Abstract
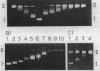
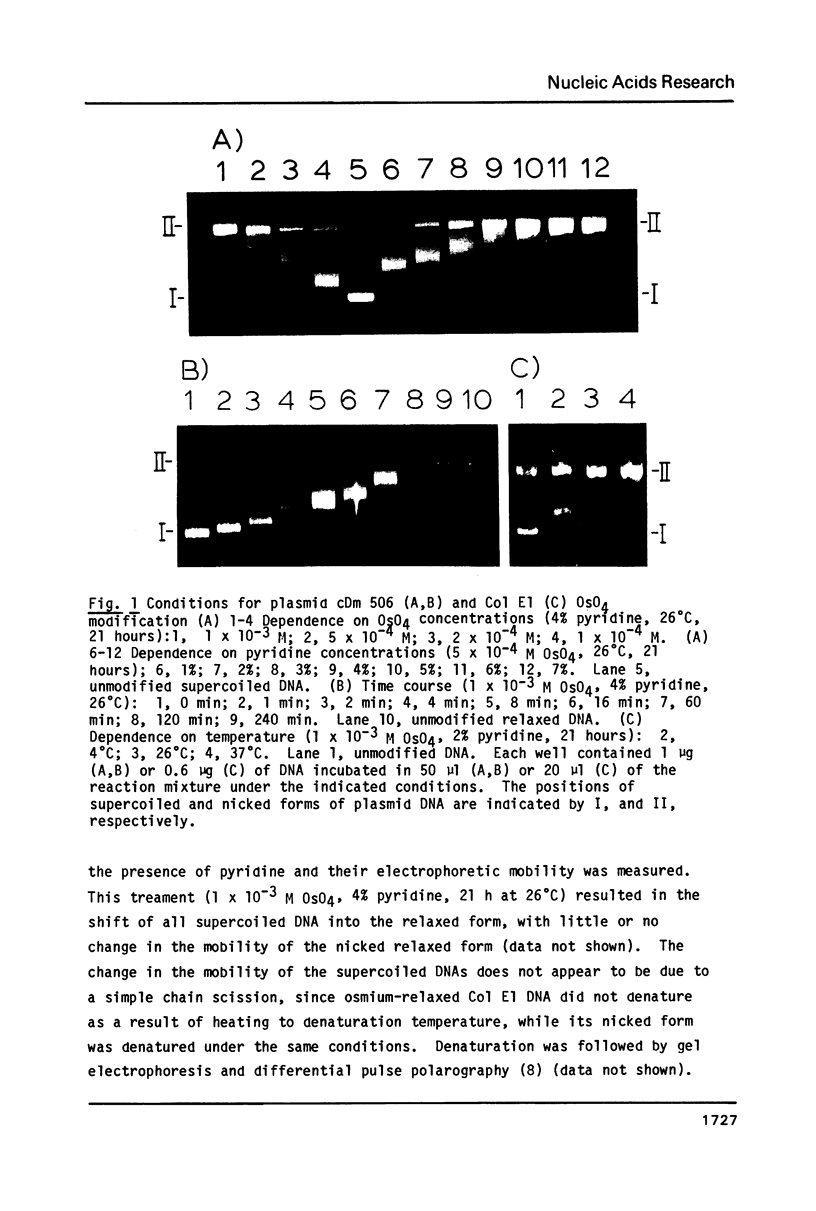
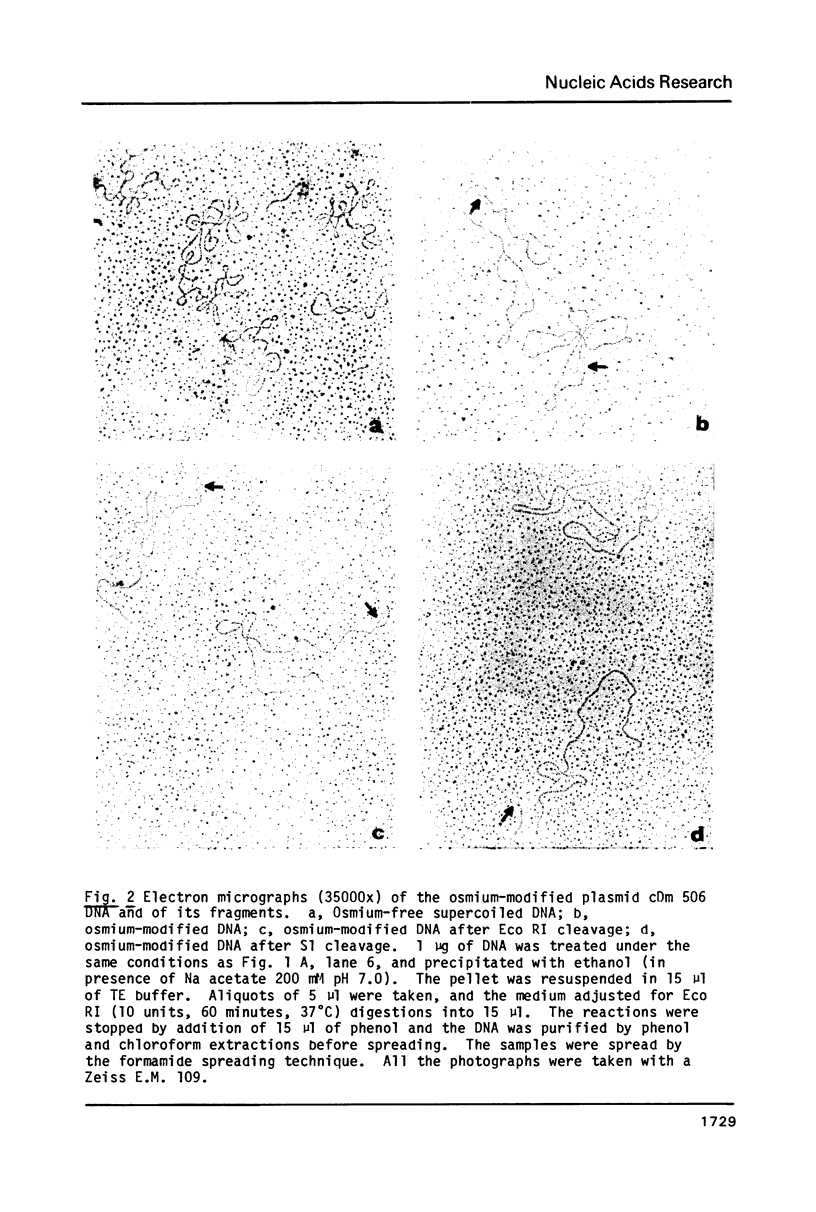
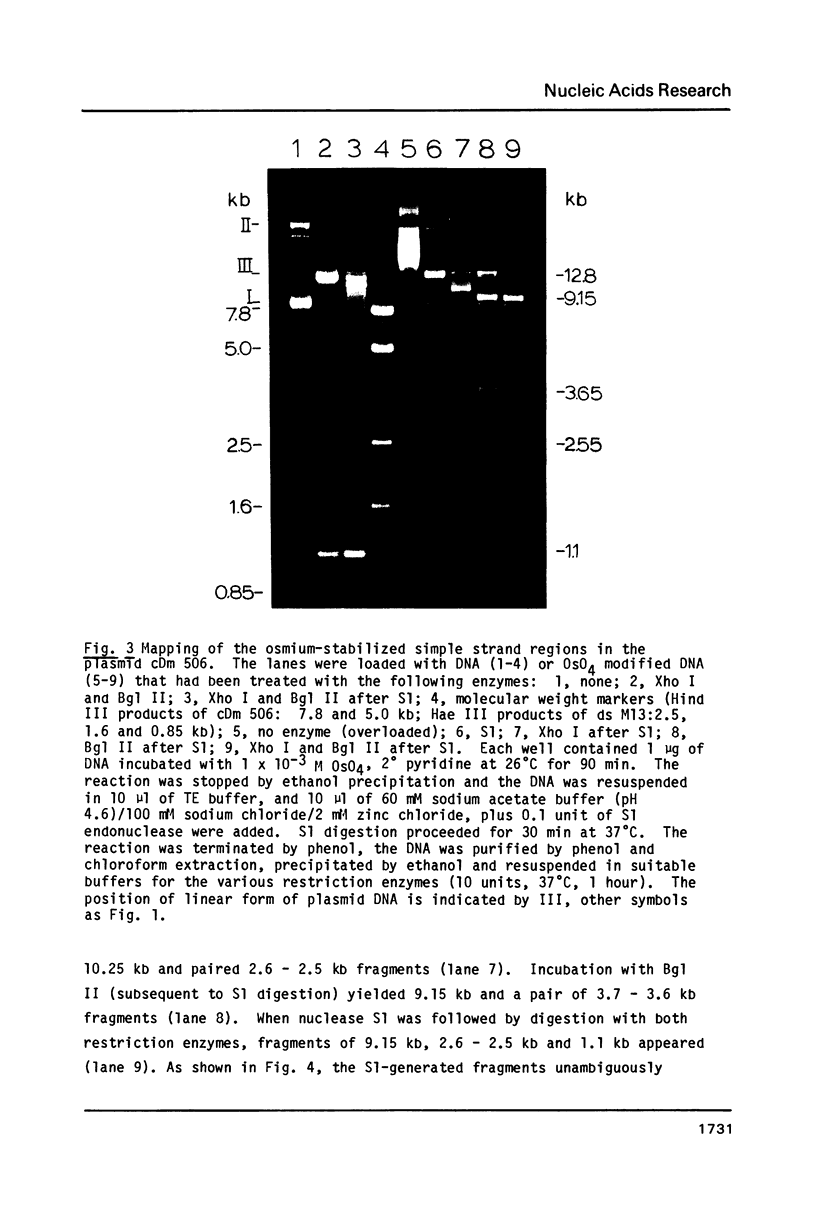
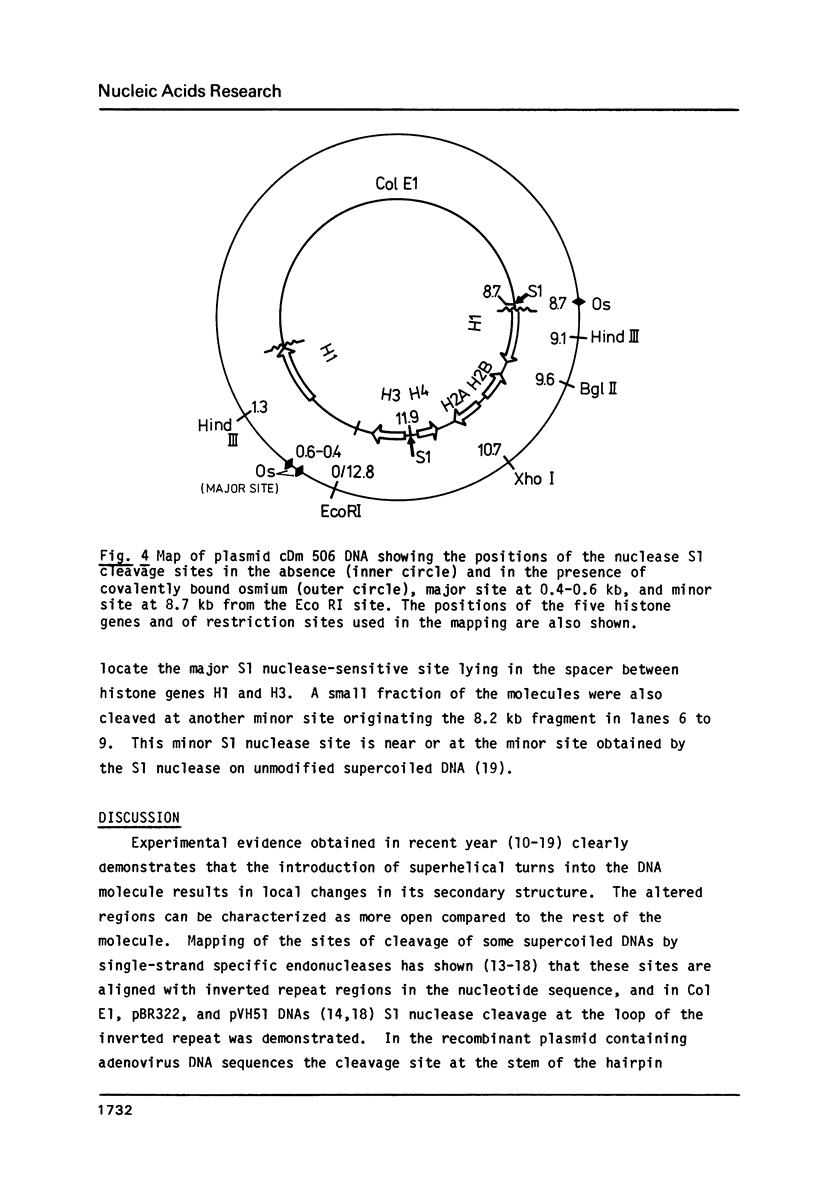
Supercoiled plasmids Col E1 and cDm 506 (a Col E1 derivative carrying the D. melanogaster histone gene repeat) were treated with OsO4 in presence of pyridine and the reaction products were analyzed using different approaches. Gel electrophoresis showed that OsO4 binding to supercoiled DNA induced its relaxation without nicking. The amount of osmium bound to DNA (as determined electrochemically) increased with the extent of DNA relaxation. As a result of osmium modification of supercoiled cDm 506, a single denaturation "bubble" was observed in the electron microscope. Mapping of the osmium binding site by S1 nuclease cleavage followed by restriction enzyme digestion has revealed one major site in the intergenic spacer between the H1 and H3 histone genes of D. melanogaster. This site differs from the site cleaved by S1 nuclease in supercoiled DNA in the absence of osmium.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer W. R. Structure and reactions of closed duplex DNA. Annu Rev Biophys Bioeng. 1978;7:287–313. doi: 10.1146/annurev.bb.07.060178.001443. [DOI] [PubMed] [Google Scholar]
- Beer M., Stern S., Carmalt D., Mohlhenrich K. H. Determination of base sequence in nucleic acids with the electron microscope. V. The thymine-specific reactions of osmium tetroxide with deoxyribonucleic acid and its components. Biochemistry. 1966 Jul;5(7):2283–2288. doi: 10.1021/bi00871a017. [DOI] [PubMed] [Google Scholar]
- Chang C. H., Beer M., Marzilli L. G. Osmium-labeled polynucleotides. The reaction of osmium tetroxide with deoxyribonucleic acid and synthetic polynucleotides in the presence of tertiary nitrogen donor ligands. Biochemistry. 1977 Jan 11;16(1):33–38. doi: 10.1021/bi00620a006. [DOI] [PubMed] [Google Scholar]
- Cole M. D., Wiggins J. W., Beer M. Molecular microscopy of labeled polynucleotides: stability of osmium atoms. J Mol Biol. 1977 Dec 5;117(2):387–400. doi: 10.1016/0022-2836(77)90134-6. [DOI] [PubMed] [Google Scholar]
- Glikin G. C., Gargiulo G., Rena-Descalzi L., Worcel A. Escherichia coli single-strand binding protein stabilizes specific denatured sites in superhelical DNA. Nature. 1983 Jun 30;303(5920):770–774. doi: 10.1038/303770a0. [DOI] [PubMed] [Google Scholar]
- Goding C. R., Russell W. C. S1 sensitive sites in adenovirus DNA. Nucleic Acids Res. 1983 Jan 11;11(1):21–36. doi: 10.1093/nar/11.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohwi-Shigematsu T., Gelinas R., Weintraub H. Detection of an altered DNA conformation at specific sites in chromatin and supercoiled DNA. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4389–4393. doi: 10.1073/pnas.80.14.4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalski D., Sanford J. P. Action of mung bean nuclease on supercoiled PM2 DNA. J Biol Chem. 1982 Jul 10;257(13):7820–7825. [PubMed] [Google Scholar]
- Kłysik J., Stirdivant S. M., Larson J. E., Hart P. A., Wells R. D. Left-handed DNA in restriction fragments and a recombinant plasmid. Nature. 1981 Apr 23;290(5808):672–677. doi: 10.1038/290672a0. [DOI] [PubMed] [Google Scholar]
- Lifton R. P., Goldberg M. L., Karp R. W., Hogness D. S. The organization of the histone genes in Drosophila melanogaster: functional and evolutionary implications. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):1047–1051. doi: 10.1101/sqb.1978.042.01.105. [DOI] [PubMed] [Google Scholar]
- Lilley D. M. Hairpin-loop formation by inverted repeats in supercoiled DNA is a local and transmissible property. Nucleic Acids Res. 1981 Mar 25;9(6):1271–1289. doi: 10.1093/nar/9.6.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley D. M. Structural perturbation in supercoiled DNA: hypersensitivity to modification by a single-strand-selective chemical reagent conferred by inverted repeat sequences. Nucleic Acids Res. 1983 May 25;11(10):3097–3112. doi: 10.1093/nar/11.10.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley D. M. The inverted repeat as a recognizable structural feature in supercoiled DNA molecules. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6468–6472. doi: 10.1073/pnas.77.11.6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palecek E., Hung M. A. Determination of nanogram quantities of osmium-labeled nucleic acids by stripping (inverse) voltammetry. Anal Biochem. 1983 Jul 15;132(2):236–242. doi: 10.1016/0003-2697(83)90002-7. [DOI] [PubMed] [Google Scholar]
- Panayotatos N., Wells R. D. Cruciform structures in supercoiled DNA. Nature. 1981 Feb 5;289(5797):466–470. doi: 10.1038/289466a0. [DOI] [PubMed] [Google Scholar]
- Singleton C. K., Klysik J., Stirdivant S. M., Wells R. D. Left-handed Z-DNA is induced by supercoiling in physiological ionic conditions. Nature. 1982 Sep 23;299(5881):312–316. doi: 10.1038/299312a0. [DOI] [PubMed] [Google Scholar]
- Singleton C. K., Wells R. D. Relationship between superhelical density and cruciform formation in plasmid pVH51. J Biol Chem. 1982 Jun 10;257(11):6292–6295. [PubMed] [Google Scholar]
- Vogt V. M. Purification and further properties of single-strand-specific nuclease from Aspergillus oryzae. Eur J Biochem. 1973 Feb 15;33(1):192–200. doi: 10.1111/j.1432-1033.1973.tb02669.x. [DOI] [PubMed] [Google Scholar]
- Vorlícková M., Kypr J., Kleinwächter V., Palecek E. Salt-induced conformational changes of poly(dA-dT). Nucleic Acids Res. 1980 Sep 11;8(17):3965–3973. doi: 10.1093/nar/8.17.3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorlícková M., Kypr J., Sklenár V. Salt-induced conformational transition of poly[d(A-T)] X poly[d(A-T)]. J Mol Biol. 1983 May 5;166(1):85–92. doi: 10.1016/s0022-2836(83)80052-7. [DOI] [PubMed] [Google Scholar]
- Zimmerman S. B. The three-dimensional structure of DNA. Annu Rev Biochem. 1982;51:395–427. doi: 10.1146/annurev.bi.51.070182.002143. [DOI] [PubMed] [Google Scholar]