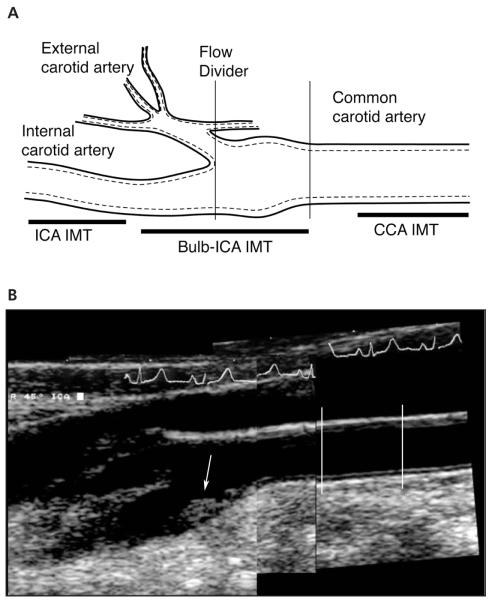
Ultrasound studies were acquired according to a standard protocol using a 7.5-MHz transducer for the CCA and a 5.0-MHz transducer (effective measured frequency, 6.7 MHz) for the ICA (SSH-140; Toshiba Medical Systems, Tustin, CA) and stored on Hi8 videotape. A trained sonographer with registered diagnostic medical sonographer certification performed all studies. The sonographer was certified in the protocol by the principal investigator (J.F.P.) after training that consisted of didactic lectures, review of the scanning technique, and proof of adherence to the protocol by performing 10 studies on volunteers. The principal investigator conducted biweekly review of the acquired studies. Doppler imaging was used to survey the carotid bifurcations and to evaluate for the presence of ICA stenosis. B-mode gray scale imaging was performed synchronized to the R-wave of an electrocardiographic signal. Images were acquired at 3 levels (Figure 1A): 1 in the CCA and 2 at the level of the carotid artery bulb and proximal ICA. A standard 45° projection from vertical was used in all cases.
Figure 1.
A, Three levels used in the Framingham carotid IMT protocol. The CCA image is taken just before the carotid artery bulb (≈5 mm). The carotid artery bulb is measured at the level of the proximal ICA sinus, typically centered on the flow divider. The ICA measurement is made in the ICA where the walls are again parallel (ICA IMT). B, Composite carotid sonograms used as part of the Framingham IMT protocol. The CCA IMT is measured between 5 and 15 mm before the carotid bulb (lines), whereas the maximum IMT is measured at the site of the thickest wall in the carotid artery bulb or proximal ICA IMT (arrow).
An additional view was taken in the carotid bulb and ICA, respectively, with the transducer centered on the largest plaque. The sonographer was instructed to change the scan plane to either a more anterior or posterior projection to better determine the maximum height of any plaque present. This judgment was purely subjective.
A certified reader performed carotid IMT measurements by drawing key interfaces on each acquired image. The maximum ICA IMT was determined by a heuristic computer algorithm that searched for the minimum distance between the lumen-intima interface to the media-adventitia interface given a constraint for curvature of the media-adventitia interface. In essence, the distance between the lumen-intima interface and the media-adventitia interface was the equivalent of a line drawn upward perpendicular to the media-adventia interface and intercepting the lumen-intima interface. A local search was then made, starting from the media-adventitia interface, to find a minimum in the calculated IMT distances over a distance of 5 points. The algorithm took into consideration images in which the far wall was not completely parallel to the transducer. The consistency of the algorithm was tested by measuring the estimated maximum IMT for generated plaque contours in a horizontal scan plane and then rotated to different degrees from horizontal. The mean CCA IMT was measured as the average of the respective IMT measurements made over a 1-cm segment of each CCA starting approximately 0.5 cm proximal to the carotid artery bulb. The maximum ICA IMT was defined as the largest IMT in either the right or left carotid artery bulb and ICA (Figure 1B).
Reproducibility was assessed by replicate measurements in 37 participants. The Pearson correlation coefficients between replicate readings were 0.94 for the mean CCA IMT and 0.76 for the maximum ICA IMT.
Prevalent Disease
All cardiovascular events in the Offspring cohort were adjudicated by a panel of 3 physicians on the basis of a review of data collected from Framingham Heart Clinic visits, inpatient hospitalizations, and office records. For the purpose of this investigation, CVD was defined according to the Framingham Heart Study as congestive heart disease (coronary death, myocardial infarction, coronary insufficiency, and angina), cerebrovascular events (ischemic stroke, hemorrhagic stroke, and transient ischemic attack), peripheral arterial disease (intermittent claudication), or heart failure.18 This constitutes a generalized score of atherosclerosis that can be readily applied to cardiovascular risk assessment.
Statistical Analysis
The analyses focused on the two key variables: mean CCA IMT and maximum ICA IMT. Associations between dichotomous cardiovascular risk factors and the presence versus absence of prevalent CVD were examined using the χ2 test; t tests were used to compare mean levels of the risk factors. Separate linear regression models with CCA IMT and ICA IMT as dependent variables were run for each of the Framingham risk factors (gender, age, systolic BP, HDL and low-density lipoprotein cholesterol, diabetes, smoking, and antihypertensive treatment).
The strength of the associations was expressed as the variance (R2) accounted for by the individual variables. This is equivalent to the percent variability in the IMT variable that can be accounted for by the risk factor. To determine which variables were stronger predictors of the IMT variables, separate multivariate linear regression models with CCA IMT and ICA IMT as dependent variables were run with all of the Framingham risk factors included. The strength of the associations between each risk factor and IMT was expressed as a semipartial R2, a metric that expresses the percent variability accounted for by the variable and also takes into account the influence of other risk factors.
Two multivariate logistic regression models were generated with prevalent CVD as the dependent variable. All Framingham risk factors were used as predictor variables to which each IMT variable was added separately. The respective odds ratios (ORs; odds of having CVD per unit value of the variable) were calculated together with their 95% confidence intervals (CIs). Odds ratios were reported on the basis of models using unitary increments for BP (millimeters of mercury) and units of 10 mg/dL for total and HDL cholesterol, The effect of lipid treatment on these models was examined by adding this variable to the two respective models.
The predictive power of the two IMT measurements was evaluated by estimating the area under the receiver operating characteristic (ROC) curve with CVD as the outcome. Sensitivity and specificity of each variable was determined at the inflection point (slope = 1) of the respective ROC curves.
All analyses were run using SAS version 9.1 software (SAS Inc, Cary, NC), P < .05 was considered statistically significant.
Results
Analyses were restricted to individuals with complete information on all risk factors and IMT (n = 3316). Demographic data are summarized in Table 1. The average age of our population was 58.8 years (SD, 9.8 years), and 1767 of the participants (52.7%) were women. Individuals with CVD tended to be older and were more likely to be men, receive hypertensive therapy, take cholesterol-lowering medications, and have diabetes (P < .0001). Total and HDL cholesterol levels were lower in individuals with CVD (P < .0001). There were no statistically significant differences in smoking status between both groups (P = .08). Both the mean CCA IMT and maximum ICA IMT were larger in individuals with prevalent CVD (P < .0001).
Table 1.
Distribution of Risk Factors as a Function of Prevalent CVD
| Risk Factor | Without CVD (n = 2949) |
With CVD (n = 367) |
Total Popu- lation (n = 3316) |
P, With vs Without CVD |
|---|---|---|---|---|
| Age, y | 57.9 (9.6) | 65.5 (8.3) | 58.8 (9.8) | <.0001 |
| Women, % | 54.9 | 34.9 | 52.7 | <.0001 |
| Systolic BP, mm Hg | 127.8 (18.7) | 133.8 (19.4) | 128.5 (18.9) | <.0001 |
| Total cholesterol, mg/dL | 206.6 (39.6) | 197.6 (42.4) | 205.6 (40.0) | <.0001 |
| HDL cholesterol, mg/dL | 51.8 (16.1) | 45.0 (15.0) | 51.0 (16.1) | <.0001 |
| Smoking, % | 14.8 | 18.3 | 15.2 | .0804 |
| Diabetes, % | 8.7 | 28.6 | 10.9 | <.0001 |
| Hypertension treatment, % | 24.5 | 58.3 | 28.2 | <.0001 |
| Lipid treatment, % | 9.7 | 38.7 | 12.9 | <.0001 |
| Mean CCA IMT, mm | 0.60 (0.13) | 0.71 (0.20) | 0.61 (0.15) | <.0001 |
| Maximum ICA IMT, mm | 1.35 (0.77) | 2.15 (1.02) | 1.44 (0.84) | <.0001 |
Values in parentheses are SDs.
Results of simple univariate linear regression models with each IMT variable as the outcome and risk factors as predictors are shown in Table 2. All risk factors were significantly associated with both measures of IMT with the exception of total cholesterol, which was not associated with CCA IMT. The association between age and the mean CCA IMT was such that the IMT increased by 0.007 mm/y, whereas the ICA IMT increased by 0.037 mm/y.
Table 2.
Univariate Linear Regression Models Summarizing Associations Between Framingham Risk Factors and IMT
| Mean CCA IMT |
Maximum ICA IMT |
|||||||
|---|---|---|---|---|---|---|---|---|
| Risk Factor | Coefficient | 95% CI | P | R2 | Coefficient | 95% CI | P | R2 |
| Age, y | 0.0066 | 0.0062, 0.0071 | <.0001 | 0.1939 | 0.0372 | 0.0345, 0.0398 | <.0001 | 0.1851 |
| Men/women | −0.0601 | −0.0699, −0.0504 | <.0001 | 0.0420 | −0.3403 | −0.3966, −0.2841 | <.0001 | 0.0407 |
| Systolic BP, mm Hg | 0.0025 | 0.0023, 0.0028 | <.0001 | 0.1039 | 0.0122 | 0.0108, 0.0137 | <.0001 | 0.0748 |
| Total cholesterol, mg/dL | 8.3 × 10−5 | −4 × 10−5, 2.1 × 10−4 | .1918 | 0.0005 | 7.6 × 10−4 | 4.6 × 10−5, 0.0015 | .0371 | 0.0013 |
| HDL cholesterol, mg/dL | −0.0018 | −0.0021, −0.0015 | <.0001 | 0.0401 | −0.0087 | −0.0105, −0.0070 | <.0001 | 0.0278 |
| Smoking, no/yes | 0.0175 | 0.0036, 0.0314 | .0137 | 0.0018 | 0.1713 | 0.0915, 0.2510 | <.0001 | 0.0053 |
| Diabetes, no/yes | 0.0955 | 0.0799, 0.1112 | <.0001 | 0.0413 | 0.5148 | 0.4245, 0.6051 | <.0001 | 0.0363 |
| Hypertension treatment, no/yes | 0.0767 | 0.0660, 0.0875 | <.0001 | 0.0555 | 0.5048 | 0.4434, 0.5662 | <.0001 | 0.0728 |
The R2 values give the strength of the association between individual risk factors and IMT. The square root is equivalent to the correlation coefficient. The coefficient is the strength of the association with the risk factor; for example, age is associated with IMT such that for every increase in 1 year of age, CCA IMT increases by 0.0066 mm, and ICA IMT increases by 0.0372 mm.
Results of multivariate linear regression models with each IMT variable as the outcome and risk factors as predictors are shown in Table 3 and Figure 2. All risk factors were significantly associated with the mean CCA IMT and maximum ICA IMT. On the basis of the percent variability explained, the order of importance of the risk factors for CCA IMT was age (19.4%), followed by gender (4.1%), systolic BP (1.9%), HDL cholesterol (1.2%), smoking (0.9%), diabetes (0.8%), hypertension treatment (0.3%), and total cholesterol (0.002%). For the ICA IMT, the order of importance of the risk factors was slightly different. Age was the strongest predictor (18.5%), followed by gender (4%), smoking (1.6%), hypertension treatment (1.1%), systolic BP (0.8%), diabetes (0.8%), HDL cholesterol (0.6%), and total cholesterol (0.1%).
Table 3.
Multivariate Linear Regression Models Summarizing Associations of Framingham Risk Factors With Mean CCA IMT and Maximum ICA IMT as Separate Dependent Variables
| Mean CCA IMT |
Maximum ICA IMT |
|||
|---|---|---|---|---|
| Risk Factor | Semipartial R2 | P | Semipartial R2 | P |
| Age, y | 0.1939 | <.0001 | 0.1851 | <.0001 |
| Men/women | 0.0408 | <.0001 | 0.0396 | <.0001 |
| Systolic BP, mm Hg | 0.0192 | <.0001 | 0.0082 | .0014 |
| Total cholesterol, mg/dL | 0.00023 | .0486 | 0.0011 | .0021 |
| HDL cholesterol, mg/dL | 0.0121 | <.0001 | 0.0060 | .0021 |
| Smoking, no/yes | 0.0091 | <.0001 | 0.0160 | <.0001 |
| Diabetes, no/yes | 0.0078 | <.0001 | 0.0079 | 0.0001 |
| Hypertension treatment, no/yes | 0.00246 | .0007 | 0.0114 | <.0001 |
| Total R2 | 0.2855 | 0.2752 | ||
Semipartial R2 values summarize the percent variability that is explained statistically by the risk factors. The sum of the semipartial R2 values gives the total R2.
Figure 2.
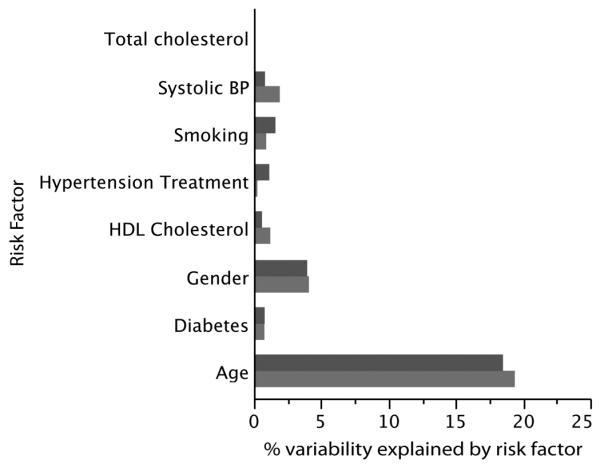
Percent variability explained by the different risk factors in the Framingham risk score for the two IMT variables: mean CCA IMT (bottom bars) and maximum ICA IMT (top bars). Note that total cholesterol has a numeric value that is too small to be visible.
Results of multivariate logistic regression models with prevalent CVD as the outcome are shown in Tables 4 and 5. Both the mean CCA IMT and maximum ICA IMT were similar predictors of prevalent CVD after adjusting for all Framingham risk factors. The standardized ORs for the mean CCA IMT were similar to those for the maximum ICA IMT. The addition of cholesterol treatment attenuated the significance of cholesterol levels and made them nonsignificant for predicted CVD. Overall, the Akaike information criterion, a standard for fitting of the overall models, was 1898.2 for the mean CCA IMT and similar at 1862.5 for the maximum ICA IMT. Results were similar for models with cholesterol treatment added: 1828.9 for CCA IMT and 1800 for ICA IMT.
Table 4.
Multivariate Logistic Regression Models for Predicting Prevalent CVD
| Mean CCA IMT |
Maximum ICA IMT |
|||||
|---|---|---|---|---|---|---|
| Risk Factor | OR | 95% CI | P | OR | 95% CI | P |
| Age, y | 1.076 | 1.059, 1.092 | <.0001 | 1.067 | 1.050, 1.083 | <.0001 |
| Men/women | 0.585 | 0.445, 0.768 | .0001 | 0.627 | 0.476, 0.827 | .0009 |
| Systolic BP, mm Hg | 0.990 | 0.984, 0.997 | .0060 | 0.991 | 0.984, 0.998 | .0079 |
| Total cholesterol, mg/dL | 0.997 | 0.994, 1.001 | .0979 | 0.997 | 0.994, 1.000 | .0672 |
| HDL cholesterol, mg/dL | 0.990 | 0.981, 0.999 | .0388 | 0.990 | 0.980, 0.999 | .0279 |
| Smoking, no/yes | 1.894 | 1.370, 2.620 | .0001 | 1.767 | 1.277, 2.445 | .0006 |
| Diabetes, no/yes | 2.192 | 1.632, 2.945 | <.0001 | 2.172 | 1.616, 2.920 | <.0001 |
| Hypertension treatment, no/yes | 2.656 | 2.063, 3.419 | <.0001 | 2.498 | 1.934, 3.226 | <.0001 |
| Mean CCA IMT | 4.950 | 2.392, 10.244 | <.0001 | |||
| Maximum ICA IMT | 1.630 | 1.430, 1.859 | <.0001 | |||
Table 5.
Multivariate Logistic Regression Models for Predicting Prevalent CVD With Cholesterol Treatment Added
| Mean CCA IMT |
Maximum ICA IMT |
|||||
|---|---|---|---|---|---|---|
| Risk Factor | OR | 95% CI | P | OR | 95% CI | P |
| Age, y | 1.074 | 1.057, 1.091 | <.0001 | 1.066 | 1.049, 1.083 | <.0001 |
| Men/women | 0.570 | 0.431, 0.753 | <.0001 | 0.608 | 0.459, 0.805 | .0005 |
| Systolic BP, mm Hg | 0.989 | 0.983, 0.996 | .0032 | 0.990 | 0.983, 0.997 | .0044 |
| Total cholesterol, mg/dL | 0.998 | 0.995, 1.002 | .3042 | 0.998 | 0.995, 1.001 | .2349 |
| HDL cholesterol, mg/dL | 0.994 | 0.984,1.003 | .1981 | 0.993 | 0.983, 1.002 | .1317 |
| Smoking, no/yes | 1.872 | 1.342, 2.610 | .0002 | 1.782 | 1.278, 2.484 | .0007 |
| Diabetes, no/yes | 2.071 | 1.530, 2.803 | <.0001 | 2.074 | 1.534, 2.805 | <.0001 |
| Hypertension treatment, no/yes | 2.211 | 1.705, 2.868 | <.0001 | 2.118 | 1.628, 2.754 | <.0001 |
| Cholesterol treatment, no/yes | 3.327 | 2.535, 4.367 | <.0001 | 3.157 | 2.402, 4.151 | <.0001 |
| Mean CCA IMT | 5.203 | 2.486, 10.888 | <.0001 | |||
| Maximum ICA IMT | 1.598 | 1.398, 1.827 | <.0001 | |||
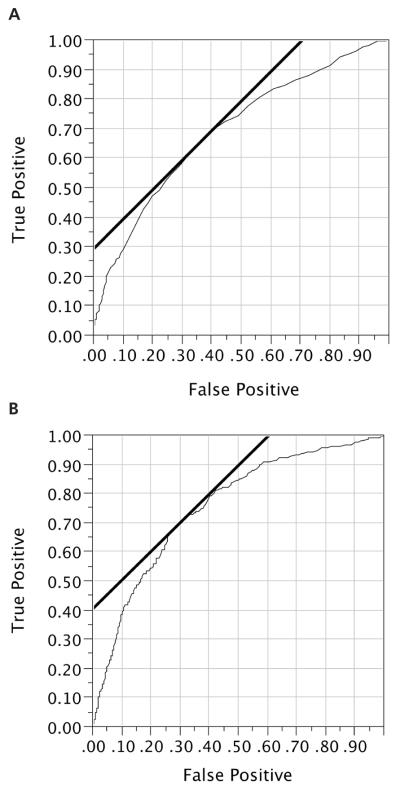
The ROC curves for the mean CCA IMT and maximum ICA IMT as single predictors of prevalent CVD are displayed in Figure 3. At the inflection point of the curve, a cut point of 1.6 mm, the sensitivity of the maximum ICA IMT for predicting prevalent CVD was 68.1% (256 of 378), and the specificity was 72.3% (2152 of 2976), whereas the cut point for CCA IMT was 0.62 mm for sensitivity of 63.5% (240 of 378) and specificity of 65.4% (1960 of 2995).
Figure 3.
A, Receiver operating characteristic curve for mean CCA IMT as a predictor of prevalent CVD (area under the curve = 0.695). B, Receiver operating characteristic curve for maximum ICA IMT as a predictor of prevalent CVD (area under the curve = 0.756).
The area under the curve was significantly (P < .0001; difference, −0.061; 95% CI, −0.091, −0.030) smaller for the mean CCA IMT (0.695) than for the maximum ICA IMT (0.756). For the models shown in Table 4, the respective statistics were significantly lower for the mean CCA IMT (0.0801) than for the maximum ICA IMT (0.820) for a difference of −0.0123 (95% CI, −0.020, −0.004). For Table 5, the respective values were 0.831 and 0.839 for a statistically significant difference of −0.009 (95% CI, −0.0152, −0.002).
Maximum ICA IMT measurements were obtained on the second view of the carotid artery bulb and proximal ICA in 53.7% of cases.
Discussion
We have found that (1) CCA and ICA IMT have similar associations with cardiovascular risk factors; (2) Framingham risk factors are associated with both measures of atherosclerosis and explain a large proportion of their variability; and (3) the maximum ICA IMT is more strongly associated with the presence of symptomatic CVD than the mean CCA IMT.
We studied the mean CCA IMT and maximum ICA IMT as measures of subclinical CVD because these measures have been proposed by two consensus groups16,21 as means of assessing global cardiovascular risk. We did not measure the mean CCA IMT from multiple projections of the CCA as is done in epidemiologic studies, yet we obtained strong associations with cardiovascular risk factors. We show that up to 29% of the variability of the mean CCA IMT is explained by cardiovascular risk factors, with a very strong association between CCA IMT and age. As in other studies, age and gender are the two strongest predictors of IMT. We also show strong associations between the maximum ICA IMT and risk factors. In difference to the mean CCA IMT, the maximum ICA IMT is an estimate of carotid artery plaque. Although it can be argued that a subjective assessment of plaque as being either present or absent might suffice for global risk assessment, the two consensus panels recommend some form of quantitative measurement.16,21 The definition of plaque varies. From absolute IMT values that vary from of 1.222 to 1.523 mm to a relative increase of 50% compared with the baseline IMT.11 However, these definitions have not been validated in large cross-sectional studies looking at associations with risk factors or in large outcomes studies. As such, our study offers a new insight into using plaque as a quantitative measurement of the maximum IMT. Consideration should also be given to the fact that we used an experienced and certified registered diagnostic medical sonographer to perform our measurements. As such, the subjective judgment of the presence or absence of plaque should have been superior to that in studies done with sonographers without years of proven experience in carotid ultrasound.
A potential limitation of our measurements is the fact that they required measurements offline on a workstation from manual tracings processed by a validated algorithm.14 They were not performed on the ultrasound device by a sonographer using calipers.24 However, because the reader was blinded to the participant demographics, our estimates of IMT are less likely to be biased than those of a sonographer because a sonographer might be influenced by the physical appearance of the individual being imaged and the clinical history.
Although cardiovascular risk factors are associated with the mean CCA IMT and maximum ICA IMT, the maximum ICA IMT is more strongly associated with prevalent CVD. This difference reflects on the fact that ICA IMT is a measure of atherosclerotic plaque and has different pathologic characteristics that the mean CCA IMT. The mean CCA IMT is a response to shear-stress25 and BP,26,27 and lesions in the CCA tend to be diffuse and composed of foam cells.28 The mean CCA IMT is a measure of wall hypertrophy,11 which responds to cholesterol-lowering interventions.10 The maximum IMT is a measure of plaque taken at the carotid bifurcation and proximal ICA, where complex oscillatory low shear stress promotes the primary deposition of low-density lipoprotein cholesterol in the wall.29,30 There is, however, a slight difference between pathologically defined plaque and ultrasound-defined plaque. The maximum ICA IMT is the distance from the lumen to the media,11,15,31 whereas plaque is a pathologic substance affecting the intima.32 In addition, plaque is not pathologically defined beyond an absolute size measurement but by a relative increase in the thickness of the intima.32
We opted to report a single maximum ICA IMT rather than average the maximum IMT measurements15,33 because this is consistent with the recommendation made by the consensus panels.11,16 Although the normally obtained mean of the maximum ICA IMT and mean of the mean ICA IMT are well suited for epidemiologic studies looking at outcomes or longitudinal changes in IMT,34–36 these measurements underestimate the presence of early plaque. An IMT measurement of 1.5 mm made at one site combined with a measurement of 0.7 mm at another site gives a mean of the maximum IMT of 1.1 mm. In this hypothetical case, 1.1 mm is below accepted thresholds for plaque, either 1.222,31,37 or 1.523,38 mm. Nevertheless, our data show that image selection should be made at the discretion of the sonographer because the maximum IMT was obtained from the additional projection selected by the sonographer in 53.7% of cases.
We find that the associations between risk factors and the maximum ICA IMT are similar to those for the mean CCA IMT. Although these differences can be argued to be of statistical significance and qualitatively different, as shown in Figure 2, they are, from a practical point of view, very similar. In fact, after accounting for age and gender, the remaining Framingham risk factors explain a small amount of the variability in IMT, approximately 5.1% for both the mean CCA IMT and maximum ICA IMT. This does not detract from the value of IMT when it comes to being a potential predictor of incident events because the variability in IMT that is not explained by risk factors likely holds some predictive value for outcomes.
We compared the overall sensitivity and specificity of both the maximum ICA IMT and mean CCA IMT based on ROC curves. These data give a coarse appreciation of the differences between both variables. In essence, they describe the relative accuracy for confirming the presence of prevalent CVD. This task, in itself, is a verification of the validity of the measurement but not a criterion for overall predictive value. The latter needs to be verified in outcomes studies. The overall correlations between each of the two variables and risk factors reflect the possible causal relationship between a given risk factor and the IMT variable of interest. As such, they may represent key differences in the way that risk factors interact with changes in the artery wall.
Our data show relatively weak associations between IMT and cholesterol levels. Much of the data studying IMT have focused on populations with hypercholesterolemia in which the association between IMT and cholesterol levels is easily determined. We, however, note an effect of modern therapy in individuals who have prevalent CVD. Lipid-lowering therapies are seen in 38.7% of individuals with prevalent CVD compared to 9.7% without. This likely explains the blunting of the association between cholesterol and IMT as well as between cholesterol and prevalent CVD.
Results looking at the associations of risk factors and IMT measured at different locations at and below the carotid bifurcation have been contradictory. Espeland et al39 showed mild segmental differences in the associations of IMT with age and hypertension in women but did not report on the overall strength of the associations between IMT at different carotid artery locations and risk factors. Tell et al40 found age, hypertension, and cigarette smoking to affect all segments in a similar fashion, but quantitative ICA IMT measurements were not made. Schott et al41 used a complex statistical approach to show that systolic BP showed similar associations with the CCA and mean ICA IMT but did not see any strong association of ICA or bulb IMT with age. The Vascular Aging Study, which was limited to women, showed results different from ours, but that study did not use quantitative ICA IMT measurements.42 In the San Antonio study, Wei et al43 showed that total cholesterol and systolic BP were more strongly associated with CCA IMT than with ICA IMT but found a much lower correlation between risk factors and IMT than ours (22% variability for CCA IMT and only 12% for ICA IMT).43 O’Leary et al44 compared CCA IMT with ICA IMT but used average values over multiple segments and found that risk factors explained 18% of the variability in CCA IMT and 17% in ICA IMT, both values lower than what we report. None of these studies reported on results for the maximum ICA IMT.
We note that although the maximum ICA IMT is more strongly associated with prevalent CVD than CCA IMT, the relative effect of a 1-mm change in CCA IMT is more significant than an equivalent 1-mm increase in ICA IMT (Table 4). This is in part explained by the larger values and wider range for ICA IMT compared with CCA IMT (Table 1).
A possible limitation to our study was the use of an older-generation ultrasound device to obtain carotid IMT measurements. However, the associations of carotid IMT with cardiovascular risk factors and prevalent CVD1,6,45 were also observed for IMT values derived from images taken with older-generation ultrasound imaging devices such the Biosound 200IISA (Atherosclerosis Risk in Communities45), ATL Ultramark 4 (Rotterdam Elderly Study1) and Toshiba SSA-270A (Cardiovascular Health Study6). Our results are consistent with these studies with the exception that we were better able to obtain estimates of ICA IMT and show slight differences between CCA and ICA IMT. Another limitation of our study was the cross-sectional nature of our data. Analyses with incident cardiovascular events are pending.
In summary, this study suggests that the maximum ICA IMT might add value to the mean CCA IMT for cardiovascular risk assessments.
Acknowledgments
This work was supported by the National Heart, Lung, and Blood Institute’s Framingham Heart Study (contract N01-HC-25195). Dr Polak is partly supported by National Heart, Lung, and Blood Institute grants R01 HL069003 and HL081352. Dr Pencina serves on a data and safety monitoring board for a study that uses common carotid artery intima-media thickness as an outcome.
Abbreviations
- BP
blood pressure
- CCA
common carotid artery
- CI
confidence interval
- CVD
cardiovascular disease
- HDL
high-density lipoprotein
- ICA
internal carotid artery
- IMT
intima-media thickness
- OR
odds ratio
- ROC
receiver operating characteristic
References
- 1.Bots ML, Breslau PJ, Briet E, et al. Cardiovascular determinants of carotid artery disease: the Rotterdam Elderly Study. Stroke. 1992;19:717–720. doi: 10.1161/01.hyp.19.6.717. [DOI] [PubMed] [Google Scholar]
- 2.Bots ML, Hoes AW, Koudstaal PJ, Hofman A, Grobbee DE. Common carotid intima-media thickness and risk of stroke and myocardial infarction: the Rotterdam Study. Circulation. 1997;96:1432–1437. doi: 10.1161/01.cir.96.5.1432. [DOI] [PubMed] [Google Scholar]
- 3.Chambless LE, Folsom AR, Clegg LX, et al. Carotid wall thickness is predictive of incident clinical stroke: the Atherosclerosis Risk in Communities (ARIC) study. Am J Epidemiol. 2000;151:478–487. doi: 10.1093/oxfordjournals.aje.a010233. [DOI] [PubMed] [Google Scholar]
- 4.Chambless LE, Heiss G, Folsom AR, et al. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: the Atherosclerosis Risk in Communities (ARIC) study, 1987–1993. Am J Epidemiol. 1997;146:483–494. doi: 10.1093/oxfordjournals.aje.a009302. [DOI] [PubMed] [Google Scholar]
- 5.Hodis HN, Mack WJ, LaBree L, et al. The role of carotid arterial intima-media thickness in predicting clinical coronary events. Ann Intern Med. 1998;128:262–269. doi: 10.7326/0003-4819-128-4-199802150-00002. [DOI] [PubMed] [Google Scholar]
- 6.O’Leary DH, Polak JF, Kronmal RA, et al. Distribution and correlates of sonographically detected carotid artery disease in the Cardiovascular Health Study: the CHS Collaborative Research Group. Stroke. 1992;23:1752–1760. doi: 10.1161/01.str.23.12.1752. [DOI] [PubMed] [Google Scholar]
- 7.O’Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK., Jr. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults: Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999;340:14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 8.Salonen JT, Seppanen K, Rauramaa R, Salonen R. Risk factors for carotid atherosclerosis: the Kuopio Ischaemic Heart Disease Risk Factor Study. Ann Medicine. 1989;21:227–229. doi: 10.3109/07853898909149939. [DOI] [PubMed] [Google Scholar]
- 9.Selzer RH, Hodis HN, Kwong-Fu H, et al. Evaluation of computerized edge tracking for quantifying intima-media thickness of the common carotid artery from B-mode ultrasound images. Atherosclerosis. 1994;111:1–11. doi: 10.1016/0021-9150(94)90186-4. [DOI] [PubMed] [Google Scholar]
- 10.Taylor AJ, Kent SM, Flaherty PJ, Coyle LC, Markwood TT, Vernalis MN. ARBITER: arterial biology for the investigation of the treatment effects of reducing cholesterol—a randomized trial comparing the effects of atorvastatin and pravastatin on carotid intima medial thickness. Circulation. 2002;106:2055–2060. doi: 10.1161/01.cir.0000034508.55617.65. [DOI] [PubMed] [Google Scholar]
- 11.Touboul PJ, Hennerici MG, Meairs S, et al. Advisory Board of the 3rd Watching the Risk Symposium 2004, 13th European Stroke Conference. Mannheim intima-media thickness consensus. Cerebrovasc Dis. 2004;18:346–349. doi: 10.1159/000081812. [DOI] [PubMed] [Google Scholar]
- 12.Touboul PJ, Prati P, Scarabin PY, Adrai V, Thibout E, Ducimetiere P. Use of monitoring software to improve the measurement of carotid wall thickness by B-mode imaging. J Hypertens Suppl. 1992;10:S37–S41. [PubMed] [Google Scholar]
- 13.Smilde TJ, van Wissen S, Wollersheim H, Trip MD, Kastelein JJ, Stalenhoef AF. Effect of aggressive versus conventional lipid lowering on atherosclerosis progression in familial hypercholesterolaemia (ASAP): a prospective, randomised, double-blind trial. Lancet. 2001;357:577–581. doi: 10.1016/s0140-6736(00)04053-8. [DOI] [PubMed] [Google Scholar]
- 14.O’Leary DH, Polak JF, Wolfson SK, Jr, et al. Use of sonography to evaluate carotid atherosclerosis in the elderly: the Cardiovascular Health Study. CHS Collaborative Research Group. Stroke. 1991;22:1155–1163. doi: 10.1161/01.str.22.9.1155. [DOI] [PubMed] [Google Scholar]
- 15.Hedblad B, Wikstrand J, Janzon L, Wedel H, Berglund G. Low-dose metoprolol CR/XL and fluvastatin slow progression of carotid intima-media thickness: main results from the Beta-Blocker Cholesterol-Lowering Asymptomatic Plaque Study (BCAPS) Circulation. 2001;103:1721–1726. doi: 10.1161/01.cir.103.13.1721. [DOI] [PubMed] [Google Scholar]
- 16.Stein JH, Korcarz CE, Hurst RT, et al. American Society of Echocardiography Carotid Intima-Media Thickness Task Force Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr. 2008;21:93–111. doi: 10.1016/j.echo.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 17.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families: The Framingham offspring study. Am J Epidemiol. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 18.D’Agostino RB, Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 19.McNamara JR, Schaefer EJ. Automated enzymatic standardized lipid analyses for plasma and lipoprotein fractions. Clin Chim Acta. 1987;166:1–8. doi: 10.1016/0009-8981(87)90188-4. [DOI] [PubMed] [Google Scholar]
- 20.Warnick GR, Benderson J, Albers JJ. Dextran sulfate-Mg2+ precipitation procedure for quantitation of high-density lipoprotein cholesterol. Clin Chem. 1982;28:1379–1388. [PubMed] [Google Scholar]
- 21.Touboul PJ, Hennerici MG, Meairs S, et al. Mannheim carotid intima-media thickness consensus (2004–2006): an update on behalf of the Advisory Board of the 3rd and 4th Watching the Risk Symposium, 13th and 15th European Stroke Conferences, Mannheim, Germany, 2004, and Brussels, Belgium, 2006. Cerebrovasc Dis. 2007;23:75–80. doi: 10.1159/000097034. [DOI] [PubMed] [Google Scholar]
- 22.Griffin M, Nicolaides A, Tyllis T. Carotid and femoral arterial wall changes and the prevalence of clinical cardiovascular disease. Vasc Med. 2009;14:227–232. doi: 10.1177/1358863X08101542. [DOI] [PubMed] [Google Scholar]
- 23.Li R, Duncan BB, Metcalf PA, et al. B-mode-detected carotid artery plaque in a general population: Atherosclerosis Risk in Communities (ARIC) Study Investigators. Stroke. 1994;25:2377–2383. doi: 10.1161/01.str.25.12.2377. [DOI] [PubMed] [Google Scholar]
- 24.Baldassarre D, Amato M, Bondioli A, Sirtori CR, Tremoli E. Carotid artery intima-media thickness measured by ultra-sonography in normal clinical practice correlates well with atherosclerosis risk factors. Stroke. 2000;31:2426–2430. doi: 10.1161/01.str.31.10.2426. [DOI] [PubMed] [Google Scholar]
- 25.Kornet L, Lambregts J, Hoeks AP, Reneman RS. Differences in near-wall shear rate in the carotid artery within subjects are associated with different intima-media thicknesses. Arterioscler Thromb Vasc Biol. 1998;18:1877–1884. doi: 10.1161/01.atv.18.12.1877. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka H, Dinenno FA, Monahan KD, DeSouza CA, Seals DR. Carotid artery wall hypertrophy with age is related to local systolic blood pressure in healthy men. Arterioscler Thromb Vasc Biol. 2001;21:82–87. doi: 10.1161/01.atv.21.1.82. [DOI] [PubMed] [Google Scholar]
- 27.Augst AD, Ariff B, McG Thom SA, Xu XY, Hughes AD. Analysis of complex flow and the relationship between blood pressure, wall shear stress, and intima-media thickness in the human carotid artery. Am J Physiol Heart Circ Physiol. 2007;293:H1031–H1037. doi: 10.1152/ajpheart.00989.2006. [DOI] [PubMed] [Google Scholar]
- 28.Dalager S, Paaske WP, Kristensen IB, Laurberg JM, Falk E. Artery-related differences in atherosclerosis expression: implications for atherogenesis and dynamics in intima-media thickness. Stroke. 2007;38:2698–2705. doi: 10.1161/STROKEAHA.107.486480. [DOI] [PubMed] [Google Scholar]
- 29.Ku DN, Giddens DP, Zarins CK, Glagov S. Pulsatile flow and atherosclerosis in the human carotid bifurcation: positive correlation between plaque location and low oscillating shear stress. Arteriosclerosis. 1985;5:293–302. doi: 10.1161/01.atv.5.3.293. [DOI] [PubMed] [Google Scholar]
- 30.Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. JAMA. 1999;282:2035–2042. doi: 10.1001/jama.282.21.2035. [DOI] [PubMed] [Google Scholar]
- 31.Salonen R, Seppanen K, Rauramaa R, Salonen JT. Prevalence of carotid atherosclerosis and serum cholesterol levels in eastern Finland. Arteriosclerosis. 1988;8:788–792. doi: 10.1161/01.atv.8.6.788. [DOI] [PubMed] [Google Scholar]
- 32.Stary HC, Chandler AB, Dinsmore RE, et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis: a report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1995;92:1355–1374. doi: 10.1161/01.cir.92.5.1355. [DOI] [PubMed] [Google Scholar]
- 33.Furberg CD, Adams HP, Jr, Applegate WB, et al. Effect of lovastatin on early carotid atherosclerosis and cardiovascular events: Asymptomatic Carotid Artery Progression Study (ACAPS) Research Group. Circulation. 1994;90:1679–1687. doi: 10.1161/01.cir.90.4.1679. [DOI] [PubMed] [Google Scholar]
- 34.Ebrahim S, Papacosta O, Whincup P, et al. Carotid plaque, intima media thickness, cardiovascular risk factors, and prevalent cardiovascular disease in men and women: the British Regional Heart Study. Stroke. 1999;30:841–850. doi: 10.1161/01.str.30.4.841. [DOI] [PubMed] [Google Scholar]
- 35.Polak JF, Person SD, Wei GS, et al. Segment-specific associations of carotid intima-media thickness with cardiovascular risk factors: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Stroke. 2010;41:9–15. doi: 10.1161/STROKEAHA.109.566596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnsen SH, Mathiesen EB. Carotid plaque compared with intima-media thickness as a predictor of coronary and cerebrovascular disease. Curr Cardiol Rep. 2009;11:21–27. doi: 10.1007/s11886-009-0004-1. [DOI] [PubMed] [Google Scholar]
- 37.Joakimsen O, Bonaa KH, Stensland-Bugge E, Jacobsen BK. Age and sex differences in the distribution and ultrasound morphology of carotid atherosclerosis: the Tromsø Study. Arterioscler Thromb Vasc Biol. 1999;19:3007–3013. doi: 10.1161/01.atv.19.12.3007. [DOI] [PubMed] [Google Scholar]
- 38.Touboul PJ, Hernández-Hernández R, Küçüko_lu S, et al. Carotid artery intima media thickness, plaque and Framingham cardiovascular score in Asia, Africa/Middle East and Latin America: the PARC-AALA study. Int J Cardiovasc Imaging. 2007;23:557–567. doi: 10.1007/s10554-006-9197-1. [DOI] [PubMed] [Google Scholar]
- 39.Espeland MA, Tang R, Terry JG, Davis DH, Mercuri M, Crouse JR., III Associations of risk factors with segment-specific intimal-medial thickness of the extracranial carotid artery. Stroke. 1999;30:1047–1055. doi: 10.1161/01.str.30.5.1047. [DOI] [PubMed] [Google Scholar]
- 40.Tell GS, Howard G, McKinney WM, Toole JF. Cigarette smoking cessation and extracranial carotid atherosclerosis. JAMA. 1989;261:1178–1180. [PubMed] [Google Scholar]
- 41.Schott LL, Wildman RP, Brockwell S, et al. Segment-specific effects of cardiovascular risk factors on carotid artery intima-medial thickness in women at midlife. Arterioscler Thromb Vasc Biol. 2004;24:1951–1956. doi: 10.1161/01.ATV.0000141119.02205.6b. [DOI] [PubMed] [Google Scholar]
- 42.Bonithon-Kopp C, Touboul PJ, Berr C, et al. Relation of intima-media thickness to atherosclerotic plaques in carotid arteries: the Vascular Aging (EVA) Study. Arterioscler Thromb Vasc Biol. 1996;16:310–316. doi: 10.1161/01.atv.16.2.310. [DOI] [PubMed] [Google Scholar]
- 43.Wei M, Gonzalez C, Haffner SM, O’Leary DH, Stern MP. Ultrasonographically assessed maximum carotid artery wall thickness in Mexico City residents and Mexican Americans living in San Antonio, Texas: association with diabetes and cardiovascular risk factors. Arterioscler Thromb Vasc Biol. 1996;16:1388–1392. doi: 10.1161/01.atv.16.11.1388. [DOI] [PubMed] [Google Scholar]
- 44.O’Leary DH, Polak JF, Kronmal RA, et al. Thickening of the carotid wall: a marker for atherosclerosis in the elderly? Cardiovascular Health Study Collaborative Research Group. Stroke. 1996;27:224–231. doi: 10.1161/01.str.27.2.224. [DOI] [PubMed] [Google Scholar]
- 45.Howard G, Sharrett AR, Heiss G, et al. Carotid artery intimal-medial thickness distribution in general populations as evaluated by B-mode ultrasound. ARIC Investigators. Stroke. 1993;24:1297–1304. doi: 10.1161/01.str.24.9.1297. [DOI] [PubMed] [Google Scholar]