Abstract
Age-related changes in proprioceptive ability and their contributions to postural instability have been well documented. In contrast, and despite the known importance of proprioceptive feedback in the control of coordinated arm and hand movement, studies focusing on upper limb proprioception in older populations are few and equivocal in their findings. This study focused on kinesthetic awareness about the wrist joint in healthy young and older adults. Passive movement detection thresholds (PMDT) were twice as high in older compared to young participants. In contrast to previous findings demonstrating asymmetries in static position sense, PMDT did not differ between the dominant and non-dominant wrist joints nor did direction of joint displacement affect PMDT as has been reported for the lower limb. Preliminary analysis indicated that PMDT was significantly higher in older adults categorized as sedentary while active older adults were able to detect passive movement as well as young adults. These findings demonstrate that upper limb kinesthesia is impaired in older adults although the degree of impairment may be influenced by one’s level of physical activity.
Introduction
The perception of limb position or joint motion in the absence of vision, collectively known as proprioception, is considered a critical component in the control of voluntary movement. Proprioceptive feedback from muscle, skin and joint mechanoreceptors is thought to play a key role in the control of muscle interaction torques [39], coordinating sequential movements involving multiple joints [8] and establishing an internal representation of body position during the production of skilled movement [24]. Further, profound disturbances in arm and hand function [38], postural control [35] and locomotion [27] occur in functionally deafferented individuals with large sensory fiber neuropathy.
In older adults, declines in proprioceptive acuity have primarily focused on balance control [10, 12] and an increased risk for falls in older adults [29, 30]. Static joint position sense about lower limb joints deteriorates with age [17] as does the ability to detect joint motion (kinesthesia) [34, 48] and integrate both static and dynamic proprioceptive feedback during more complex lower limb motor tasks [33, 46]. Despite the demonstrated importance of proprioceptive feedback for coordinated hand and arm control, surprisingly few studies have focused on age-related changes in proprioceptive acuity in the upper limb and those which have been conducted are equivocal in their findings. Kokmen et al [26] found no differences in passive detection of finger joint motion in young and older individuals while later studies demonstrated age-related impairments in the ability to reproduce a passively determined joint position of the index finger [11] and arm [40]. In contrast, Lovelace and Aikens [31] found no age-related differences in proprioceptively-guided pointing accuracy, supporting the conclusion that “proprioception in the arm and hand is relatively well preserved in healthy older adults” [23]. More recently, however, we demonstrated that upper limb static position sense is impaired in older adults. Using a limb position reproduction task, we demonstrated that matching errors about the elbow [2] and wrist [1] were significantly greater in older compared to young participants.
While evidence exists that position sense is impaired in the upper limbs of older adults, it is not known whether more dynamic aspects of proprioception show similar declines. Kinesthetic awareness relies heavily on the dynamic responsiveness of larger diameter muscle spindle afferents and, in terms of assessing this aspect of proprioception, does not require position matching-related muscle activation which can alter spindle sensitivity via the fusimotor system. The present study was conducted to determine if the ability to perceive passive movement about the wrist, as reflected by passive movement detection thresholds (PMDT), is altered in older individuals. The experiment focused on wrist joint kinesthetic ability given the importance of this joint in controlling finger force production during prehension tasks [21, 28]. The influence of movement direction (flexion vs extension) was also examined as it has been shown that knee flexion movements are more easily perceived than extension movements in both young [47] and older [49] individuals. Muscle spindle numbers are greater in muscles with larger cross sectional areas [3, 25] and wrist flexor volume is twice that of the wrist extensors [22]. Thus, it was hypothesized that wrist extension-directed movements which stretch the wrist flexors and thereby activate muscle spindles would be more easily detected than flexion-directed movements. Passive movement detection thresholds were also examined at both the dominant and non-dominant wrist joints to determine if a non-dominant hand advantage for proprioceptive processing, shown to exist in young individuals [17, 19] persisted with aging. Alternatively, long term, use-dependent superiority of the dominant hand in the performance of fine motor skills [44] may lead to enhanced perception of movement about the dominant wrist. Lastly, a preliminary exploration of the effects of general physical activity on detection of passive movement was conducted where it was hypothesized that PMDT would be higher in sedentary compared to physically active older adults.
Methods
Participants
Ten older, independent community dwelling adults (6 males, 4 females, mean age: 79.5 +/− 2.2 yrs) participated in the study. All were in general good health with no reports of neurological or musculoskeletal disorders. Range of motion about the shoulder, elbow and wrist joints was normal as was cognitive function with MMSE scores greater than 26 [9]. Ten young (24.0 +/−2.3 yrs), gender matched participants served as a control group. All participants were right-handed as determined by the Edinburgh Handedness Inventory and none were engaged in intensive physical activity or had a history of music/dance training. Written informed consent was obtained from each participant following procedures established by the University of Michigan Institutional Review Board.
Functional, Cognitive and Physical Activity Assessments
Each age group was characterized using standardized measures of manual dexterity (Nine Hole Peg Test), attention and information processing speed during cognitive-motor tasks (Trail Making Test, Parts A and B), and maximum grip force (average of two trials) using a hand held dynamometer. In order to obtain preliminary evidence related to the effects of general physical activity levels on PMDTs, older adults completed the Community Healthy Activities Model Program for Seniors (CHAMPS) survey which determines the frequency and duration of 41 physical activities ranging from light to moderately heavy tasks [42]. Based on this survey, estimated energy expenditure in kcal/per week was determined for the older participants and they were sub-classified into an active (n=5) and sedentary (n=5) group. Using a mean split of energy expenditure values, mean (+/− 1 SD) energy expenditure for the active group was 4098 +/− 683 kcal/week and 1553 +/− 846 kcal/week for the sedentary group.
Testing Paradigm
Blindfolded participants were seated with their forearms resting on fixed lever arms of a manipulandum apparatus. Shoulder position was 20–30 deg flexion and 45–55 deg abduction, with the elbow joint flexed at approximately 100 deg. The forearms were semipronated and stabilized by neoprene straps. The hands and fingers were supported by a custom made splint attached to the moving lever arms of the manipulanda with the digits in a relaxed, slightly flexed position. These supports provided light contact and stability of the hand to limit cutaneous information during slow displacement at the wrist. The axis of wrist joint rotation was aligned to the axis of rotation of the manipulandum for a neutral starting position (0 deg extension). Two servo-motors (Smartmotor™) passively displaced the wrist at 0.5 deg/s in a random flexion or extension direction. During wrist displacements, the participants were instructed to press an indicator switch held in the opposite hand when they first perceived any passive movement at the wrist being tested. Once the indicator switch was pressed, the servomotor was automatically turned off. Following movement detection, the wrist was passively returned to the starting position. Participants were instructed to completely relax throughout wrist displacement and test trials were repeated if any evidence of active movement was observed in the real-time wrist displacement records.
Data Collection and Analysis
Trials involving the right dominant and left non-dominant wrists were counter-balanced across participants for a total of 16 possible trials per participant. Data were acquired for 15 s and a 30 s rest was allowed between each trial. Wrist joint position was recorded from potentiometers mounted beneath the pivot point of each manipulandum and digitized at 100 Hz. Data were low pass filtered with a 4th order Butterworth filter (zero phase lag, 6 Hz cut off freq) and analyzed using customized software (LabVIEW™, National Instruments). The PMDT was defined as the amount of wrist displacement (deg) which occurred when the participant first perceived movement, and pressed the switch.
Statistical Analysis
A two-way (young vs older group, dominant vs non-dominant hand) repeated measures analysis of variance (ANOVA) was used to determine differences in the nine hole peg test and maximium grip force. In order to determine changes in PMDT, a three-way (group, hand, direction of displacement) ANOVA was used to determine main and interaction effects. A secondary, one way ANOVA using data collapsed across nonsignificant factors was performed to examine the effect of physical activity on PMDT in older individuals. Post-hoc comparisons were performed using Sidak adjustments for multiple comparisons. All statistical analyses were conducted using PASW18 software. All tests were two-tailed and the significance level was set at p< 0.05. Effect size (ES) was calculated using Cohen’s D method where an effect size of 0.2 is considered a small effect, 0.5 is a moderate effect, and over 0.8 a strong effect [7].
Results
Manual dexterity scores were significantly lower in the older compared to young group for both the dominant (p<.01, ES: 1.62) and non-dominant (p<.001, ES: 1.97) hands (Table 1, Nine-Hole Peg Test). While hand differences were not significant in the young group (p=.16, ES: .52), asymmetries existed in the older group with better right dominant than left non-dominant hand performance (p<.001, ES: .65). Maximum grip force was also asymmetric with greater grip forces seen for the dominant hand in both the young (p<.01, ES: .22) and older groups (p<.01, ES: .38). Maximum grip force did not reach statistical significance between groups although effect sizes were moderate (dominant hand: p=.23, ES: .57; non-dominant hand: p=.18, ES: .61). Trail Making Test scores were normal for age and education [45]. Correlations between manual dexterity scores and PMDT were not significant for either age group or hand (p>.5).
Table 1.
Performance on functional and cognitive assessments in young (Y) and older (O) adults (mean +/− 1 SD). Right (R) and left (L) hand data are shown for the Nine-hole peg test and maximum grip force. Trail-making test data were obtained using the dominant hand.
| Age (y) | Nine-Hole Peg Test (s) | Grip Force (kg) | Trail-Making Test (s) | ||||
|---|---|---|---|---|---|---|---|
| R | L | R | L | Aa | Bb | ||
| Y | 24.0 ± 3.0 | 16.9 ± 2.3c | 17.9 ± 1.9d | 41.8 ± 12.4 | 38.9 ± 13.5 | 26.0 ± 7.8 | 50.6 ± 16.4 |
| O | 79.5 ± 6.4 | 22.4 ± 4.5 | 25.9 ± 6.2 | 35.4 ± 9.71 | 31.8 ± 9.43 | 41.7 ± 20.5 | 130.9 ± 90.5 |
Trail-Making Test Part A reflects time taken to quickly draw lines to connect 25 circled numbers (1–25) in ascending order distributed over a sheet of paper.
Trail-Making Test Part B reflects time taken to draw lines to connect circled numbers (1–13) and letters (A–L) by alternating between the numbers and letters
significant group differences p<.01
significant group differences p<.001
Passive Movement Detection Thresholds
Passive movement detection thresholds in older participants were twice that of young participants (p< .01, ES: 1.25). Young adults were able to detect joint movement following approximately 1 deg of passive displacement while, in the older group, a mean displacement of 2 deg was required. However, the direction of displacement (i.e. flexion vs extension) did not affect PMDT in either group (Y-right: p=.46, ES: .43; Y-left: p=.88, ES: .11; O-right: p=.43, ES: .21; O-left: p=.20, ES: .73)
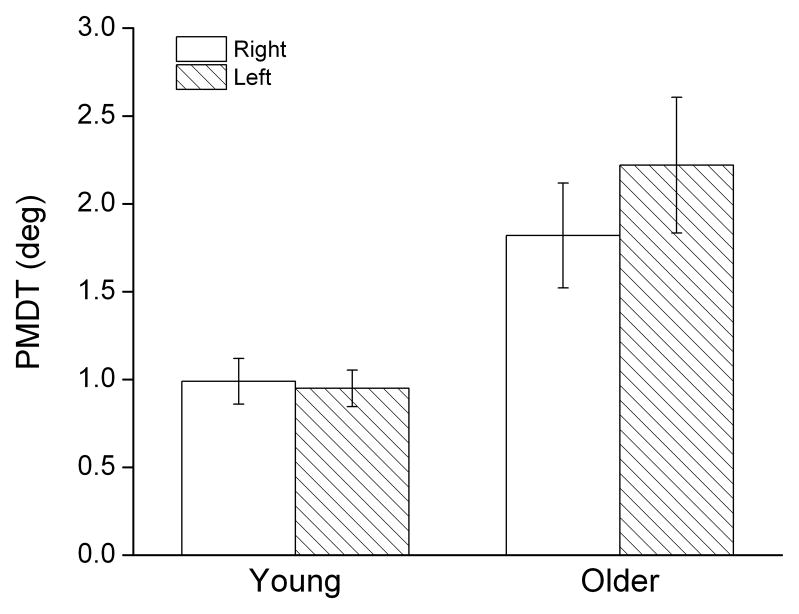
No asymmetries between the dominant and nondominant limbs were observed in either age group. In young participants, the ability to perceive joint motion was virtually identical for both the right and left wrists (PMDT right: 0.99 deg, left: 0.95 deg; Fig. 1). While there was a trend towards increased PMDT for the left, non-dominant wrist in the older group (PMDT right: 1.82 deg, left: 2.22 deg), differences were not statistically significant and the effect size was small(p=.087, ES: .22).
Figure 1.
Mean (+/− 1 SE) passive movement detection thresholds (PMDT) in young and older adults. Open bars represent data obtained from movements made about the right dominant wrist joint; hatched bars, left, nondominant wrist joint (flexion and extension data collapsed). Threshold measures reflect the magnitude of passive joint displacement required to detect movement.
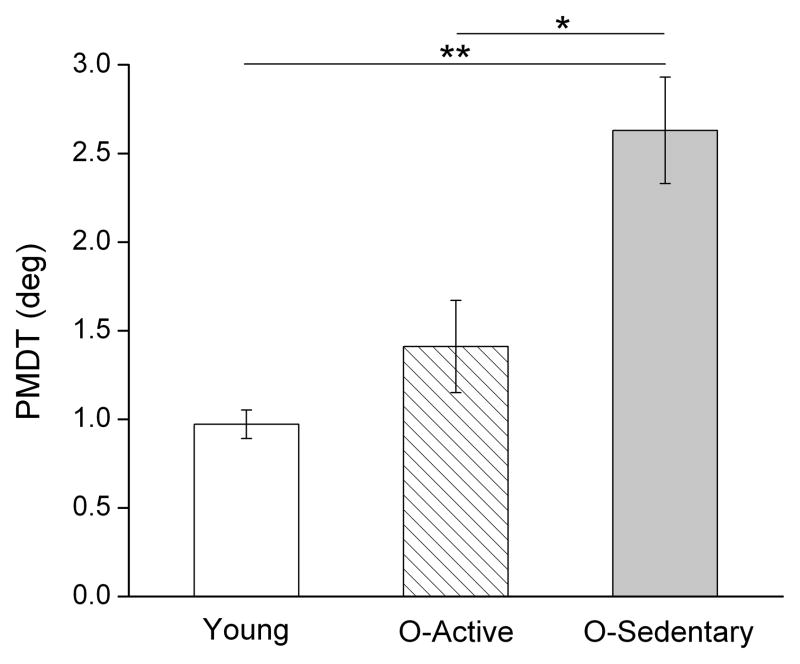
A secondary analysis based on energy expenditure revealed that older adults classified as sedentary showed greater impairments in the ability to detect passive movement compared to the older active group (p<.05, ES: 1.15) and the young (p<.01, ES: 2.01) groups (Fig. 2). In contrast, no differences in PMDT were seen between the young and older active groups (p=.078, ES. 63). Despite the small elderly sample, effect size calculations revealed a very strong effect of physical activity on PMDT when comparing the older active and sedentary groups.
Figure 2.
Mean (+/− 1 SE) passive movement detection thresholds (PMDT) in young, older-active, and older-sedentary adults. (*p<.05, **p<.01)
Discussion
The results presented here support the view that impaired proprioceptive ability in older adults is a generalized phenomenon and not restricted to lower limb balance control. These data are the first to provide strong evidence that the ability to detect wrist joint motion is significantly impaired in healthy older adults with clinically normal cognitive function, a finding which extends our previous observations showing declines in elbow and wrist static position sense in older adults [1, 2]. Further, declines in kinesthetic ability were comparable to that reported for the ankle joint [48, 49], suggesting that age-related proprioceptive impairment may be independent of limb function. This is consistent with findings in middle-aged adults with knee osteoarthritis where declines in the ability to detect passive movement occurred at both the knee and elbow joints [32].
While lower limb proprioceptive deficits have been implicated in increased risk of falls in the elderly [29, 30], the functional consequences of impaired elbow or wrist proprioceptive ability relate more to precise dynamic or static control of goal-directed arm movements. Previously, we found that mean elbow position matching errors in older adults were almost 40 percent greater than errors made by young adults which, functionally, could lead to an end-point hand position error of 4–5 cm [2]. Proprioceptive feedback may also compensate for changes in wrist position due to interactional torques developed during multi-joint arm movements–a control strategy shown to be impaired in functionally deafferented individuals [39]. In the case of wrist PMDT, it is well established that maximum finger force production is highly dependent on maintaining an optimal wrist position which facilitates the force producing capability of the extrinsic finger muscles [21, 36]. Deviations from an optimal neutral or slightly extended wrist position biomechanically decrease muscle tendon moment arms and also affect finger force sharing capability during different types of precision grasp tasks [28]. Thus, proprioceptive monitoring of wrist position may assist in maintaining an optimal wrist position during object grasping and manipulation tasks. The lack of an observed correlation between hand manipulation ability and PMDT in the present study may be due to the low forces required to manipulate small pins and thus future studies should address the relationship between proprioceptive deficits and a broader range of upper limb functional activities.
Directional Sensitivity in Passive Movement Detection Thresholds
Passive movement detection thresholds were comparable regardless of the direction of wrist movement in both young and older groups a finding which contrasts previous studies demonstrating a directional sensitivity in the perception of lower limb motion. Weiler and Awiszus [47] reported that, in young adults, flexion movements about the knee were perceived at lower thresholds than extension movements, a finding subsequently confirmed in an older population [49]. More recently, it was shown that at movement speeds greater than 10 deg/s, a similar asymmetry in directional sensitivity was observed for the knee. In this case, however, the effect was reversed with extension movements being more easily detected than flexion movements [4]. It has been postulated that such directional differences may be due to asymmetries in antagonistic muscle mass, strength, and density and distribution of muscle spindles [47].
Given that wrist flexor cross-sectional area is approximately twice that of wrist extensors [22] and, assuming comparable muscle spindle densities, it was hypothesized that extension directed motion would be more easily detected, particularly in young adults, as a result of stretching the flexor muscles. It is possible that the lack of directional sensitivity seen here may be related to the relatively slow speed of joint displacement used in the present study since it is known that the stretch response of muscle spindles is speed sensitive [5]. It is also possible that positioning the wrist in a neutral position may have minimized any directional sensitivity to passive joint displacement. Thus, future studies examining potential differences between flexion and extension displacements across the range of wrist joint movement are warranted.
Asymmetries in Passive Movement Detection Thresholds
In younger individuals, static position sense about the elbow is asymmetric with the non-dominant arm exhibiting an enhanced ability to utilize limb position feedback [16, 18]. This has been interpreted as reflecting specialization of the non-dominant arm/hemisphere system for the processing of position-related proprioceptive feedback [15] and is also seen in left-handed individuals [19]. In older adults, asymmetries in wrist [1] and elbow [2] position sense have been also reported but primarily in conditions requiring interhemispheric transfer of position-related information (i.e. matching with the opposite limb). Less is known regarding kinesthetic asymmetries although the ability to reproduce the dynamic characteristics of a proprioceptively-guided movement does not differ between the two arms in young adults [14]. In the present study, it was expected that, as a result of a lifetime of dominant hand use, detection of passive movement about the right wrist would be enhanced. The lack of hand asymmetry in kinesthetic awareness may be the consequence of increased bilateral activation of somatosensory cortical areas [17] which also supports recent findings demonstrating a reduction in kinematic asymmetries during the performance of visually-guided reaching movements [37]. These observations extend the view that bilateral activation of sensorimotor areas may be a hallmark of the aging process, reflecting neurodegenerative processes such as a reduction in cortical inhibition and/or compensation for less efficient contralateral function [13, 43]. However, it should be noted that, in the present study, age-related motor asymmetries were observed for manual dexterity tasks which underscores the influence of task when assessing asymmetries in upper limb performance [44].
Role of Physical Activity and Proprioception
Although based on a small sample, general physical activity levels influenced the ability of older adults to perceive passive wrist motion. Older active adults performed almost as well as young adults while movement detection thresholds in older sedentary adults were almost twice as great as those seen in older active adults. It is well established that physical activity contributes to improved health in elderly individuals [6, 41], but it is unclear whether similar benefits apply to proprioceptive function. There is some evidence that the ability to use proprioceptive feedback about the ankle joint is enhanced in physically active older adults. For example, Xu et al. [49] showed that kinesthetic awareness was greater in elderly Tai Chi practitioners compared to sedentary older adults. Recently, we demonstrated a similar effect for upper limb static position sense where physically active older adults performed as well as young individuals [1]. Preliminary evidence presented here provides further support for the notion that age-related declines in proprioceptive function may be ameliorated by a physically active lifestyle. These findings also underscore the importance of taking into account general physical activity levels when measuring sensorimotor performance in older populations.
Conclusions
The results of this study provide valuable information changes in regarding movement-related proprioception in older adults. Not only do the findings extend our understanding regarding the extent of age-related proprioceptive declines but provide preliminary evidence of the value of physical activity in maintaining somatosensory function. These results also have clinical value. As recently noted by Hagert et al [20], there is a paucity of both basic and applied research focused on wrist proprioceptive rehabilitation. This is particularly relevant for our aging society where declines in hand function due to long term neuromuscular degeneration and/or acute trauma such as colles fractures are common.
Highlights.
Determine if upper limb kinesthetic awareness declines with aging
Passive movement detection thresholds twice as great in older adults
No asymmetries between the arms in young or older adults
Thresholds greater in older sedentary compared to older active adults
Arm kinesthetic sense declines with age but may be improved by physical activity
Acknowledgments
This research was supported by the National Institutes of Health, National Institute on Aging (AG025120-01) to SB, National Institute on Aging T32 training grant (AG00114) to DA, and Dept. of Education OSERS leadership training fellowship (H325D020028) to MW. The authors wish to thank K. Kern for assistance with data analysis and manuscript preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adamo DE, Alexander NB, Brown SH. The influence of age and physical activity on upper limb proprioceptive ability. J Aging Phys Act. 2009;17:272–293. doi: 10.1123/japa.17.3.272. [DOI] [PubMed] [Google Scholar]
- 2.Adamo DE, Martin BJ, Brown SH. Age-related differences in upper limb proprioceptive acuity. Percep Mot Skills. 2007;104:1297–1309. doi: 10.2466/pms.104.4.1297-1309. [DOI] [PubMed] [Google Scholar]
- 3.Banks RW. An allometric analysis of the number of muscle spindles in mammalian skeletal muscles. J Anat. 2006;208:753–768. doi: 10.1111/j.1469-7580.2006.00558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brindle TJ, Mizelle JC, Lebiedowska MK, Miller JL, Stanhope SJ. Visual and proprioceptive feedback improves knee joint position sense. Knee Surg Sports Traumatol Arthrosc. 2009;17:40–47. doi: 10.1007/s00167-008-0638-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burke D, Hagbarth KE, Lofstedt L. Muscle-spindle responses in man to changes in load during accurate position maintenance. J Physiol-London. 1978;276:159–164. doi: 10.1113/jphysiol.1978.sp012225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chodzko-Zajko WJ, Proctor DN, Singh MAF, Minson CT, Nigg CR, Salem GJ, Skinner JS. Exercise and physical activity for older adults. Med Science Sports Exer. 2009;41:1510–1530. doi: 10.1249/MSS.0b013e3181a0c95c. [DOI] [PubMed] [Google Scholar]
- 7.Cohen J. A power primer. Psychol Bull. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 8.Cordo PJ. Kinesthetic control of a multijoint movement sequence. J Neurophysiol. 1990;63:161–172. doi: 10.1152/jn.1990.63.1.161. [DOI] [PubMed] [Google Scholar]
- 9.Crum RM, Anthony JC, Bassett SS, Folstein MF. Population-based norms for the mini-mental-state-examination by age and educational-level. JAMA. 1993;269:2386–2391. [PubMed] [Google Scholar]
- 10.Diener HC, Dichgans J, Guschlbauer B, Mau H. The significance of proprioception on postural stabilization as assessed by ischemia. Brain Res. 1984;296:103–109. doi: 10.1016/0006-8993(84)90515-8. [DOI] [PubMed] [Google Scholar]
- 11.Ferrell WR, Crighton A, Sturrock RD. Position sense at the proximal interphalangeal joint is distorted in patients with rheumatoid arthritis of finger joints. Exp Physiol. 1992;77:675–680. doi: 10.1113/expphysiol.1992.sp003633. [DOI] [PubMed] [Google Scholar]
- 12.Fitzpatrick R, McCloskey DI. Proprioceptive, visual and vestibular thresholds for the perception of sway during standing in humans. J Physiol. 1994;478(Pt 1):173–186. doi: 10.1113/jphysiol.1994.sp020240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fling BW, Peltier SJ, Bo J, Welsh RC, Seildler RD. Age differences in interhemispheric interactions: callosal structure, physiolgical function, and behavior. Front Neurosci. 2011;21:38, 1–8. doi: 10.3389/fnins.2011.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goble DJ, Brown SH. Dynamic proprioceptive target matching behavior in the upper limb: Effects of speed, task difficulty and arm/hemisphere asymmetries. Behav Brain Res. 2009;200:7–14. doi: 10.1016/j.bbr.2008.11.034. [DOI] [PubMed] [Google Scholar]
- 15.Goble DJ, Brown SH. The biological and behavioral basis of upper limb asymmetries in sensorimotor performance. Neuro Biobehav Rev. 2008;32:598–610. doi: 10.1016/j.neubiorev.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 16.Goble DJ, Brown SH. Upper limb asymmetries in the matching of proprioceptive versus visual targets. J Neurophysiol. 2008;99:3063–3074. doi: 10.1152/jn.90259.2008. [DOI] [PubMed] [Google Scholar]
- 17.Goble DJ, Coxon JP, Wenderoth N, Van Impe A, Swinnen SP. Proprioceptive sensibility in the elderly: Degeneration, functional consequences and plastic-adaptive processes. Neuros Biobehav Rev. 2009;33:271–278. doi: 10.1016/j.neubiorev.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 18.Goble DJ, Lewis CA, Brown SH. Upper limb asymmetries in the utilization of proprioceptive feedback. Exp Brain Res. 2006;168:307–311. doi: 10.1007/s00221-005-0280-y. [DOI] [PubMed] [Google Scholar]
- 19.Goble DJ, Noble BC, Brown SH. Proprioceptive target matching asymmetries in left-handed individuals. Exp Brain Res. 2009;197:403–408. doi: 10.1007/s00221-009-1922-2. [DOI] [PubMed] [Google Scholar]
- 20.Hagert E. Proprioception of the Wrist Joint: A Review of Current Concepts and Possible Implications on the Rehabilitation of the Wrist. J Hand Ther. 2010;23:2–16. doi: 10.1016/j.jht.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 21.Hazelton FT, Smidt GL, Flatt AE, Stephens RI. Influence of wrist postiion on force produced by finger flexors. J Biomech. 1975;8:301. doi: 10.1016/0021-9290(75)90082-2. [DOI] [PubMed] [Google Scholar]
- 22.Holzbaur KRS, Murray WM, Gold GE, Delp SL. Upper limb muscle volumes in adult subjects. J Biomech. 2007;40:742–749. doi: 10.1016/j.jbiomech.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 23.Jones LA, Lederman SJ, ebrary I. Human hand function. Oxford University Press; Oxford; New York: 2006. [Google Scholar]
- 24.Kawato M. Internal models for motor control and trajectory planning. Curr Opin Neurobiol. 1999;9:718–727. doi: 10.1016/s0959-4388(99)00028-8. [DOI] [PubMed] [Google Scholar]
- 25.Kokkorogiannis T. Somatic and intramuscular distribution of muscle spindles and their relation to myscular angiotypes. J Theor Biol. 2004;2:229, 263–280. doi: 10.1016/j.jtbi.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 26.Kokmen E, Bossemeyer RW, Jr, Williams WJ. Quantitative evaluation of joint motion sensation in an aging population. J Gerontol. 1978;33:62–67. doi: 10.1093/geronj/33.1.62. [DOI] [PubMed] [Google Scholar]
- 27.Lajoie Y, Teasdale N, Cole JD, Burnett M, Bard C, Fleury M, Forget R, Paillard J, Lamarre Y. Gait of a deafferented subject without large myelinated sensory fibers below the neck. Neurology. 1996;47:109–115. doi: 10.1212/wnl.47.1.109. [DOI] [PubMed] [Google Scholar]
- 28.Li ZM. The influence of wrist position on individual finger forces during forceful grip. J Hand Sur-American Volume. 2002;27A:886–896. doi: 10.1053/jhsu.2002.35078. [DOI] [PubMed] [Google Scholar]
- 29.Lord SR, Clark RD, Webster IW. Physiological factors associated with falls in an elderly population. J Am Geriatr Soc. 1991;39:1194–1200. doi: 10.1111/j.1532-5415.1991.tb03574.x. [DOI] [PubMed] [Google Scholar]
- 30.Lord SR, Rogers MW, Howland A, Fitzpatrick R. Lateral stability, sensorimotor function and falls in older people. J Am Geriatr Soc. 1999;47:1077–1081. doi: 10.1111/j.1532-5415.1999.tb05230.x. [DOI] [PubMed] [Google Scholar]
- 31.Lovelace EA, Aikens JE. Vision, kinesthesis, and control of hand movement by young and old adults. Percept Mot Skills. 1990;70:1131–1137. doi: 10.2466/pms.1990.70.3c.1131. [DOI] [PubMed] [Google Scholar]
- 32.Lund H, Weile U, Christensen R, Rostock B, Downey A, Bartels EM, Danneskiold-Samsoe B, Bliddal H. A randomized controlled trial of aquatic and land-based exercise in patients with knee osteoarthritis. J Rehabil Med. 2008;40:137–144. doi: 10.2340/16501977-0134. [DOI] [PubMed] [Google Scholar]
- 33.Madhavan S, Shields RK. Influence of age on dynamic position sense: evidence using a sequential movement task. Exp Brain Res. 2005;164:18–28. doi: 10.1007/s00221-004-2208-3. [DOI] [PubMed] [Google Scholar]
- 34.McChesney JW, Woollacott MH. The effect of age-related declines in proprioception and total knee replacement on postural control. J Gerontol A Biol Sci Med Sci. 2000;55:M658–666. doi: 10.1093/gerona/55.11.m658. [DOI] [PubMed] [Google Scholar]
- 35.Messier J, Adamovich S, Berkinblit M, Tunik E, Poizner H. Influence of movement speed on accuracy and coordination of reaching movements to memorized targets in three-dimensional space in a deafferented subject. Exp Brain Res. 2003;150:399–416. doi: 10.1007/s00221-003-1413-9. [DOI] [PubMed] [Google Scholar]
- 36.ODriscoll SW, Horii E, Ness R, Cahalan TD, Richards RR, An KN. The relationship between wrist position, grasp size, and grip strength. J Hand Sur-American. 1992;17A:169–177. doi: 10.1016/0363-5023(92)90136-d. [DOI] [PubMed] [Google Scholar]
- 37.Przybyla A, Haaland KY, Bagesteiro LB, Sainburg RL. Motor asymmetry reduction in older adults. Neuros Lett. 2011;489:99–104. doi: 10.1016/j.neulet.2010.11.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rothwell JC, Traub MM, Day BL, Obeso JA, Thomas PK, Marsden CD. Manual motor-performance in a deafferented ban. Brain. 1982;105:515–542. doi: 10.1093/brain/105.3.515. [DOI] [PubMed] [Google Scholar]
- 39.Sainburg RL, Poizner H, Ghez C. Loss of proprioception produces deficits in interjoint coordination. J Neurophysiol. 1993;70:2136–2147. doi: 10.1152/jn.1993.70.5.2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stelmach GE, Sirica A. Aging and proprioception. Age. 1986;9:99–103. [Google Scholar]
- 41.Stewart AL, Grossman M, Bera N, Gillis DE, Sperber N, Castrillo M, Pruitt L, McLellan B, Milk M, Clayton K, Cassady D. Multilevel perspectives on diffusing a physical activity promotion program to reach diverse older adults. J Aging Phy Act. 2006;14:270–287. doi: 10.1123/japa.14.3.270. [DOI] [PubMed] [Google Scholar]
- 42.Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med Sci Sports Exerc. 2001;33:1126–1141. doi: 10.1097/00005768-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 43.Talelli P, Waddingham W, Ewas A, Rothwell JC, Ward NS. The effect of age on task-related modulation of interhemispheric balance. Exp Brain Res. 2008;186:59–66. doi: 10.1007/s00221-007-1205-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Teixeira LA. Categories of manual asymmetry and their variation with advancing age. Cortex. 2008;44:707–716. doi: 10.1016/j.cortex.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 45.Tombaugh TN. Trail Making Test A and B: Normative data stratified by age and education. Arch Clin Neuropsychol. 2004;19:203–214. doi: 10.1016/S0887-6177(03)00039-8. [DOI] [PubMed] [Google Scholar]
- 46.Verschueren SM, Brumagne S, Swinnen SP, Cordo PJ. The effect of aging on dynamic position sense at the ankle. Behav Brain Res. 2002;136:593–603. doi: 10.1016/s0166-4328(02)00224-3. [DOI] [PubMed] [Google Scholar]
- 47.Weiler HT, Awiszus F. Differences between motion-direction perception and unspecific motion perception in the human knee joint. Exp Brain Res. 2000;132:523–530. doi: 10.1007/s002210000378. [DOI] [PubMed] [Google Scholar]
- 48.Westlake KP, Wu YS, Culham EG. Sensory-specific balance training in older adults: Effect on position, movement, and velocity sense at the ankle. Phys Ther. 2007;87:560–568. doi: 10.2522/ptj.20060262. [DOI] [PubMed] [Google Scholar]
- 49.Xu D, Hong Y, Li J, Chan K. Effect of tai chi exercise on proprioception of ankle and knee joints in old people. British J of Sports Med. 2004;38:50–54. doi: 10.1136/bjsm.2002.003335. [DOI] [PMC free article] [PubMed] [Google Scholar]