Summary
Ca2+ signaling in nonexcitable cells is typically initiated by receptor-triggered production of inositol-1,4,5-trisphosphate and the release of Ca2+ from intracellular stores [1]. An elusive signaling process senses the Ca2+ store depletion and triggers the opening of plasma membrane Ca2+ channels [2–5]. The resulting sustained Ca2+ signals are required for many physiological responses, such as T cell activation and differentiation [6]. Here, we monitored receptor-triggered Ca2+ signals in cells transfected with siRNAs against 2,304 human signaling proteins, and we identified two proteins required for Ca2+-store-depletion-mediated Ca2+ influx, STIM1 and STIM2 [7–9]. These proteins have a single transmembrane region with a putative Ca2+ binding domain in the lumen of the endoplasmic reticulum. Ca2+ store depletion led to a rapid translocation of STIM1 into puncta that accumulated near the plasma membrane. Introducing a point mutation in the STIM1 Ca2+ binding domain resulted in prelocalization of the protein in puncta, and this mutant failed to respond to store depletion. Our study suggests that STIM proteins function as Ca2+ store sensors in the signaling pathway connecting Ca2+ store depletion to Ca2+ influx.
Results and Discussion
Identification of STIM1 and STIM2 as Potential SOC Influx Mediators
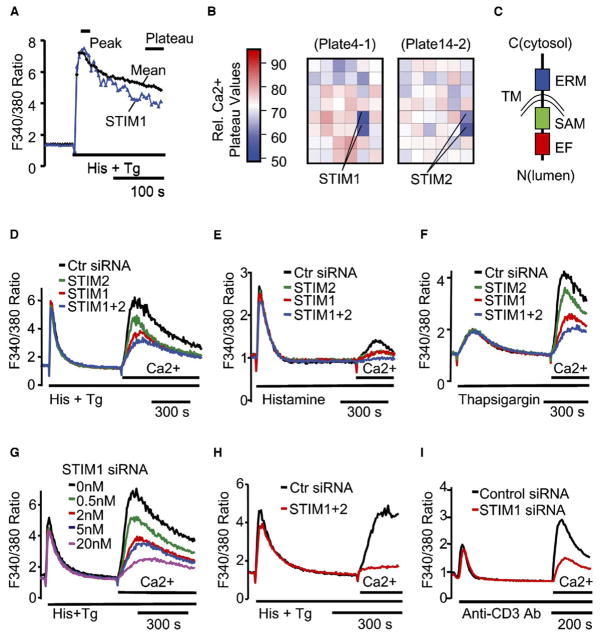
Stimulation of cells with a variety of physiological stimuli leads to an inositol-1,4,5-trisphosphate (IP3)-mediated release of Ca2+ from intracellular stores, which in turn triggers an influx of Ca2+ across the plasma membrane [1–6, 10, 11]. Many of the signaling pathways leading from cell stimulation to Ca2+ store depletion have been defined, but the pathways leading from Ca2+ store depletion to Ca2+ influx through the plasma membrane (also termed store-operated Ca2+ [SOC] influx or capacitative Ca2+ entry pathway [2–5]) have remained elusive. To identify proteins involved in the SOC influx pathway, we have selected 2,304 proteins that contain known signaling domains from the National Center for Biotechnology Information (NCBI) database and generated in vitro-diced siRNAs against each of the targets. We then tested their role in Ca2+ signaling with a kinetic Ca2+ screen in an automated microplate reader. Stimulation of HeLa cells with histamine and thapsigargin leads to an initial peak in cytosolic Ca2+ levels and then a plateau. The sustained plateau level is indicative of induced SOC influx. Thus, by monitoring which siRNA reduces the sustained plateau phase without changing the initial peak response, we could identify proteins that mediate SOC influx.
With visual inspection of the Ca2+ time courses (Figure 1A) and comparison of the relative Ca2+ plateau values (Figure 1B), the siRNAs targeting the gene products STIM (stromal interaction molecule) 1 and STIM2 stood out in their ability to suppress the sustained Ca2+ signals while showing little effect on the peak amplitude. Although STIM1 and STIM2 were identified previously as potential tumor growth suppressors [8, 12, 13], they had not been suspected of having a role in Ca2+ signaling. Nevertheless, both proteins have been biochemically characterized and have been shown to form homo- and hetero-oligomers as well as to have a transmembrane domain and a putative luminal single EF-hand Ca2+ binding domain [9, 13, 14] (Figure 1C).
Figure 1. STIM1 and STIM2 Are Identified in a Kinetic Ca2+ Screen for Suppression of SOC Influx with 2,304 Human Signaling siRNAs.
(A) Comparison of the Ca2+ time course in STIM1 knockdown cells to the averaged reference time course. HeLa cells were transfected with the siRNA signaling set at an average concentration of 10 nM for 2 days. Fura-2 Ca2+ time courses were measured in a microplate reader with automated stimulus addition.
(B) Positional heat-map analysis of relative Ca2+ plateau values in the two microplates containing STIM1 and STIM2 siRNA-transfected cells (24 duplicate siRNAs per plate). Relative Ca2+ plateau values were calculated by dividing the plateau (the average of the last 5 data points) by the peak (the average of 3 peak points) fluorescence.
(C) Domain structure of STIM1 and STIM2. Domains include an EF-hand motif (EF), a SAM domain, a single transmembrane domain (TM), and an ERM domain arranged from N to C terminus of both proteins with the C terminus in the cytosol.
(D–F) Suppression of Ca2+ influx by STIM siRNAs measured by “Ca2+ add-back” in HeLa cells transfected with 10 nM STIM1 and/or 10 nM STIM2 siRNA for 2 days. One hundred micromolars histamine plus two micromolars thapsigargin (D), histamine alone (E), or thapsigargin alone (F) was used to deplete Ca2+ stores.
(G) Titration of STIM1 siRNA. Total siRNA concentration was kept at 20 nM for all samples with control siRNA.
(H) Near-complete inhibition of Ca2+ influx in cells transfected for 3 days with 20 nM STIM1 plus 20 nM STIM2 siRNA. Data shown in (D)–(H) are the average of 3 bulk-cell Ca2+ measurements obtained with a microplate reader.
(I) Suppression of T-cell-receptor-triggered Ca2+ influx by STIM1 siRNA. Ca2+ add-back experiments were done in Jurkat T cells transfected with pYFP-Nuc (transfection marker) plus 72 nM STIM1 or control siRNA for 2 days. Ca2+ stores were depleted with 20 μg/ml anti-human CD3 antibody. Shown are the average Ca2+ responses of 48 control and 84 STIM1 siRNA-transfected (YFP-positive) single cells.
Suppression of Ca2+ Influx in STIM Knockdown Cells
To confirm that STIM1 and STIM2 are required for the SOC influx pathway, we performed a “Ca2+ add-back” experiment, in which Ca2+ stores were first depleted in the absence of extracellular Ca2+ with histamine and thapsigargin. Extracellular Ca2+ was then added back to monitor Ca2+ influx. In HeLa cells transfected with siRNA against STIM1, STIM2, or both, we observed a significant suppression of Ca2+ influx (Figure 1D). The same results were obtained when knockdown cells were stimulated with either histamine (Figure 1E) or thapsigargin alone (Figure 1F). We also found that siRNAs against the 3′ untranslated regions (UTR) of both STIM1 and STIM2 as well as three synthesized individual siRNAs against STIM1 led to a similar suppression of Ca2+ influx (see Figure S1A in the Supplemental Data available with this article online; data not shown). As additional controls, we found that there was no significant difference in membrane potential changes or residual Ca2+ in the store between control and STIM1 knockdown cells after stimulation (Figures S1B and S1C). The suppression of Ca2+ influx by STIM siRNAs could be titrated as a function of the siRNA concentration (shown for STIM1 in Figure 1G). Remarkably, when HeLa cells were treated for 3 days with a combination of 20 nM STIM1 and 20 nM STIM2 siRNA, a significant 6-fold reduction of the SOC influx (to 15% of control siRNA) was observed (Figure 1H). This nearly complete inhibition of Ca2+ influx provides support for a key role of STIM proteins in mediating SOC influx.
Suppression of NF-AT Translocation and TCR-Triggered Ca2+ Influx by STIM1 siRNA
To test for a functional consequence of the reduction in SOC influx, we monitored the activation of NF-AT, a transcription factor that is activated in response to sustained Ca2+ signals [15, 16]. Consistent with a functional relevance of STIM1 in NF-AT activation, the translocation of YFP-NF-ATc1 to the nucleus was nearly completely reduced in Ca2+-store-depleted HeLa cells transfected with siRNA against STIM1 in comparison to control siRNA (Figure S1D). Because SOC influx has been shown to be required for T cell activation [6, 17], we also examined the effect of STIM1 on Ca2+ influx in a Jurkat T lymphocyte model. As shown in Figure 1I, the SOC influx triggered by T-cell-receptor (TCR) stimulation was effectively suppressed by siRNA against STIM1.
Regulation of SOC Influx by STIM
The suppression of Ca2+ signals in STIM knockdown cells could in principle result from an accelerated plasma membrane Ca2+ extrusion instead of a reduced influx. In order to distinguish between these two possibilities, we monitored the Ca2+ influx directly with an Mn2+ quench assay [18]. This method is based on the earlier findings that SOC influx channels are permeant to Mn2+ and that Fura-2 becomes nonfluorescent when complexed with Mn2+. Consistent with a role of STIM in regulating Ca2+ influx and not Ca2+ extrusion, siRNAs against a combination of both STIM isoforms suppressed the Mn2+ quench-rate increase triggered by Ca2+ store depletion (Figure 2A).
Figure 2. STIM Regulates SOC Influx.

(A) Direct measurement of Ca2+ influx with an Mn2+ quench assay. HeLa cells were transfected for 2 days with 20 nM control or a mix of 10 nM STIM1 and 10 nM STIM2 siRNAs. Two millimolars Mn2+ was added before image acquisition, and 100 μM histamine plus 2 μM thapsigargin was added 50 s after. The left panel shows the relative Fura-2 fluorescence intensity measured with 360 nm excitation as a function of time in control and STIM knockdown cells. Each trace represents the average quench response of three separate experiments, with 660–1300 individual cells analyzed per experiment. A Matlab program was used to calculate the ΔF/F quench rate in each cell before (4–44 s) and after (100–140 s) stimulus addition. The average ΔF/F is shown in a bar graph on the right. (B) Overexpression of YFP-STIM1 enhances SOC influx. HeLa cells transfected with 40 ng YFP-STIM1 or control vector for 1 day were subjected to Mn2+ quench assays as described in (A). A SOC influx inhibitor, SKF 96365, was used at 20 μM. Over 150 individual cells were analyzed for each data set. Error bars are 95% confidence bounds.
We then cloned human STIM1, conjugated the protein with an N-terminal YFP-tag (after the signal peptide), and tested the effect of STIM1 overexpression on SOC influx. As shown in Figure 2B, overexpression of YFP-STIM1 significantly increased the SOC influx. We also observed a small increase in basal Ca2+ influx in unstimulated cells; this increase might be an artifact of the transient overexpression or may indicate that STIM1, at high expression levels, can partially induce Ca2+ influx in the absence of store depletion. The increases of SOC and also the basal influx resulting from STIM1 overexpression were almost completely blocked by SKF 96365, an inhibitor of SOC influx [19], providing further support that STIM1 has a role in activating SOC influx.
Redistribution of YFP-STIM1 into Puncta after Ca2+ Store Depletion
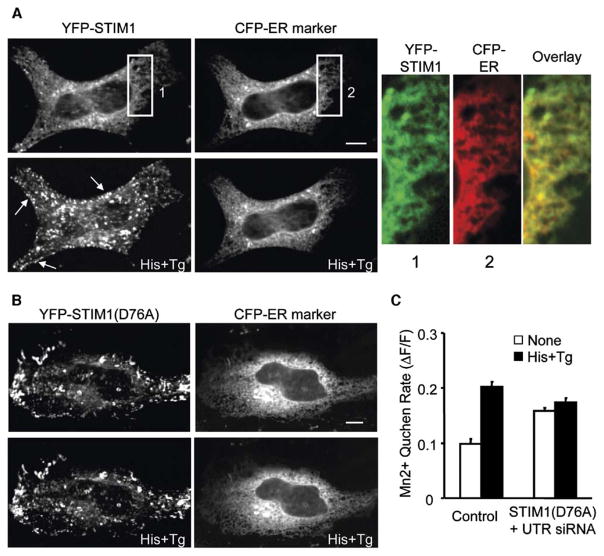
To investigate the mechanism by which STIM proteins regulate the SOC influx pathway, we first determined the localization of STIM1 in unstimulated HeLa cells. As shown in Figure 3A, YFP-STIM1 mostly colocalized with a marker of the endoplasmic reticulum (ER). Strikingly, Ca2+ store depletion triggered a redistribution of YFP-STIM1 into puncta that accumulated inside the cell and also appeared to be enriched near the cell periphery (left bottom panel, Figure 3A). Initial translocation of YFP-STIM1 could be observed in less than 1 min (Figure S2). To test whether this translocation process was reversible, we used BHQ instead of thapsigargin to deplete Ca2+ stores and found that most YFP-STIM1 puncta disappeared within 3 min after the removal of histamine and BHQ (Figure S3). We next examined whether some of these puncta are domains within the plasma membrane. We stained the cells for extracellular YFP-STIM1 with anti-GFP antibodies and found no significant insertion of YFP-STIM1 to the plasma membrane after Ca2+ store depletion (data not shown). Furthermore, we found that the redistribution of YFP-STIM1 occurred in the absence of extracellular Ca2+ (Figure S4), suggesting that the redistribution is likely a cause rather than a consequence of Ca2+ influx.
Figure 3. STIM1 Senses ER Ca2+ Depletion with Its Luminal EF-Hand.
(A) YFP-STIM1 redistributes into punctate structures after Ca2+ store depletion. HeLa cells were cotransfected with YFP-STIM1 and a CFP-tagged ER marker. CFP/YFP confocal images of the same cell were taken before (top two panels) and 8 min after (bottom two panels) histamine plus thapsigargin stimulation. The arrows point to peripheral sites rich in puncta. The magnified panels on the right show the colocalization of STIM1 with the ER marker before stimulation.
(B) The EF-hand mutant of STIM1 is already localized to puncta and does not respond to store depletion. HeLa cells were cotransfected with YFP-STIM1(D76A) and a CFP-ER marker. CFP/YFP confocal images of the same cell were taken before (top two panels) and 8 min after (bottom two panels) histamine plus thapsigargin stimulation. The scale bar represents 10 μm.
(C) STIM1 knockdown cells expressing the EF-hand mutant show elevated influx prior to stimulation and are unresponsive to Ca2+ store depletion. Mn2+ quench assays were performed in HeLa cells transfected with 40 nM STIM1 UTR or control siRNA plus YFP-STIM1(D76A) or the YFP vector as described in Figure 2A. Error bars are 95% confidence bounds.
The EF-Hand of STIM1 Senses ER Ca2+ Store Depletion
Because STIM1 and STIM2 are type-I transmembrane proteins, their unpaired EF-hand domains are predicted to be in the lumen of the ER. This led us to the hypothesis that STIM proteins may function as Ca2+ sensors that use their EF-hand domains to monitor the loading of Ca2+ stores. Interestingly, when the first Ca2+ binding aspartic acid residue in the EF-hand [20, 21] was mutated to alanine (D76A), this YFP-STIM1 EF-hand mutant was already significantly prelocalized in puncta before stimulation, and Ca2+ store depletion had no significant additional effect on the localization of the EF-hand mutant (Figure 3B). We further tested the function of the EF-hand mutant by examining its effect on SOC influx in STIM1 knockdown cells, in which the effects of the endogenous STIM1 are minimized. Consistent with the imaging results, expression of the EF-hand mutant increased Ca2+ influx even if the Ca2+ stores were filled, and it failed to further promote SOC influx in response to Ca2+ store depletion (Figure 3C). Notably, the EF-hand mutant could not completely restore SOC influx level in knockdown cells, which may indicate that prolonged activation of STIM leads to a partial desensitization of SOC influx.
Two Localization States of YFP-STIM1 Regulated by Ca2+ Binding
In order to directly compare the puncta formed by wild-type and the EF-hand mutant of STIM1, we constructed a CFP-conjugated wild-type STIM1 that can be imaged in the same cell as the YFP-conjugated STIM1 EF-hand mutant. We monitored the cells by confocal microscopy and focused near the cell adhesion surface, where the largest numbers of puncta formed by the EF-hand mutant could be seen. Remarkably, store depletion triggered a translocation of the initially ER-distributed wild-type STIM1 into the same puncta already marked by the EF-hand mutant (Figure 4A). This suggests that STIM1 can exist in two states, a relatively uniform ER distribution when Ca2+ is bound and a punctate distribution when Ca2+ dissociates or when the EF-hand Ca2+ binding site is mutated.
Figure 4. STIM1 Exists in Two Localization States and Is Rapidly Redistributed into Puncta Near the Plasma Membrane after Ca2+ Store Depletion.
(A) Colocalization of wild-type and the EF-hand mutant of STIM1 in puncta after Ca2+ store depletion. HeLa cells were cotransfected with CFP-STIM1 and YFP-STIM1(D76A). CFP/YFP confocal images were taken near the adhesion surface of the same cell before (top two panels) and 2.5 min after (bottom two panels) histamine plus thapsigargin stimulation. The magnified panels on the right show STIM1 puncta formation and the colocalization of wild-type and the EF-hand mutant of STIM1 after Ca2+ store depletion.
(B) TIRF microcopy shows that many YFP-STIM1 puncta are rapidly formed within 100 nm of the plasma membrane. HeLa cells were cotransfected with YFP-STIM1 and CFP-CAAX. CFP/YFP TIRF images were taken in the same cells at different time points after histamine plus thapsigargin stimulation. The scale bar represents 10 μm.
(C) Kinetic analysis of the average relative fluorescence intensity in near-plasma-membrane YFP-STIM1 puncta (n = 212; an exponential fit is shown).
Rapid Redistribution of YFP-STIM1 into Puncta Near the Plasma Membrane
An “induced coupling” model has been proposed previously for activation of plasma membrane SOC channels [22]. In this model, Ca2+ store depletion may induce the formation of new conformationally coupled junctions between ER and the plasma membrane. To investigate whether some of the STIM1 puncta are formed near the plasma membrane after Ca2+ store depletion, we used total internal reflection fluorescence (TIRF) microscopy, which selectively excites fluorescence within 100 nm of the plasma membrane [23], to measure STIM1 puncta formation. When comparing YFP-STIM1 to a CFP-conjugated plasma membrane marker, CFP-CAAX, in the same cell with TIRF microscopy, we found a fast increase in near-plasma-membrane punctate fluorescence intensity after Ca2+ store depletion (Figure 4B). The kinetics of YFP-STIM1 puncta formation near the plasma membrane (t1/2 = 52 s; Figure 4C) is also consistent with previously reported times required for the activation of SOC influx (tens or hundreds of seconds [23]). Although the resolution of the TIRF measurements cannot definitely prove that there is a physical link between STIM1 and the plasma membrane, they show that a fraction of the STIM1 puncta rapidly form within 100 nm of the plasma membrane. This is consistent with a hypothesis that short-range signaling or direct coupling might be involved in the activation of the SOC influx pathway by STIM proteins.
Conclusions
Our study shows that STIM proteins are necessary for the signaling process that links Ca2+ store depletion to the induction of Ca2+ influx. On the basis of the EF-hand mutant data, it is likely that STIM proteins function as Ca2+ sensors that monitor the loading level of intracellular Ca2+ stores. In a plausible model, the loss of luminal Ca2+ binding by STIM proteins triggers a conformational change that leads to its translocation to local sites (puncta) that are enriched near the plasma membrane. STIM proteins in these puncta may then directly or indirectly trigger Ca2+ influx.
Experimental Procedures
Cell Transfection, Plasmids, and Reagents
HeLa (human epithelial) cells and Jurkat (human T lymphocyte) E6-1 line were purchased from ATCC. DNA plasmids and siRNA were cotransfected into HeLa or Jurkat cells with Genesilencer reagent (Gene Therapy Systems, San Diego, CA). Full-length human STIM1 cDNA was isolated by PCR, sequenced, and cloned in to pDS_XB-YFP vector (ATCC). Enhanced yellow fluorescent protein (YFP) or cyan fluorescent protein (CFP) (Clontech, Palo Alto) was inserted immediately downstream of the predicted signal-peptide region of human STIM1. The YFP-conjugated EF-hand mutant of STIM1, YFP-STIM1(D76A), was made by site-directed mutagenesis with the QuikChange Site-Directed Mutagenesis Kit (Stratagene). Nucleotide sequences of constructs were verified by sequencing. NF-ATc1-YFP was provided by Dr. Won Do Heo. pECFP-Nuc, pECFP-ER, and pEYFP-Nuc plasmids were obtained from Clontech. pECFP-CAAX plasmid was provided by Dr. Mary Teruel. Thapsigargin, histamine, and BHQ (2,5-di-(t-Butyl)-1,4-benzohydroquinone) were purchased from Calbiochem. Anti-human CD3 (T cell receptor) antibody (BD BioSciences) was used at 20 μg/ml. SKF 96365 (Sigma) was used at 20 μM.
siRNA Library of Signaling Proteins
Two thousand, three hundred and four human signaling-related proteins were selected from the NCBI (RefSeq database) on the basis of the presence of signaling domains, such as protein kinase, SH2, SAM, EF, and PH domains, as well as by text searches of signaling-related terms. Gene-specific primers for the selected signaling proteins were designed with an in-house primer program and were used to generate ~600 bp cDNA fragments immediately upstream of the stop codon of each mRNA by PCR. An additional set of nested primers was designed to add T7 promoters at both ends of the final cDNA fragment. Nested PCR products were subjected to in vitro transcription, in vitro dicing, and purification to produce siRNA as described previously [24]. The siRNA signaling set was sorted according to the NCBI RefSeq Protein accession number and was stored in 24 96-well plates. The screen for SOC influx regulators was done by transfecting HeLa cells with the siRNA signaling set at an average concentration of 10 nM for 2 days in the 96-well format. Twenty-four siRNAs present in duplicates were screened at a time in an experimental microplate. The whole screen was performed twice.
Ca2+ Measurements
Ca2+ measurements were made with a fluorescence microplate reader (FlexStation, Molecular Devices). HeLa cells were loaded with 2 μM Fura-2-AM in extracellular buffer (125 mM NaCl, 5 mM KCl, 1.5 mM MgCl2, 20 mM HEPES, 10 mM glucose, and 1.5 mM CaCl2 [pH 7.4]) for 30 min at room temperature. Fura-2 fluorescence was measured by illuminating the cells with an alternating 340/380 nm light every 5 s. Fluorescence intensity was measured at 510 nm. Changes in intracellular Ca2+ concentration are presented as the change in the ratio of fluorescence intensity for excitation at 340 and 380 nm. For Ca2+ add-back experiments, 3 mM EGTA was added together with histamine and thapsigargin to remove extracellular Ca2+, and 10 mM Ca2+ was added back after Ca2+ store depletion. Imaging-based single-cell Ca2+ measurements of HeLa or Jurkat cells were performed with a 4× (HeLa) or 10× (Jurkat) objective on an automated fluorescent microscope ImageXpress 5000A (Molecular Devices) by loading cells with 0.5 μM Fura-2-AM. Fluorescence intensities of single cells were measured with the ImageXpress analysis software.
Mn2+ Quench Assays
Two millimolars Mn2+ was added to cells immediately before image acquisition. Histamine and thapsigargin were added 50 s after, and image acquisition was continued for another 90 s. Quenching of Fura-2 fluorescence was measured by illuminating cells with 360 nm light every 4 s, and fluorescence intensity was measured at 510 nm with ImageXpress.
Fluorescence Imaging
NF-ATc1 translocation was monitored in HeLa cells cotransfected with NF-ATc1-YFP and pECFP-Nuc plasmids with ImageXpress 5000A with a 10× objective. Live-cell confocal imaging experiments were performed with transfected HeLa cells with a 40× objective on a spinning-disk confocal microscope (Nipkow Wallac system). Live-cell TIRF imaging was done with transfected HeLa cells with a 60× objective on a Nikon TIRF microscopy system. Images were analyzed with MetaMorph software (Universal Imaging).
Supplementary Material
Acknowledgments
We acknowledge the support of the members in the Alliance for Cellular Signaling (AfCS) microscopy laboratory, the Meyer laboratory, other researchers in the Clark/Bio-X building, and Dr. David Solow-Cordero from the Stanford High Throughput Biosciences Center for stimulating discussions and for help in the troubleshooting of the siRNA library and the Ca2+ assays. We also thank Dr. Annette Salmeen for critically reading the manuscript. This work was supported by the National Institutes of Health and the Sandler Program for Asthma Research.
Footnotes
Supplemental Data
Four supplemental figures are available at http://www.current-biology.com/cgi/content/full/15/13/1235/DC1/.
Note Added in Proof
After submission of this manuscript, a paper was published showing that siRNAs targeting the Drosophila STIM homolog and human STIM1 both suppress SOC influx [25].
References
- 1.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: Dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 2.Putney JW., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 3.Mikoshiba K, Hattori M. IP3 receptor-operated calcium entry. Sci STKE. 2000;2000:PE1. doi: 10.1126/stke.2000.51.pe1. [DOI] [PubMed] [Google Scholar]
- 4.Putney JW, Jr, Broad LM, Braun FJ, Lievremont JP, Bird GS. Mechanisms of capacitative calcium entry. J Cell Sci. 2001;114:2223–2229. doi: 10.1242/jcs.114.12.2223. [DOI] [PubMed] [Google Scholar]
- 5.Prakriya M, Lewis RS. CRAC channels: Activation, permeation, and the search for a molecular identity. Cell Calcium. 2003;33:311–321. doi: 10.1016/s0143-4160(03)00045-9. [DOI] [PubMed] [Google Scholar]
- 6.Lewis RS. Calcium signaling mechanisms in T lymphocytes. Annu Rev Immunol. 2001;19:497–521. doi: 10.1146/annurev.immunol.19.1.497. [DOI] [PubMed] [Google Scholar]
- 7.Oritani K, Kincade PW. Identification of stromal cell products that interact with pre-B cells. J Cell Biol. 1996;134:771–782. doi: 10.1083/jcb.134.3.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sabbioni S, Barbanti-Brodano G, Croce CM, Negrini M. GOK: A gene at 11p15 involved in rhabdomyosarcoma and rhabdoid tumor development. Cancer Res. 1997;57:4493–4497. [PubMed] [Google Scholar]
- 9.Williams RT, Manji SS, Parker NJ, Hancock MS, Van Stekelenburg L, Eid JP, Senior PV, Kazenwadel JS, Shandala T, Saint R, et al. Identification and characterization of the STIM (stromal interaction molecule) gene family: Coding for a novel class of transmembrane proteins. Biochem J. 2001;357:673–685. doi: 10.1042/0264-6021:3570673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Missiaen L, De Smedt H, Pary JB, Oike M, Casteels R. Kinetics of empty store-activated Ca2+ influx in HeLa cells. J Biol Chem. 1994;269:5817–5823. [PubMed] [Google Scholar]
- 11.Montero M, Barrero MJ, Torrecilla F, Lobaton CD, Moreno A, Alvarez J. Stimulation by thimerosal of histamine-induced Ca(2+) release in intact HeLa cells seen with aequorin targeted to the endoplasmic reticulum. Cell Calcium. 2001;30:181–190. doi: 10.1054/ceca.2001.0224. [DOI] [PubMed] [Google Scholar]
- 12.Parker NJ, Begley CG, Smith PJ, Fox RM. Molecular cloning of a novel human gene (D11S4896E) at chromosomal region 11p15.5. Genomics. 1996;37:253–256. doi: 10.1006/geno.1996.0553. [DOI] [PubMed] [Google Scholar]
- 13.Williams RT, Senior PV, Van Stekelenburg L, Layton JE, Smith PJ, Dziadek MA. Stromal interaction molecule 1 (STIM1), a transmembrane protein with growth suppressor activity, contains an extracellular SAM domain modified by N-linked glycosylation. Biochim Biophys Acta. 2002;1596:131–137. doi: 10.1016/s0167-4838(02)00211-x. [DOI] [PubMed] [Google Scholar]
- 14.Manji SS, Parker NJ, Williams RT, van Stekelenburg L, Pearson RB, Dziadek M, Smith PJ. STIM1: A novel phosphoprotein located at the cell surface. Biochim Biophys Acta. 2000;1481:147–155. doi: 10.1016/s0167-4838(00)00105-9. [DOI] [PubMed] [Google Scholar]
- 15.Crabtree GR, Olson EN. NFAT signaling: Choreographing the social lives of cells. Cell. 2002;109:S67–S79. doi: 10.1016/s0092-8674(02)00699-2. [DOI] [PubMed] [Google Scholar]
- 16.Beals CR, Clipstone NA, Ho SN, Crabtree GR. Nuclear localization of NF-ATc by a calcineurin-dependent, cyclosporin-sensitive intramolecular interaction. Genes Dev. 1997;11:824–834. doi: 10.1101/gad.11.7.824. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 18.Kass GE, Llopis J, Chow SC, Duddy SK, Orrenius S. Receptor-operated calcium influx in rat hepatocytes. Identification and characterization using manganese. J Biol Chem. 1990;265:17486–17492. [PubMed] [Google Scholar]
- 19.Putney JW., Jr Pharmacology of capacitative calcium entry. Mol Interv. 2001;1:84–94. [PubMed] [Google Scholar]
- 20.Kawasaki H, Nakayama S, Kretsinger RH. Classification and evolution of EF-hand proteins. Biometals. 1998;11:277–295. doi: 10.1023/a:1009282307967. [DOI] [PubMed] [Google Scholar]
- 21.Gulati J, Babu A, Putkey JA. Down-regulation of fast-twitch skeletal muscle fiber with cardiac troponin-C and recombinant mutants. Structure/function studies with site-directed mutagenesis. FEBS Lett. 1989;248:5–8. doi: 10.1016/0014-5793(89)80420-x. [DOI] [PubMed] [Google Scholar]
- 22.Venkatachalam K, van Rossum DB, Patterson RL, Ma HT, Gill DL. The cellular and molecular basis of store-operated calcium entry. Nat Cell Biol. 2003;4:E263–E272. doi: 10.1038/ncb1102-e263. [DOI] [PubMed] [Google Scholar]
- 23.Steyer JA, Almers W. A real-time view of life within 100 nm of the plasma membrane. Nat Rev Mol Cell Biol. 2001;2:268–275. doi: 10.1038/35067069. [DOI] [PubMed] [Google Scholar]
- 24.Myers JW, Jones JT, Meyer T, Ferrell JE., Jr Recombinant Dicer efficiently converts large dsRNAs into siRNAs suitable for gene silencing. Nat Biotechnol. 2003;21:324–328. doi: 10.1038/nbt792. [DOI] [PubMed] [Google Scholar]
- 25.Roos J, Digregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.