Abstract
Microtubules are critical components of the cytoskeleton. Perturbing their function arrests the growth of a broad spectrum of cancer cell lines, making microtubules an excellent and established target for chemotherapy. All of the U.S. Food and Drug Administration-approved antitubulin agents bind to paclitaxel or vinblastine binding sites in tubulin. Because of the complexity of their structures, it is difficult to structurally modify the vinca alkaloids and taxanes and develop orally bioavailable agents. Antitubulin agents that target the colchicine-binding site in tubulin may provide a better opportunity to be developed for oral use because of their relatively simple structures and physicochemical properties. A potent antitubulin agent, 4-(3,4,5-trimethoxybenzoyl)-2-phenyl-thiazole (SMART-H), binding to the colchicine-binding site, was discovered in our laboratory. However, the bioavailability of SMART-H was low because of its poor solubility. Structural modification of SMART-H led to the development of 2-aryl-4-benzoyl-imidazole analog (ABI-274), with improved bioavailability and potency but still considerable first-pass metabolism. A chlorine derivative (ABI-286), replacing the methyl site of ABI-274, resulted in 1.5-fold higher metabolic stability in vitro and 1.8-fold lower clearance in rats in vivo, indicating that metabolic stability of ABI-274 can be extended by blocking benzylic hydroxylation. Overall, ABI-274 and ABI-286 provided 2.4- and 5.5-fold increases in exposure (area under the curve) after oral dosing in rats compared with SMART-H. Most importantly, the structural modifications did not compromise potency. ABI-286 exhibited moderate clearance, moderate volume of distribution, and acceptable oral bioavailability. This study provided the first evidence that ABI-286 may be the first member of a new class of orally bioavailable antitubulin agents.
Introduction
Microtubules are critical components of the cytoskeleton and play a pivotal role in spindle formation, cellular shape maintenance, and intracellular transportation (Jordan and Wilson, 2004; Jordan and Kamath, 2007). Because of their critical role in mitosis and cell division, microtubules are regarded as an excellent chemotherapeutic target to treat proliferative oncogenic disorders. Three unique small-molecule binding sites are known in tubulin and are responsible for the interaction and pharmacologic effect of paclitaxel, vinblastine, and colchicine (Nogales et al., 1998; Gigant et al., 2005). Numerous drugs that target microtubule function and bind to either the paclitaxel or vinca alkaloid binding site have been approved by the U.S. Food and Drug Administration for the treatment of cancer, including paclitaxel, docetaxel, ixabepilone, and vinca alkaloids such as vinblastine, vincristine, and vinorelbine. All of these exhibit high potency, but they are poorly soluble, require intravenous administration, and become less effective in tumors expressing drug efflux transporters. The search for potent antitubulin agents that are poor P-glycoprotein substrates has intensified (Bollag et al., 1995; Wagner et al., 1999; Sampath et al., 2003, 2006). However, most of these agents also suffer from poor oral bioavailability because of their high molecular weight and low permeability. Compared with compounds binding the paclitaxel or vinca alkaloid binding site, antitubulin agents that bind to the colchicine binding site usually exhibit relatively simple structures, thus providing a better opportunity for structural optimization as orally bioavailable agents. Although several anticancer agents that bind to the colchicine binding site are being developed, none of them are approved for clinical use (Lakhani et al., 2003; Rustin et al., 2010). A few such agents demonstrate potent tubulin inhibitory properties with a potential for oral use (Tahir et al., 2003; Hande et al., 2006; Liou et al., 2007). In addition, most of these drugs appear to circumvent P-glycoprotein-mediated drug resistance.
Oral bioavailability is a complex parameter involving many chemical and physiological processes, such as solubility, permeability, and metabolic stability. A potential orally bioavailable drug candidate is expected to have sufficient aqueous solubility to enable it to dissolve and be absorbed in the gastrointestinal tract. Permeability is another key component because high permeability is necessary for molecules to penetrate across the intestinal lumen into the systemic circulation (Tan et al., 2008). Low clearance is also desired to avoid hepatic first-pass metabolism (Li et al., 2006). When bioavailability is poor, strategies to address these three major factors must be considered to identify and overcome the major barriers to oral drug delivery.
Our group recently developed a series of 4-substituted methoxybenzoyl-aryl-thiazoles (SMART) that bind to the colchicine binding site and inhibit tubulin polymerization and cancer cell growth at low nanomolar concentrations (Lu et al., 2009). In addition, the SMART compounds circumvented P-glycoprotein-mediated drug resistance and retained potent anticancer activity in vitro and in vivo (Li et al., 2011). The SMART compounds demonstrated potency comparable to paclitaxel and exhibited favorable pharmacokinetic properties in rats and dogs when administered intravenously (Li et al., 2010). However, the bioavailability of 4-(3,4,5-trimethoxybenzoyl)-2-phenyl-thiazole (SMART-H) was poor because of its low solubility. In this study, we investigated the low bioavailability of SMART-H and proposed strategies, including enhancement of solubility and metabolic stability, to improve oral bioavailability. We identified a new series of 2-aryl-4-benzoyl-imidazoles (ABI) with improved aqueous solubility. ABI-182 demonstrated improved bioavailability as a result of replacing the thiazole with an imidazole ring and enhanced aqueous solubility. One of the most potent molecules in the series, ABI-274, was used as a lead to examine the metabolic stability of the ABI scaffold in human, mouse, rat, and dog liver microsomes. Three major metabolites were identified by liquid chromatography/tandem mass spectrometry (LC-MS/MS). ABI-286 was then designed and synthesized to improve metabolic stability on the basis of metabolite identification studies with ABI-274. In vivo pharmacokinetic studies were also performed and were in good agreement with in vitro metabolic stability. The optimized compound, ABI-286, demonstrated potent in vitro activity and an acceptable bioavailability after oral dosing. These studies shed light on the potential of ABI-286 as a potent and orally bioavailable tubulin inhibitor.
Materials and Methods
Cell Culture and Cytotoxicity Assay.
We examined the antiproliferative activity of the test compounds in a human PC-3 prostate cancer cell lines (American Type Culture Collection, Manassas, VA). Cells were cultured in RPMI 1640 (Cellgro Mediatech, Inc., Manassas, VA) medium supplemented with 10% fetal bovine serum (Cellgro Mediatech) and were maintained at 37°C in a humidified atmosphere containing 5% CO2. Depending on cell types, 3000 cells were plated into each well of 96-well plates and exposed to different concentrations of the compounds of interest for 96 h. At the end of the treatments, cell viability was measured using the sulforhodamine B assay. Percentage of cell survival was plotted against drug concentrations, and the IC50 values (concentration that inhibited cell growth by 50% of untreated control) were obtained by nonlinear regression analysis with SigmaPlot (Systat Software Inc., San Jose, CA) using the standard four-parameter logistic curve.
Metabolic Incubations.
Metabolic stability studies were conducted by incubating 0.5 μM of test compounds in a total reaction volume of 1 ml containing 1 mg/ml microsomal protein in reaction buffer [0.2 M of phosphate buffer solution (pH 7.4), 1.3 mM NADP+, 3.3 mM glucose 6-phosphate, and 0.4 U/ml glucose-6-phosphate dehydrogenase] at 37°C in a shaking water bath. Female rat liver microsomes and pooled human, mouse, and dog liver microsomes were used to examine metabolic stability. ABI-274, at a concentration of 50 μM was used for metabolite identification studies under the previously mentioned conditions. The NADPH regenerating system (solution A and B) was obtained from BD Biosciences (San Jose, CA). The total dimethyl sulfoxide (DMSO) concentration in the reaction solution was approximately 0.5% (v/v). Aliquots (100 μl) from the reaction mixtures used to determine metabolic stability were sampled at 5, 10, 20, 30, 60, and 90 min. Acetonitrile (150 μl) containing 200 nM of the internal standard was added to quench the reaction and to precipitate the proteins. Samples were then centrifuged at 4000g for 15 min at room temperature, and the supernatant was analyzed directly by LC-MS/MS.
Prediction of In Vivo Clearance of ABI-274 and ABI-286 in Rat and Human.
In vivo clearance was predicted using the data obtained from in vitro metabolic stability studies (i.e., half-life values in liver microsomes). The intrinsic hepatic clearance (Cli, in vitro) was determined using the equation Cli, in vitro = [0.693/(t1/2 × protein concentration)], where t1/2 is in minutes and protein concentration is in milligrams per milliliter. The intrinsic clearance was then scaled to predict clearance that would occur in the liver in vivo. Scaling factors [(mg protein/g liver) × (g liver/kg body weight)] were 2400 and 1980 for rat and human, respectively (Li et al., 2010). In vivo intrinsic hepatic clearance (Cli,h, ml · min−1 · kg−1 body weight) in liver was estimated by multiplying Cli, in vitro by the scaling factors. In vivo hepatic clearance (Clh) was estimated by incorporating estimates of Cli,h, and Qh into the well stirred model (venous equation): Clh = [Qh × Cli,h/(Qh + Cli,h)] (Chiba et al., 2009), where Qh represented hepatic blood flow.
Aqueous Solubility.
The solubility of drugs was determined by Multiscreen Solubility Filter Plate (Millipore Corporation, Billerica, MA) coupled with LC-MS/MS. In brief, 198 μl of phosphate-buffered saline buffer (pH 7.4) was loaded into a 96-well plate, and 2 μl of 10 mM test compounds (in DMSO) was dispensed and mixed with gentle shaking (200–300 rpm) for 1.5 h at room temperature (n = 3). The plate was centrifuged at 800g for 10 min, and the filtrate was used to determine its concentration and solubility of test compound by LC-MS/MS as described under Pharmacokinetic Study.
Pharmacokinetic Study.
Female Sprague-Dawley rats (n = 3 or 4; 254 ± 4 g) were purchased from Harlan (Indianapolis, IN). Rat thoracic jugular vein catheters were purchased from Braintree Scientific Inc. (Braintree, MA). On arrival at the animal facility, the animals were acclimated for 3 days in a temperature-controlled room (20–22°C) with a 12-h light/dark cycle before any treatment. All animals were fed before dosing and had access to water ad libitum. SMART-H is a crystalline white solid with a purity of 99% as determined by high-performance liquid chromatography. All ABI compounds are also solids with >95% purity. Differential scanning calorimetry studies showed that SMART-H, ABI-182, and ABI-286 are crystalline, whereas ABI-274 is amorphous. Dosing volumes for intravenous bolus and oral solutions were 2 and 4 ml/kg, respectively. SMART-H was administered intravenously into the thoracic jugular vein at a dose of 2.5 mg/kg (in DMSO/PEG300, 2:8, v/v), whereas ABI-182, ABI-274, and ABI-286 were dosed at 5 mg/kg (in DMSO/PEG300, 1:9, v/v). Catheters were flushed with 1 ml of heparinized saline after intravenous bolus. An equal volume of heparinized saline was injected to replace the removed blood, and blood samples (250 μl) were collected via the jugular vein catheter at 10, 20, and 30 min and 1, 2, 4, 8, 12, and 24 h. Area under the curve (AUC) values were calculated using the trapezoid rule and extrapolated backward from 10 min to time 0 on the basis of the half-life estimated in each animal to minimize the error associated with the delay between dosing and the first sampling time. SMART-H, ABI-182, and ABI-286 were also given by oral gavage at 10 mg/kg (in Tween80/DMSO/H2O, 2:1:7, v/v/v) to evaluate their oral bioavailability. ABI-274 (in DMSO/PEG300/H2O, 2:2:6, v/v/v) was given orally at a dose of 10 mg/kg. All blood samples (250 μl) after oral administration were collected via the jugular vein catheter at 30, 60, 90, 120, 150, 180, 210, and 240 min and 8, 12, and 24 h. Heparinized syringes and vials were prepared before blood collection. Plasma samples were prepared by centrifuging the blood samples at 8000g for 5 min. All plasma samples were stored immediately at −80°C until analyzed.
Analytes were extracted from 100 μl of plasma with 200 μl of acetonitrile containing 200 nM of the internal standard (Fig. 1). The samples were thoroughly mixed, centrifuged, and the organic extract was transferred to autosampler for LC-MS/MS analysis. Multiple reaction monitoring mode, scanning m/z 356 → 188 (SMART-H), m/z 339 → 171 (ABI-182), m/z 353 → 185 (ABI-274), m/z 373 → 205 (ABI-286), and m/z 309 → 171 (the internal standard), was used to obtain the most sensitive signals. The pharmacokinetic parameters were determined using noncompartmental analysis (WinNonlin; Pharsight Corporation, Mountain View, CA).
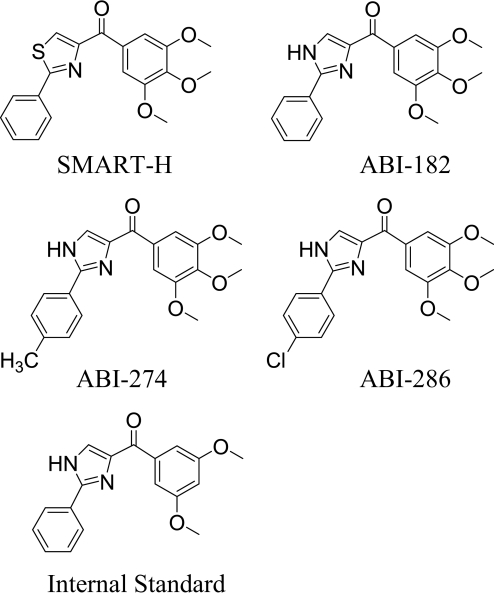
Fig. 1.
Chemical structures of SMART-H, ABI-182, ABI-274, ABT-286, and the internal standard.
Analytical Method.
Sample solution (10 μl) was injected into an Agilent series high-performance liquid chromatography system (Agilent 1100 Series Agilent 1100 Chemstation; Agilent Technologies, Santa Clara, CA). All analytes were separated on a narrow-bore C18 column (Alltech Alltima HP, 2.1 × 100 mm, 3 μm; Thermo Fisher Scientific, Waltham, MA). Two gradient modes were used. For metabolic stability studies, the gradient mode was used to achieve the separation of analytes using mixtures of mobile phase A [acetonitrile/H2O (5:95%, v/v) containing 0.1% formic acid] and mobile phase B [acetonitrile/H2O (95:5%, v/v) containing 0.1% formic acid] at a flow rate of 300 μl/min. Mobile phase A was used at 15% from 0 to 1 min, followed by a linearly programmed gradient to 100% of mobile phase B within 4 min; 100% of mobile phase B was maintained for 0.5 min before a quick ramp to 15% mobile phase A. Mobile phase A was continued for another 10 min toward the end of analysis. For metabolite identification studies of ABI-274, a slower gradient mode was used to achieve the separation of analytes by using the same flow rate and mobile phase A and B as described. Mobile phase A was used at 5% from 0 to 0.5 min, followed by a linearly programmed gradient to 100% of mobile phase B within 9.5 min; 100% of mobile phase B was maintained for 0.5 min before a quick ramp to 5% mobile phase A. Mobile phase A was continued for another 15 min toward the end of analysis.
A triple-quadruple mass spectrometer, API Qtrap 4000 (Applied Biosystems/MDS Sciex Foster City, CA), operating with a TurboIonSpray source was used. The spraying needle voltage was set at 5 kV for positive mode. Curtain gas was set at 10 psi; gas 1 and gas 2 were set at 50 psi. Collision-assisted-dissociation gas was set at medium, and the source heater probe temperature was set at 500°C. Data acquisition and quantitative processing were accomplished using Analyst software, version 1.4.2 (Applied Biosystems).
Results
SMART-H Exhibits Favorable Pharmacokinetic Properties, but Low Bioavailability.
Previous studies (Li et al., 2010, 2011) showed that SMART-H (Fig. 1) demonstrates potent in vitro and in vivo antiproliferative activity. SMART-H inhibited the proliferation of various cancer cells in vitro with subnanomolar IC50 and in vivo, in nude mice xenografts, with near 100% tumor growth inhibition. In addition, SMART-H exhibited favorable pharmacokinetic properties in rats and dogs, including low clearance, moderate volume of distribution, and long half-life values. SMART-H was predicted to have low clearance in humans on the basis of the in vitro-in vivo correlation model developed in our laboratory. Herein, bioavailability was tested to determine the oral bioavailability of SMART-H. Pharmacokinetic parameters of SMART-H in female rats are summarized in Table 1. The pharmacokinetic parameters for SMART-H in female rats in this study were similar to the pharmacokinetic parameters previously reported for male rats (Li et al., 2010). The oral bioavailability of SMART-H was extremely low, averaging only 3.3% at a dose of 10 mg/kg. The aqueous solubility of SMART-H was also low (1.1 ± 0.1 μg/ml; Table 2), suggesting that the poor oral bioavailability of SMART-H may due to low solubility.
TABLE 1.
Pharmacokinetic parameters of SMART-H, ABI-182, ABI-274, and ABI-286 in rats
| Parameter | Unit | SMART-H |
ABI-182 |
ABI-274 |
ABI-286 |
||||
|---|---|---|---|---|---|---|---|---|---|
| IV | PO | IV | PO | IV | PO | IV | PO | ||
| n | 4 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | |
| Dose | mg/kg | 2.5 | 10 | 5 | 10 | 5 | 10 | 5 | 10 |
| Cl | ml · min−1 · kg−1 | 7.7 ± 1.0 | N.R. | 16 ± 0.2 | N.R. | 29 ± 4 | N.R. | 16 ± 3 | N.R. |
| Vss | l/kg | 4.9 ± 1.9 | N.R. | 1.1 ± 0.2 | N.R. | 2.1 ± 0.5 | N.R. | 1.9 ± 0.2 | N.R. |
| t1/2 | min | 925 ± 115 | N.R. | 1088 ± 267 | N.R. | 703 ± 341 | N.R. | 326 ± 190 | N.R. |
| AUC | min · μg/ml | 279 ± 53 | 37 ± 20 | 306 ± 3 | 146 ± 12 | 172 ± 24 | 87 ± 53 | 321 ± 77 | 205 ± 43 |
| Cmax | ng/ml | 3816 ± 509 | 212 ± 65 | 3877 ± 124 | 1181 ± 150 | 2494 ± 272 | 861 ± 1053 | 2519 ± 231 | 1088 ± 403 |
| Apparent Tmax | min | 10 | 60 | 10 | 30 | 10 | 30 | 10 | 60 |
| F | % | 3.3 | 24 | 25 | 32 | ||||
Cl, clearance; N.R., not reported; IV, intravenous; PO, oral.
TABLE 2.
Cytotoxicity (n = 3, mean ± S.E.) in PC-3 cancer cell line and aqueous solubility (n = 3, mean ± S.D.) of SMART-H, ABI-182, ABI-274, and ABI-286
| IC50 Value in PC-3 Cell Line | Aqueous Solubility | |
|---|---|---|
| nM | μg/ml | |
| SMART-H | 21 ± 1a | 1.1 ± 0.1 |
| ABI-182 | 288 ± 30b | >30 |
| ABI-274 | 8.7 ± 0.4b | >30 |
| ABI-286 | 35 ± 1b | 12 ± 2 |
Previously reported in Lu et al., 2009.
We considered three major barriers that may contribute to the low oral bioavailability of SMART-H, including aqueous solubility, permeability, and metabolic stability. SMART-H demonstrated low systemic plasma clearance after intravenous dosing, suggesting that hepatic first-pass metabolism was not a major factor that would limit oral bioavailability. Permeability studies using Caco-2 cells confirmed that SMART-H has excellent permeability because the Papp value was 33 × 10−6 cm/s (A→B) compared with the positive control, propranolol (Papp 12 × 10−6 cm/s) (data not shown). The aqueous solubility of SMART-H was low (1.1 μg/ml). Therefore, SMART-H qualifies as a Biopharmaceutics Classification System class II compound, which exhibits low solubility and high permeability. Overall, these data suggested that the low solubility of SMART-H contributed to its limited absorption.
ABI-182 and ABI-274 Demonstrates That Improved Solubility Results in Improvement of Oral Bioavailability.
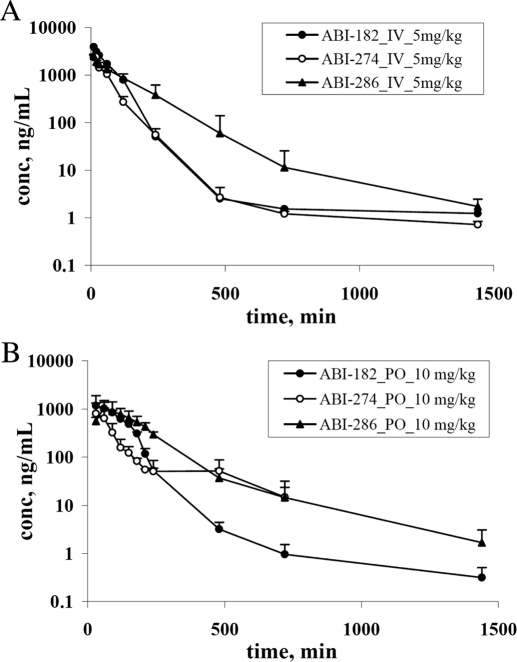
We structurally modified the thiazole moiety of the SMART compounds to an imidazole in an attempt to improve solubility. ABI-182 (Fig. 1), an imidazole derivative, exhibited greatly improved solubility (>30 μg/ml) compared with SMART-H and was selected for pharmacokinetic studies. Figure 2 shows the concentration-time profile of ABI-182 in rats, exhibiting a moderate volume of distribution (1.1 l/kg), but higher systemic plasma clearance (16 ml · min−1 · kg−1) (Table 1). This structural modification resulted in a 4-fold higher systemic exposure (AUC) of ABI-182 compared with SMART-H when administered orally. The oral bioavailability dramatically increased to 24% (ABI-182) compared with SMART-H (3.3%), suggesting that aqueous solubility was crucial for absorption (Table 1).
Fig. 2.
Pharmacokinetics of ABI-182, ABI-274, and ABI-286. Female Sprague-Dawley rats (n = 3) were dosed with 5 mg/kg by intravenous administration with formulation DMSO/PEG300 (1/9). A, Sprague-Dawley rats (n = 3) were dosed with 10 mg/kg by oral administration of ABI-274 [in DMSO/PEG300/H2O (2/2/6)], ABI-182, and ABI-286 [in Tween 80/DMSO/H2O (2:1:7)] B, bar, S.D.
Although ABI-182 demonstrated acceptable bioavailability, it demonstrated 10-fold less in vitro potency in the PC-3 cancer cell line (Table 2) compared with SMART-H. ABI-274 was identified as a promising compound in our previous structure activity relationship studies with this class of compounds (Chen et al., 2010). ABI-274 with imidazole ring also exhibited increased solubility and improved systemic exposure and bioavailability (Fig. 2; Table 1) compared with SMART-H.
Benzylic Hydroxylation and O-Demethylation Are the Major Metabolic Pathways for ABI-274.
It was important to note that the systemic plasma clearance of ABI-274 was over 3 times greater than that observed for SMART-H (Table 1), suggesting that its systemic exposure and bioavailability were limited because of first-pass hepatic metabolism. We performed in vitro metabolism studies to identify the metabolites and labile sites of ABI-274. Table 3 shows the metabolic stability (half-lives) of ABI-274 in human, mouse, rat, and dog liver microsomes. ABI-274 had comparable metabolic stability in human, rat, and dog liver microsomes, but it was more quickly metabolized in mouse liver microsomes. Because these incubations did not include UDP-glucuronic acid, these data suggest that phase I metabolism plays a significant role in ABI-274 metabolism.
TABLE 3.
In vitro metabolic stability
The percentage of drug remaining was determined by LC-MS/MS. Metabolic stability is presented as half-lives in liver microsomes. Incubations were conducted with 0.5 μM test compounds and 1 mg/ml liver microsomal proteins in the presence of NADPH at 37°C (n = 3, mean ± S.D.).
| Liver Microsomes | Half-Life (min) |
|
|---|---|---|
| ABI-274 | ABI-286 | |
| Human | 21 ± 1 | 44 ± 6 |
| Mouse | 9 ± 1 | 23 ± 2 |
| Rat | 30 ± 2 | 46 ± 4 |
| Dog | 20 ± 3 | 26 ± 2 |
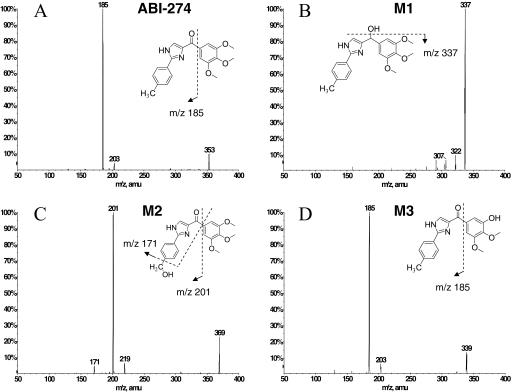
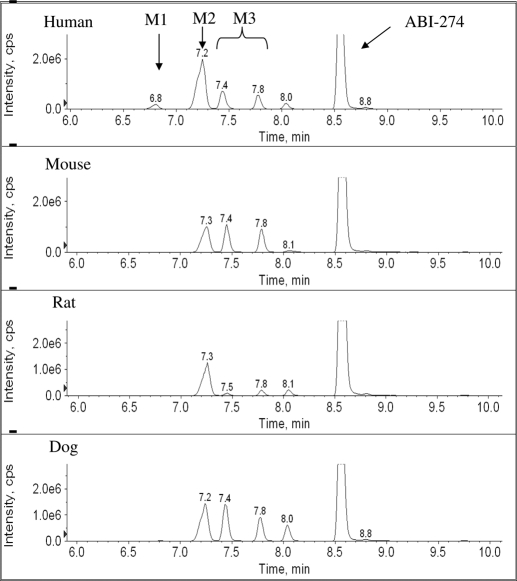
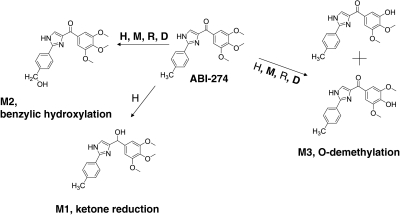
In vitro metabolite identification studies were performed using ABI-274 (50 μM) and liver microsomes (1 mg/ml) from four species (human, mouse, rat, and dog) to obtain its metabolite profile and to determine metabolically labile sites (i.e., soft spots) in the ABI-274 pharmacophore. LC-MS/MS was used for proposed metabolite identification based on mass shifts compared with the molecular ion [M+H]+ of ABI-274 and retention time shifts to the parent ABI-274. To identify the potential metabolites, the product ion scan for each peak of interest was examined to obtain structural information. Three major metabolites of ABI-274 were identified in human liver microsomes. The product ion spectrum of ABI-274 (Fig. 3A) has an abundant product ion m/z 185 (loss of trimethoxy benzene). The metabolite, M1 (m/z 355, mass shifts + 2 Da, ketone reduction) (Fig. 3B), resulted in the fragment ion m/z 337 (loss of H2O). The major and the most abundant metabolite in all species, M2 (m/z 369, mass shifts + 16 Da, benzylic hydroxylation) (Fig. 3C), has an abundant product ion m/z 201 (loss of trimethoxy benzene) and a minor product ion m/z 171 (loss of trimethoxy benzene and CH2OH). M3 (m/z 339, O-demethylation) (Fig. 3D) was present as either the 3- or 4-demethylated metabolite of ABI-274. The mass spectrum could not distinguish between these metabolites because they exhibit identical precursor ion and the same pattern of product ions. Figure 4 shows the multiple-reaction-monitoring chromatography of ABI-274 with its three metabolites (M1–M3) after the 1-h incubation of ABI-274 (50 μM) in liver microsomes from four different species. Because of higher polarity, all of the metabolites had shorter retention times than ABI-274. M1 (with a retention time of 6.8 min) was only found in human liver microsomes (Fig. 4), a result consistent with our previous study (Li et al., 2010). However, it was not the major metabolite in human liver microsomes in this case. M2 (with the retention time at 7.2 or 7.3 min) was the major metabolite in all species. M3 [with the retention time of (7.4 or 7.5) or 7.8 min] presented as positional isomers and could be identified in all species, but with different relative proportions of the 3- or 4-desmethyl metabolite between species. The isomers (M3) were separated based on the gradient chromatographic condition. However, we were unable to determine which of the methoxy groups was demethylated. The peak eluted at 8.0 to 8.1 was another hydroxylated metabolite; however, we were unable to determine the hydroxylation site. The overall proposed metabolic pathway for ABI-274 in four species is summarized in Fig. 5. Ketone reduction (M1) was apparently unique to human liver microsomes, whereas benzylic hydroxylation (M2) was the major pathway for all four species. O-demethylation (M3) was also a major pathway in mouse and dog, but not in human and rat liver microsomes. Overall, these studies suggest that benzylic hydroxylation and O-demethylation were the two predominant mechanisms for ABI-274 metabolism.
Fig. 3.
Proposed metabolites of ABI-274 in human liver microsomes. The parent, ABI-274 (A); M1, ketone reduction (B); M2, benzylic hydroxylation (C); and M3, O-demethylation (D).
Fig. 4.
Chromatography of ABI-274 and its metabolites. Fifty micromoles of ABI-274 was incubated with 1 mg/ml microsomal proteins for 1 h at 37°C. Metabolic profile was conducted in human, mouse, rat, and dog liver microsomes. Peak eluted at 8.8 min was spiked internal standard.
Fig. 5.
Proposed metabolites and metabolic pathway of ABI-274 in different species. H, M, R, and D represent human, mouse, rat, and dog liver microsomes, respectively, and bold font indicates the major metabolites.
Blocking Benzylic Hydroxylation Increased the Metabolic Stability of ABI-286 in Liver Microsomes.
The two major metabolites of ABI-274 in liver microsomes, M2 (benzylic hydroxylation) and M3 (O-demethylation), indicated the methyl and methoxy groups as the most labile (i.e., soft spots) to metabolism. We thus designed ABI-286 (Fig. 1) to incorporate substitutions aimed at improving the metabolic stability of these metabolic soft spots. ABI-286 was designed with a 4-chloro phenyl substituent attached to the imidazole ring to block benzylic hydroxylation. Metabolic stability studies with ABI-286 indicated that blocking benzylic hydroxylation successfully improved the stability in human, mouse, and rat liver microsomes compared with ABI-274 (Table 3). In dog liver microsomes, metabolic stability was slightly increased, but not as much as observed in the other species. The metabolites of ABI-286 were identified and showed that the hydroxylation peak was abolished in all species, confirming that the methyl substituent was the major labile site (data not shown). Most importantly, structural modification in ABI-286 exhibited similar potency but 12-fold higher solubility compared with SMART-H (Table 2).
Structural Changes in ABI-286 Improved Systemic Exposure and Oral Bioavailability.
Rat pharmacokinetic studies were performed to study whether ABI-286 exhibits reduced systemic clearance and increased exposure as suggested by the increase in in vitro metabolic stability compared with ABI-274. In Fig. 2A, the plasma concentration-time curve of ABI-286 is shown and compared with ABI-274 by intravenous administration. ABI-286 was eliminated more slowly than ABI-274. The systemic plasma clearance of ABI-286 was 16 ml · min−1 · kg−1, which was 1.8-fold less than ABI-274 (29 ml · min−1 · kg−1) (Table 1). ABI-274 and ABI-286 exhibited similar volumes of distributions (1.9 and 2.1 l/kg, respectively). Table 4 summarizes the predicted clearance values based on in vitro metabolic stability and scaling methods (described under Materials and Methods). The result suggested that in vivo clearance correlated well with the data from in vitro metabolic stability studies conducted using rat liver microsomes, suggesting that the liver microsome system was an excellent tool to facilitate pharmacokinetic optimization. The predicted in vivo clearance values in human are also shown in Table 4. Next, we determined the oral bioavailability of ABI-286. The plasma concentration-time curve of ABI-286 is shown in Fig. 2B. The data clearly show that ABI-286 exhibited more than 2-fold increased exposure (AUC) by the oral route as compared with ABI-274. Overall, by oral administration, the exposure (AUC) of ABI-286 was 2.4- and 5.5-fold higher than ABI-274 and SMART-H, respectively. The maximal concentration (Cmax) and bioavailability of ABI-286 were 1088 ng/ml and 32%, respectively (Table 1).
TABLE 4.
Prediction of in vivo hepatic clearance of ABI-274 and ABI-286 in rat and human from in vitro metabolic stability
| Rat ABI-274 | Rat ABI-286 | Human ABI-274 | Human ABI-286 | |
|---|---|---|---|---|
| Hepatic blood flow (ml · min−1 · kg−1) | 55 | 55 | 21 | 21 |
| t1/2, in liver microsomes, min | 30 | 46 | 21 | 44 |
| SF | 2400 | 2400 | 1980 | 1980 |
| SF (mg protein/g liver · g liver/kg b.wt.) | (54 · 45) | (54 · 45) | (77 · 25.7) | (77 · 25.7) |
| Cli, in vitro | 0.023 | 0.015 | 0.033 | 0.016 |
| Cli, h | 55.44 | 36.16 | 65.34 | 31.19 |
| Clh, ml · min−1 · kg−1 (predicted) | 28 | 22 | 16 | 13 |
| Clin vivo | 29 | 16 | N.A. | N.A. |
N.A., not available; SF, scaling factor.
Discussion
The studies and compounds reported in this paper were designed in an attempt to improve the aqueous solubility, metabolic stability, and oral bioavailability of a novel series of thiazoles and imidazoles with potent anticancer activity.
Studies with ABI-182 demonstrated a proof of concept that enhanced aqueous solubility resulted in improved oral bioavailability (3.3 versus 24% for SMART-H and ABI-182, respectively). However, ABI-182 exhibited approximately 11-fold less potency than SMART-H. ABI-274 (a 4-methyl phenyl imidazole derivative) exhibited higher aqueous solubility and greater potency and was selected for further characterization. Pharmacokinetic studies of ABI-274 in rats revealed that ABI-274 had higher clearance (29 ml · min−1 · kg−1) than SMART-H (7.7 ml · min−1 · kg−1). High systemic clearance usually leads to limited oral bioavailability and exposure (AUC) because of first-pass hepatic metabolism. Metabolite identification provided a metabolic perspective to guide synthetic modification and enhance in vivo metabolic stability. It is interesting to note that metabolite identification studies of ABI-274 showed that ketone reduction was only found in human liver microsomes, a finding consistent with our previous study (Li et al., 2010). However, it was not the major metabolic pathway for ABI-274 as was the case for SMART-H. Benzylic hydroxylation was identified as a major in vitro metabolic pathway in all four species (human, mouse, rat and dog), whereas the extent of O-demethylation varied between species, suggesting that preventing or slowing these processes may improve metabolic stability in all species. Therefore, ABI-286 was designed and synthesized with a 4-chloro substituent replacing the 4-methyl substituent of ABI-274 to block benzylic hydroxylation and demonstrated improved metabolic stability in liver microsomes of the four species. In addition, ABI-286 did not generate new metabolites in human liver microsomes (data not shown). We also examined the pharmacokinetics of ABI-286 in rats and showed that in vivo disposition data correlated well with metabolic stability determined using in vitro liver microsome data. These results confirmed that liver microsome systems provide an accurate and facile method to predict systemic clearance in animals. Similar results were obtained by oral administration. The exposure (AUC) of ABI-286 was more than twice that observed for ABI-274, demonstrating that hepatic first-pass metabolism was less for ABI-286 because of reduced benzylic hydroxylation.
Optimization of pharmacokinetic properties is a key to the further improvement of an identified lead compound. An ideal orally bioavailable candidate should exhibit acceptable drug-like properties, including solubility, permeability, and metabolic stability (Vacca et al., 1994 Stegemann et al., 2007). Liver microsomes, which contain several key enzymes such as cytochromes P450, flavin monooxygenases, and glucuronosyltransferases required for drug metabolism, are used for in vitro metabolic stability studies. Liver microsome systems are also useful tools to perform metabolite identification studies (Watt et al., 2003; Baranczewski et al., 2006). There are several ways to enhance metabolic stability during molecular design (Humphrey and Smith, 1992). In general, metabolic stability may be improved by introduction of a more stable functional group. For example, introduction of a halogen to prevent benzylic or allylic hydroxylation is commonly attempted (Palani et al., 2002). Fortunately, this strategy was successfully applied to the optimization of the SMART and ABI scaffolds. However, the optimization process sometimes provides unexpected results. Structural modifications at one labile site may result in an increase in the rate of metabolism at another position in the molecule, a phenomenon known as “metabolic switching.” Thus, the lead optimization process for solving a metabolic stability problem is often iterative and time-consuming.
This study showed that the solubility-limited bioavailability (3.3%) of SMART-H could be improved by replacing the thiazole linker with an imidazole and protecting the phenyl ring from oxidation. Importantly, the refined compounds maintained potent anticancer activity. The optimized compound, ABI-286, showed increased metabolic stability and improved oral bioavailability, albeit with less in vitro potency than ABI-274 and similar to SMART-H. Future studies will examine the influence of other substitutions at the 4-phenyl position to further improve the pharmacokinetic properties of ABI compounds and gain a more comprehensive understanding of their in vivo disposition and potential as anticancer agents. Suitable formulations and salt forms of ABI-286 will be also tested in an attempt to further enhance the oral bioavailability of the ABI compounds.
Acknowledgments
We thank Terrence A. Costello, Katie N. Kail, and Stacey L. Barnett for providing technical support for animal studies at GTx Inc. We also thank Dr. Tai Ahn for helping with the differential scanning calorimetry studies.
This work was supported in part by the National Institutes of Health National Cancer Institute [Grant R01-CA148706-01A1].
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
doi:10.1124/dmd.110.036616.
- SMART
- 4-substituted methoxybenzoyl-aryl-thiazoles
- SMART-H
- 4-(3,4,5-trimethoxybenzoyl)-2-phenyl-thiazole
- ABI
- 2-aryl-4-benzoyl-imidazoles
- AUC
- area under the curve
- LC-MS/MS
- liquid chromatography-tandem mass spectrometry
- DMSO
- dimethyl sulfoxide
- PEG300
- polyethylene glycol 300.
Authorship Contributions
Participated in research design: C.M. Li, Chen, Lu, Narayanan, W. Li, Miller, and Dalton.
Conducted experiments: C.M. Li, Chen, Lu, Narayanan, and Parke.
Contributed new reagents or analytic tools: C.M. Li, Chen, Lu, W. Li, Ahn, and Miller.
Performed data analysis: C.M. Li and Dalton.
Wrote or contributed to the writing of the manuscript: C.M. Li.
Other: Dalton edited and finalized the written manuscript.
References
- Baranczewski P, Stańczak A, Kautiainen A, Sandin P, Edlund PO. (2006) Introduction to early in vitro identification of metabolites of new chemical entities in drug discovery and development. Pharmacol Rep 58:341–352 [PubMed] [Google Scholar]
- Bollag DM, McQueney PA, Zhu J, Hensens O, Koupal L, Liesch J, Goetz M, Lazarides E, Woods CM. (1995) Epothilones, a new class of microtubule-stabilizing agents with a taxol-like mechanism of action. Cancer Res 55:2325–2333 [PubMed] [Google Scholar]
- Chen J, Wang Z, Li CM, Lu Y, Vaddady PK, Meibohm B, Dalton JT, Miller DD, Li W. (2010) Discovery of novel 2-aryl-4-benzoyl-imidazoles targeting the colchicines binding site in tubulin as potential anticancer agents. J Med Chem 53:7414–7427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba M, Ishii Y, Sugiyama Y. (2009) Prediction of hepatic clearance in human from in vitro data for successful drug development. AAPS J 11:262–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigant B, Wang C, Ravelli RB, Roussi F, Steinmetz MO, Curmi PA, Sobel A, Knossow M. (2005) Structural basis for the regulation of tubulin by vinblastine. Nature 435:519–522 [DOI] [PubMed] [Google Scholar]
- Hande KR, Hagey A, Berlin J, Cai Y, Meek K, Kobayashi H, Lockhart AC, Medina D, Sosman J, Gordon GB, et al. (2006) The pharmacokinetics and safety of ABT-751, a novel, orally bioavailable sulfonamide antimitotic agent: results of a phase 1 study. Clin Cancer Res 12:2834–2840 [DOI] [PubMed] [Google Scholar]
- Humphrey MJ, Smith DA. (1992) Role of metabolism and pharmacokinetic studies in the discovery of new drugs–present and future perspectives. Xenobiotica 22:743–755 [DOI] [PubMed] [Google Scholar]
- Jordan MA, Kamath K. (2007) How do microtubule-targeted drugs work? An overview. Curr Cancer Drug Targets 7:730–742 [DOI] [PubMed] [Google Scholar]
- Jordan MA, Wilson L. (2004) Microtubules as a target for anticancer drugs. Nat Rev Cancer 4:253–265 [DOI] [PubMed] [Google Scholar]
- Lakhani NJ, Sarkar MA, Venitz J, Figg WD. (2003) 2-Methoxyestradiol, a promising anticancer agent. Pharmacotherapy 23:165–172 [DOI] [PubMed] [Google Scholar]
- Li CM, Lu Y, Narayanan R, Miller DD, Dalton JT. (2010) Drug metabolism and pharmacokinetics of 4-substituted methoxybenzoyl-aryl-thiazole (SMART). Drug Metab Dispos 38:2032–2039 [DOI] [PubMed] [Google Scholar]
- Li CM, Wang Z, Lu Y, Ahn S, Narayanan R, Kearbey JD, Parke DN, Li W, Miller DD, Dalton JT. (2011) Biological activity of 4-substituted methoxybenzoyl-aryl-thiazole: an active microtubule inhibitor. Cancer Res 71:216–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HL, Zhang WD, Zhang C, Liu RH, Wang XW, Wang XL, Zhu JB, Chen CL. (2006) Bioavailability and pharmacokinetics of four active alkaloids of traditional Chinese medicine Yanhuanglian in rats following intravenous and oral administration. J Pharm Biomed Anal 41:1342–1346 [DOI] [PubMed] [Google Scholar]
- Liou JP, Hsu KS, Kuo CC, Chang CY, Chang JY. (2007) A novel oral indoline-sulfonamide agent, N-[1-(4-methoxybenzenesulfonyl)-2,3-dihydro-1H-indol-7-yl]-isonicotinamide (J30), exhibits potent activity against human cancer cells in vitro and in vivo through the disruption of microtubule. J Pharmacol Exp Ther 323:398–405 [DOI] [PubMed] [Google Scholar]
- Lu Y, Li CM, Wang Z, Ross CR, 2nd, Chen J, Dalton JT, Li W, Miller DD. (2009) Discovery of 4-substituted methoxybenzoyl-aryl-thiazole as novel anticancer agents: synthesis, biological evaluation, and structure-activity relationships. J Med Chem 52:1701–1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogales E, Wolf SG, Downing KH. (1998) Structure of the alpha beta tubulin dimer by electron crystallography. Nature 391:199–203 [DOI] [PubMed] [Google Scholar]
- Palani A, Shapiro S, Josien H, Bara T, Clader JW, Greenlee WJ, Cox K, Strizki JM, Baroudy BM. (2002) Synthesis, SAR, and biological evaluation of oximino-piperidino-piperidine amides. 1. Orally bioavailable CCR5 receptor antagonists with potent anti-HIV activity. J Med Chem 45:3143–3160 [DOI] [PubMed] [Google Scholar]
- Rustin GJ, Shreeves G, Nathan PD, Gaya A, Ganesan TS, Wang D, Boxall J, Poupard L, Chaplin DJ, Stratford MR, et al. (2010) Phase Ib trial of CA4P (combretastatin A-4 phosphate), carboplatin, and paclitaxel in patients with advanced cancer. Br J Cancer 102:1355–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath D, Discafani CM, Loganzo F, Beyer C, Liu H, Tan X, Musto S, Annable T, Gallagher P, Rios C, et al. (2003) MAC-321, a novel taxane with greater efficacy than paclitaxel and docetaxel in vitro and in vivo. Mol Cancer Ther 2:873–884 [PubMed] [Google Scholar]
- Sampath D, Greenberger LM, Beyer C, Hari M, Liu H, Baxter M, Yang S, Rios C, Discafani C. (2006) Preclinical pharmacologic evaluation of MST-997, an orally active taxane with superior in vitro and in vivo efficacy in paclitaxel- and docetaxel-resistant tumor models. Clin Cancer Res 12:3459–3469 [DOI] [PubMed] [Google Scholar]
- Stegemann S, Leveiller F, Franchi D, de Jong H, Lindén H. (2007) When poor solubility becomes an issue: from early stage to proof of concept. Eur J Pharm Sci 31:249–261 [DOI] [PubMed] [Google Scholar]
- Tahir SK, Nukkala MA, Zielinski Mozny NA, Credo RB, Warner RB, Li Q, Woods KW, Claiborne A, Gwaltney SL, 2nd, Frost DJ, et al. (2003) Biological activity of A-289099: an orally active tubulin-binding indolyloxazoline derivative. Mol Cancer Ther 2:227–233 [PubMed] [Google Scholar]
- Tan W, Chen H, Zhao J, Hu J, Li Y. (2008) A study of intestinal absorption of bicyclol in rats: active efflux transport and metabolism as causes of its poor bioavailability. J Pharm Pharm Sci 11:97–105 [DOI] [PubMed] [Google Scholar]
- Vacca JP, Dorsey BD, Schleif WA, Levin RB, McDaniel SL, Darke PL, Zugay J, Quintero JC, Blahy OM, Roth E. (1994) L-735,524: an orally bioavailable human immunodeficiency virus type 1 protease inhibitor. Proc Natl Acad Sci USA 91:4096–4100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner MM, Paul DC, Shih C, Jordan MA, Wilson L, Williams DC. (1999) In vitro pharmacology of cryptophycin 52 (LY355703) in human tumor cell lines. Cancer Chemother Pharmacol 43:115–125 [DOI] [PubMed] [Google Scholar]
- Watt AP, Mortishire-Smith RJ, Gerhard U, Thomas SR. (2003) Metabolite identification in drug discovery. Curr Opin Drug Discov Devel 6:57–65 [PubMed] [Google Scholar]