Abstract
K2, a synthetic cannabinoid (SC), is an emerging drug of abuse touted as “legal marijuana” and marketed to young teens and first-time drug users. Symptoms associated with K2 use include extreme agitation, syncope, tachycardia, and visual and auditory hallucinations. One major challenge to clinicians is the lack of clinical, pharmacological, and metabolic information for the detection and characterization of K2 and its metabolites in human samples. Information on the metabolic pathway of SCs is very limited. However, previous reports have shown the metabolites of these compounds are excreted primarily as glucuronic acid conjugates. Based on this information, this study evaluates nine human recombinant uridine diphosphate-glucuronosyltransferase (UGT) isoforms and human liver and intestinal microsomes for their ability to glucuronidate hydroxylated metabolites of 1-naphthalenyl-1(1-pentyl-1H-indol-3-yl)-methanone (JWH-018) and (1-butyl-1H-indol-3-yl)-1-naphthalenyl-methanone (JWH-073), the two most common SCs found in K2 products. Conjugates were identified and characterized using liquid chromatography/tandem mass spectrometry, whereas kinetic parameters were quantified using high-performance liquid chromatography-UV-visible methods. UGT1A1, UGT1A3, UGT1A9, UGT1A10, and UGT2B7 were shown to be the major enzymes involved, showing relatively high affinity with Km ranging from 12 to 18 μM for some hydroxylated K2s. These UGTs also exhibited a high metabolic capacity for these compounds, which indicates that K2 metabolites may be rapidly glucuronidated and eliminated from the body. Studies of K2 metabolites will help future development and validation of a specific assay for K2 and its metabolites and will allow researchers to fully explore their pharmacological actions.
Introduction
Cannabis sativa, or marijuana, has been used medicinally and recreationally for centuries, and today, it remains one of the most abused drugs in the world (Tims et al., 2002; EMCDDA, 2009). The principal active and addictive constituent of marijuana, Δ-9-tetrahydrocannabinol (Δ9-THC), induces its psychoactive properties through agonistic interaction with cannabinoid CB1 receptors, which are located primarily in the central nervous system (Herkenham et al., 1990; Ishac et al., 1996). Since Gaoni and Mechoulam (1964) first discovered Δ9-THC in 1964 as the primary psychoactive ingredient in marijuana, many other classical and nonclassical cannabinoids have been investigated for therapeutic use. It is generally thought that cannabinoid-based drugs offer great promise for the treatment of epilepsy, inflammation, cancer, anxiety, neurodegeneration, depression, and osteoporosis (Lee et al., 2009).
Amnioakylindoles (AAIs) such as 1-naphthalenyl-1(1-pentyl-1H-indol-3-yl)-methanone (JWH-018) and (1-butyl-1H-indol-3-yl)-1-naphthalenyl-methanone (JWH-073) are synthetic cannabinoids that have recently emerged as new drugs of abuse being touted as “legal” marijuana and sold under brand names such as K2 and SPICE. These products are typically vegetative material laced with these potent CB-receptor agonists, and recent clinical data provide evidence of development of anxiety (Schneir et al., 2011), tolerance (Zimmermann et al., 2009), and enhancement of psychoses (Every-Palmer, 2010) in individuals using these products. There is also an evolving body of information available on the metabolism of synthetic cannabinoids, JWH-018 and JWH-073, in body fluids (Moller et al., 2010; Sobolevsky et al., 2010; Beuck et al., 2011; Grigoryev et al., 2011; Moran et al., 2011) and in vitro (Wintermeyer et al., 2010). Continued characterization of these metabolic pathways is important because recent studies have shown that oxidized products of JWH-018 retain significant in vitro and in vivo activity (L. K. Brents, E. E. Reichard, S. M. Zimmerman, J. H. Moran, W. E. Fantegrossi, and P. L. Prather, unpublished data). The unique characteristics of K2-AAIs relative to Δ9-THC, coupled with a virtual lack of information concerning K2 metabolism, pharmacology, or toxicology, suggests that a critical need exists to understand the metabolic profile of K2-AAIs. In vitro metabolic studies are of fundamental importance in the study of the metabolism of drugs and endobiotics. Without this information, the toxicity and pharmacokinetics of these compounds cannot be fully understood.
Oxidative metabolism (Phase I) often serves to functionalize compounds for subsequent conjugation reactions (Phase II), and this process generally terminates biological activity and facilitates excretion. Whereas as many as 10 different K2-AAIs are reported to be present in various K2 preparations, the two commonly observed derivatives are JWH-018 and JWH-073 (Vardakou et al., 2010). There are indications that both Phase I and Phase II are involved in the metabolism of these two compounds in vivo (Sobolevsky et al., 2010; Wintermeyer et al., 2010; Moran et al., 2011).
In this study, we investigated and characterized the glucuronidation of JWH-018, JWH-073, and several hydroxylated products of each (Fig. 1) using human hepatic and intestinal microsomes and 12 human recombinant UGTs. Data show that primary hydroxylated metabolites excreted in human urine are substrates for both hepatic and extrahepatic UGTs. Mass spectrometric analysis of the product mixtures of the hydroxylated and carboxylated metabolites of JWH-018 and JWH-073 identified and confirmed the presence of glucuronide conjugates. Comparison of reactions and evaluation of active sites available for glucuronidation suggests that glucuronidation is occurring on either the hydroxyl group of the indole ring or the hydroxyl or carboxyl terminus of the alkyl side chain.
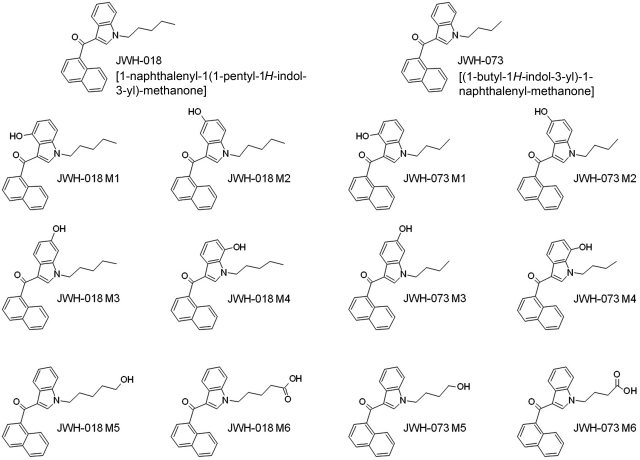
Fig. 1.
Structures of compounds. JWH-018 and JWH-073 differ only in the length of the alkyl side chain attached to the indole ring. The hydroxylated metabolites shown are as follows: JWH-018 M1 [(4-hydroxyl-1-pentyl-1H-indol-3-yl)(napthalen-1-yl)methanone], JWH-018 M2 [(5-hydroxyl-1-pentyl-1H-indol-3-yl)(napthalen-1-yl)methanone], JWH-018 M3 [(6-hydroxyl-1-pentyl-1H-indol-3-yl)(napthalen-1-yl)methanone], JWH-018 M4 [(7-hydroxyl-1-pentyl-1H-indol-3-yl)(napthalen-1-yl)methanone], JWH-018 M5 [(1-(5-hydroxypentyl)-1H-indol-3-yl)(napthalen-1-yl)methanone], JWH-018 M6 [5-(3-(1-napthoyl)-1H-indol-1-yl)pentanoic acid], JWH-073 M1 [(1-butyl-4-hydroxy-1H-indol-3-yl)(napthalen-1-yl)methanone], JWH-073 M2 [(1-butyl-5-hydroxy-1H-indol-3-yl)(napthalen-1-yl)methanone], JWH-073 M3 [(1-butyl-6-hydroxy-1H-indol-3-yl)(napthalen-1-yl)methanone], JWH-073 M4 [(1-butyl-7-hydroxy-1H-indol-3-yl)(napthalen-1-yl)methanone], JWH-073 M5 [(1-(4-hydroxybutyl)-1H-indol-3-yl)(napthalen-1-yl)methanone], and JWH-073 M6 [4-(3-(1-napthoyl)-1H-indol-1-yl)butanoic acid] (Moran et al., 2011).
Materials and Methods
Materials.
All chemicals used for this study were of at least reagent grade. Analytical standards of JWH-018, JWH-073, and the metabolites M1–M6 (Moran et al., 2011) for each tested AAI (Fig. 1) were provided by Cayman Chemical (Ann Arbor, MI). Recombinant UGT1A1, UGT1A3, UGT1A4, and UGT 1A6–1A10 expressed in baculovirus-infected Sf9 insect cells as His-tagged proteins were provided by our collaborator, Dr. M. Finel (Drug Discovery and Development Technology Center, Faculty of Pharmacy, University of Helsinki, Helsinki, Finland). Recombinant UGT2B7 was expressed in our laboratory using stably transfected human embryonic kidney (HEK) 293 cells. Recombinant UGT2B4, UGT2B15, and UGT2B17 were provided by BD Biosciences (San Diego, CA). ACS spectrophotometric-grade dimethyl sulfoxide (>99.9% pure) was purchased from Sigma-Aldrich (St. Louis, MO). Optima LC-MS-grade acetonitrile and methanol were purchased from Thermo Fisher Scientific (Waltham, MA). Reagent-grade formic acid (99% pure) was purchased from Acros Organics (Pittsburgh, PA). Deionized water was purified to 18.2 MΩ-cm resistivity using an PURELAB Ultra laboratory water purification system (Elga, Woodridge, IL). UDP-glucuronic acid and all other chemicals and reagents were purchased from Sigma-Aldrich, unless specified otherwise.
Equipment.
Sample analysis was performed using an API-4000 Q TRAP tandem mass spectrometer (Applied Biosystems, Foster City, CA) interfaced with an Agilent Series 1200 quaternary liquid chromatography system (Agilent Technologies, Santa Clara, CA). Analyst software (version 1.5; Invitrogen, Carlsbad, CA) was used to control the overall operation of the HPLC system and the mass spectrometer.
Kinetic studies were evaluated using an HP1050 HPLC system equipped with a UV-Vis diode array detector. Instrument operation and data acquisition were controlled through the ChemStation software (Agilent Technologies).
Screening of Human Liver and Recombinant UGT Isoforms and Steady-State Enzyme Kinetics Assays.
Human liver microsomes (HLMs) and human intestinal microsomes (HIMs) from a single donor (HL2 and HI46, respectively) or UGT recombinant membrane protein were assayed for activity toward JWH-018, JWH-073, and each tested metabolite (Fig. 1). The cloning and expression of UGT1A1, UGT1A3, UGT1A4, and UGT1A6–10 in baculovirus-infected Sf9 insect cells as His-tagged proteins and the preparation of enriched membrane fractions have been reported previously (Kurkela et al., 2003; Kuuranne et al., 2003). These preparations have been shown to contain similar amounts of protein by Western blot analysis using an antibody directed at the His-tag modification present on each of these recombinant isoforms. Human recombinant UGT2B7 was expressed in HEK293 cells as reported previously (Coffman et al., 1997). Other 2B enzymes were obtained from BD Biosciences and assayed according to manufacturer protocols. Each enzyme tested in this study is known to be active toward substrates specific for that isoform.
Kinetic parameters were determined by incubating recombinant UGT membrane protein in the presence of varying concentrations of substrate (10–1000 μM) at a fixed concentration of UDP-GlcUA (4 mM) for 90 min. All other conditions were identical to those of the screening experiments.
Membrane protein was incubated in 100 μM Tris-HCl (pH 7.4)/5 mM MgCl2/5 mM saccharolactone with 500 μM substrate in a total volume of 30 μl. Substrates were added in dimethyl sulfoxide with a final concentration of 2% as an activator for the membranes, and controls omitting substrates/cosubstrate were run with each assay. All incubations were performed in duplicate, and no additional detergents or other activators were used in the incubations. The UGT2B isoforms were obtained from BD Biosciences and assayed according to manufacturer protocols. Reactions were started by the addition of UDP-GlcUA (4 mM) and incubated for 90 min at 37°C. The rates of glucuronidation with these enzymes have been shown to be linear for up to 3 h (data not shown). The reactions were stopped by the addition of 30 μl of ethanol, followed by centrifugation at 14,000 rpm for 8 min to spin down the protein.
Analysis of Glucuronidated Products.
The presence of potential glucuronidated products were first screened using liquid chromatography/tandem mass spectrometry (LC-MS/MS) methods described previously (Moran et al., 2011). In brief, analytes of interest were chromatographically separated using a Zorbax Eclipse XDB-C18 analytical column (150 × 4.6 mm, 5 μm; Agilent Technologies) heated to 40°C. Mobile phases consisted of 0.1% formic acid in water (mobile phase A) and 0.1% formic acid in acetonitrile (mobile phase B). The gradient used consisted of 50% B (0–4.5 min), a linear gradient from 50% B to 90% B (4.5–7.1 min), and 50% B (7.1–10 min). MS data were acquired in positive ion mode by electrospray ionization. The Turbo IonSpray source voltage was 2500 V, and the source temperature was maintained at 600°C. Nitrogen gas pressures for the GS1 and GS2 source gases, curtain gas, and collision gases were 55.0, 55.0, and 35.0 cm/S and “high,” respectively. Molecule-specific parameters for specific reaction monitoring-information-dependent acquisition experiments are listed in Table 1. The specific reaction monitoring-information-dependent acquisition transition threshold that triggered enhanced product ion experiments was set to an intensity of 4000 cps. Specific enhanced product ion parameters are summarized in Table 1.
TABLE 1.
Glucuronidation activity for JWH-018 and JWH-073 and their metabolites as identified by LC-MS/MS
| Compound | Analyte Number | UGT Isoform |
HL2 | HI46 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1A1 | 1A3 | 1A4 | 1A6 | 1A7 | 1A8 | 1A9 | 1A10 | 2B7 | ||||
| JWH-018 | 1 | − | − | − | − | − | − | − | − | − | − | − |
| JWH-018 M1 | 2 | − | − | − | − | − | − | − | − | − | − | − |
| JWH-018 M2 | 3 | + | + | − | + | + | + | + | + | + | + | + |
| JWH-018 M3 | 4 | ++ | + | − | − | + | + | + | ++ | + | ++ | + |
| JWH-018 M4 | 5 | + | + | + | + | + | ++ | ++ | ++ | + | +++ | +++ |
| JWH-018 M5 | 6 | + | + | + | − | − | − | − | + | ++ | +++ | + |
| JWH-018 M6 | 7 | + | + | − | − | − | − | − | + | ++ | ++ | + |
| JWH-073 | 8 | − | − | − | − | − | − | − | − | − | − | − |
| JWH-073 M1 | 9 | − | − | − | − | − | − | − | − | − | − | − |
| JWH-073 M2 | 10 | + | + | − | − | + | + | + | + | − | + | + |
| JWH-073 M3 | 11 | ++ | + | − | − | + | + | + | +++ | + | + | + |
| JWH-073 M4 | 12 | + | ++ | + | + | + | ++ | ++ | +++ | + | ++ | +++ |
| JWH-073 M5 | 13 | + | − | + | − | − | − | − | + | + | + | + |
| JWH-073 M6 | 14 | + | + | − | − | − | − | + | + | + | + | + |
+++, peak height ≥2.5E7; ++, peak height ≥1.0E7 and ≤2.5E7; +, peak height ≤1.0E7; —, glucuronidated product not detected.
Quantitative measurements necessary for assessing kinetic parameters were performed using HPLC-UV-Vis techniques. In brief, samples were separated using a Supelcosil LC-18 (25 cm × 4.6 mm, 5 mm) at 37°C. The solvent system used consisted of 0.1% acetic acid in water (A) and methanol (B) at a flow rate 1 ml/min, with an elution gradient of 100% A (5 min), a linear gradient from 100% A to 100% B (5–25 min), and 100% B (25–31 min). The column was re-equilibrated at initial conditions for 10 min between runs. The elution of each K2 metabolite was monitored at 330 nm. Primary standards for the glucuronidated metabolites are not available; therefore, product concentrations were calculated using the external standard response for each substrate. It has been shown previously that the addition of the glucuronic acid moiety does not alter the extinction coefficients of the unreacted substrates (Doerge et al., 2000).
Data Analysis.
Curve-fitting and statistical analyses were conducted using GraphPad Prism version 4.0b (GraphPad Software Inc., San Diego, CA). Kinetic constants were obtained by fitting experimental data to the following kinetic models using the nonlinear regression (curve fit) function:
- Michaelis-Menten equation for one-enzyme model

- Hill equation, which describes sigmoidal autoactivation kinetics, where S50 is the substrate concentration at 50% Vmax (analogous to Km in Michaelis-Menten kinetics) and n is the Hill coefficient, which can be considered to be a measure of autoactivation and reflects the extent of cooperativity among multiple binding sites (Weiss, 1997).

- Uncompetitive substrate inhibition model, where Ki is the inhibition constant describing the reduction in rate.

The fit of the data for each model was assessed from the standard error, 95% confidence intervals, and r2 values. Kinetic curves were also analyzed as Eadie-Hofstee plots to support kinetic models. Kinetic constants were reported as the mean ± S.E. of at least duplicate experiments.
Results
Screening of Human Hepatic and Intestinal Microsomes and Recombinant UGTs for Activity toward Hydroxylated Products of K2.
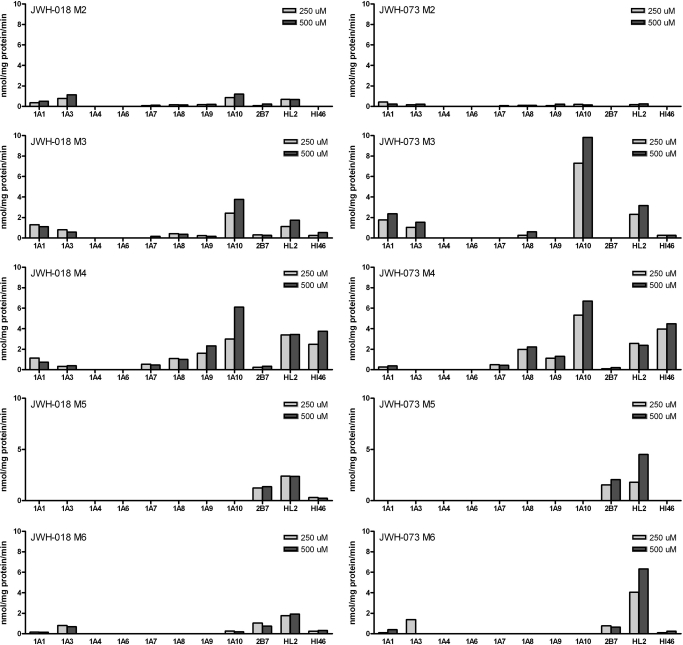
Eight human recombinant UGT1A isoforms expressed as His-tag proteins in baculovirus-infected Sf9 insect cells, UGT2B7 expressed in HEK293 cells; UGT2B4, UGT2B15, and UGT2B17 (BD Biosciences); and human liver and intestinal microsomes (each from a single donor; HL2 and HI46) were evaluated for their ability to glucuronidate JWH-018, JWH-073, and six metabolites of each AAI. M1–M4 each contained a hydroxyl group at unique positions on the indole ring (Fig. 1), whereas M5 and M6 each have a modification on the alkyl side chain. M5 possesses a terminal hydroxyl group, and M6 has a terminal carboxyl group. Specific activities at two substrate concentrations (250 and 500 μM) were calculated using HPLC-UV/Vis analysis, and these data are shown in Fig. 2.
Fig. 2.
Screening of UGT isoforms analyzed by HPLC-UV/Vis. Recombinant UGT1A1, UGT1A3, UGT1A4, UGT1A6, UGT1A7, UGT1A8, UGT1A9, UGT1A10, UGT2B4, UGT2B15, and UGT2B17 (5 μg), UGT2B7 from HEK293 cells (50 μg), human liver microsomes (one donor; 50 μg), and human intestinal microsomes (one donor; HI46; 50 μg) were evaluated for their ability to glucuronidate JWH-018 and JWH-073 and their hydroxylated metabolites (M1–M6). No activity seen was observed with UGT1A4, UGT1A6, UGT2B4, UGT2B15, or UGT2B17. No activity was seen for the parent compounds, JWH-018 and JWH-073, or for the their M1 metabolites.
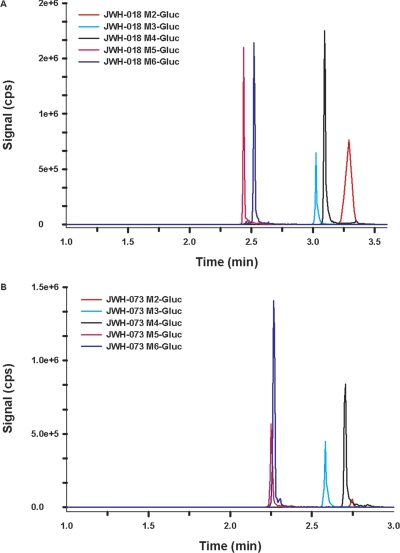
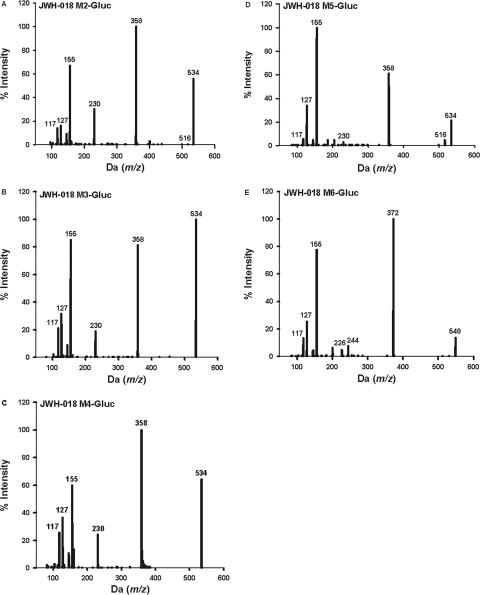
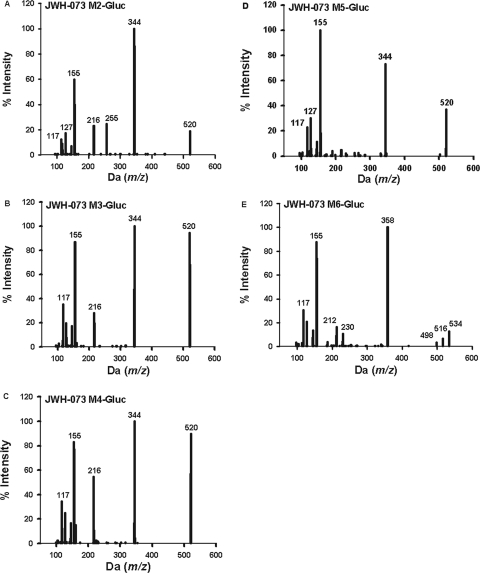
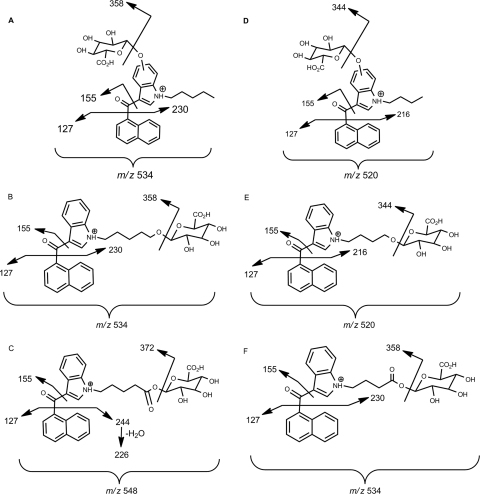
For preliminary LCMS/MS assessments, only the higher substrate concentrations (500 μM) were used to maximize the potential for identifying any and all glucuronide conjugates. The relative enzymatic activity was estimated by evaluating peak heights using LC-MS/MS and by estimating initial enzymatic velocities to identify the most active isoforms. These data are summarized in Table 1. Comparison of LC-MS/MS chromatographic retention times and mass spectra of products produced by HLMs and HIMs to the retention times and mass spectra from metabolites generated by recombinant enzymes showed no differences. No interfering products were identified in negative control reactions in which either substrates or cofactor were omitted. All glucuronidated products eluted within 3.5 min (Fig. 3) and product ion spectra have signals consistent with the predicted glucuronidated metabolites (Figs. 4 and 5). Molecular ions [MH+] were observed for each glucuronidated product (m/z 520, 534, and 548; Figs. 4 and 5). Loss of glucuronic acid (m/z 176) produced charged species representative of the respective hydroxylated and carboxylated substrates (m/z 358 or 344; Figs. 4 and 5). Product ion spectra also showed characteristic fragments for aminoalkylindoles (m/z of 127 and 155) (Zhang et al., 2006; Sobolevsky et al., 2010). Because UGTs showed no activity toward the parent compounds, the regiochemical assignments for glucuronidation sites were based on the positions of the active hydroxyl and carboxyl moieties (Fig. 6).
Fig. 3.
LC chromatograms of the glucuronides of JWH-018 M2-M6 (A) and JWH-073 M2-M6 (B). Different color tracings are representative of multiple reaction monitoring experiments used to detect the M2-M6 glucuronides of JWH-018 (A) and JWH-073 (B). Signals for m/z 520/344 (JWH-073 M2-M5), 534/358 (JWH-018 M2-M5 and JWH-073 M6), and 548/372 (JWH-018 M6) were used to monitor each glucuronide product.
Fig. 4.
MS/MS spectra of the JWH-018 M2-glucuronide (A), JWH-018 M3-glucuronide (B), JWH-018 M4-glucuronide (C), JWH-018 M5-glucuronide (D), and JWH-018 M6-glucuronide (E). Spectra are representative of the glucuronidated products shown in Figure 3A. Data were obtained in positive ion mode as described under Materials and Methods.
Fig. 5.
MS/MS spectra of the JWH-073 M2-glucuronide (A), JWH-073 M3-glucuronide (B), JWH-073 M4-glucuronide (C), JWH-073 M5-glucuronide (D), and JWH-073 M6-glucuronide (E). Spectra are representative of the glucuronidated products shown in Figure 3B. Data were obtained in positive ion mode as described under Materials and Methods.
Fig. 6.
Proposed MS/MS fragmentation pathways for glucuronidated metabolites of JWH-018 and JWH-073 substrates. Shown are JWH-018 M2-M4 (A), JWH-018 M5 (B), JWH-018 M6 (C), JWH-073 M2-M4 (D), JWH-073 M5 (E), and JWH-073 M6 (F).
HLM, HIM, and several human recombinant isoforms were active toward the M2–M6 metabolites of JWH-018 and JWH-073 (Fig. 2; Table 1). However, no activity was observed toward native JWH-018, JWH-073, or either M1. Unique product profiles are seen for each enzyme source, and similar patterns of activity are seen with the corresponding metabolites of JWH-018 and JWH-073 (Fig. 2; Table 1). The major isoforms involved in the metabolism of these compounds were predominantly the hepatic isoforms, UGT1A1, UGT1A9, and UGT2B7, and the extrahepatic isoform, UGT1A10. Activity was also seen with isoforms known to be expressed in the lung (UGT1A7) and brain (UGT1A3 and UGT2B7). However, no activity was seen with UGT2B4, UGT2B15, or UGT2B17. As expected, LC-MS/MS analysis, which has much higher sensitivity, identified additional isoforms with low levels of activity toward some tested substrates that were not seen using HPLC-UV/Vis analysis.
Kinetic Analysis.
The kinetic constants of human recombinant UGT1A isoforms, UGT2B7, and HLMs from a single donor (HL2) were evaluated. Kinetic profiles for the majority of the enzymes analyzed followed classical Michaelis-Menten kinetics, and reliable kinetics parameters were determined (Table 2). For JWH-073 M2, the kinetic parameters were not measured because of the very low activity toward this substrate.
TABLE 2.
Glucuronidation kinetics for JWH-018 and JWH-073 metabolites
Km and Vmax values are given in micromoles and nmol/mg protein/min, respectively.
| JWH-018 Metabolites |
JWH-073 Metabolites |
|||||
|---|---|---|---|---|---|---|
| Km | Vmax | Kinetic Fit | Km | Vmax | Kinetic Fit | |
| JWH-018/073-M2 | ||||||
| HL2 | 18.5 ± 2.8 | 0.6 ± 0.02 | M-M | – | – | – |
| UGT1A3 | 12.1 ± 2.8 | 0.5 ± 0.02 | M-M | – | – | – |
| UGT1A10 | 18.5 ± 4.5 | 0.8 ± 0.04 | M-M | – | – | – |
| JWH-018/073-M3 | ||||||
| HL2 | 122 ± 11 | 2.7 ± 0.1 | M-M | 95.4 ± 12 | 5.0 ± 0.2 | M-M |
| UGT1A1 | 28.4 ± 3.4 | 1.5 ± 0.05 | M-M | 12.3 ± 3.7 | 1.8 ± 0.1 | Biphasic |
| UGT1A10 | 285 ± 65 | 11 ± 0.7 | M-M | 156 ± 18 | 13 ± 0.7 | Biphasic |
| JWH-018/073-M4 | ||||||
| HL2 | 149 ± 26 | 4.7 ± 0.3 | M-M | 59.1 ± 9.1 | 4.2 ± 0.2 | M-M |
| UGT1A9 | 33.9 ± 5.1 | 1.8 ± 0.05 | M-M | 11.8 ± 2.0 | 1.2 ± 0.4 | M-M |
| UGT1A10 | 291 ± 61 | 10 ± 0.6 | M-M | 172 ± 28 | 12 ± 0.5 | M-M |
| JWH-018/073-M5 | ||||||
| HL2 | 80.6 ± 10 | 2.3 ± 0.09 | M-M | 181 ± 13 | 5.8 ± 0.2 | M-M |
| UGT2B7 | 26.2 ± 2.0 | 1.0 ± 0.02 | Hill; n = 2.3 | 112 ± 6.3 | 3.9 ± 0.1 | M-M |
| JWH-018/073-M6 | ||||||
| HL2 | 236 ± 14 | 3.3 ± 0.07 | M-M | 207 ± 20 | 6.4 ± 0.3 | M-M |
| UGT1A3 | 125* | 1.5* | * | 63.8 ± 13 | 0.7 ± 0.03 | M-M |
| UGT2B7 | 125* | 0.9* | * | 41.5 ± 7.2 | 1.9 ± 0.1 | M-M |
, atypical inhibition kinetics; –, very low activity, no kinetic analysis done; M-M, Michaelis-Menten; Vmax, values represent the highest activity measured before inhibition is seen; Km, values represent the lowest concentration of substrate that results in an activity of half “observed Vmax.”
HLMs showed significant activity (Vmax values from 2.7 to 6.4 nmol/mg protein/min) toward the M3, M4, M5, and M6 metabolites of both JWH-018 and JWH-073, whereas minimal activity was observed toward the M2 metabolites. HLMs showed a large range of affinities for the substrates analyzed (Km values from 18.5 to 236 μM). The highest affinity was observed toward JWH-018 M3 and the lowest with JWH-018 M6.
Because of the high activity observed with HLMs, the hepatic recombinant isoforms that were most active toward each compound (UGT1A1, UGT1A3, UGT1A9, and UGT2B7) were selected for kinetic analysis. The hepatic UGT1A isoforms with the highest activity toward the JWH-018 and JWH-073 metabolites with hydroxyl groups located on the indole ring also had very high affinity for these compounds, with apparent Km values between 12 and 34 μM. When the Km values of the recombinant enzymes were compared to those found for HLMs, which are generally much higher (5–10-fold), it was evident that other hepatic isoforms must contribute to this activity in the liver. The sole exception was the glucuronidation of JWH-018 M2 by UGT1A3 (HL2, Km = 18.5 μM; 1A3, Km = 12.1 μM); UGT2B7 was the most active isoform toward the four compounds with modified alkyl side chains. This is in agreement with numerous studies showing this isoform to have high specificity for compounds containing nonphenolic carboxyl and hydroxyl functions (Jude et al., 2001; Little et al., 2004).
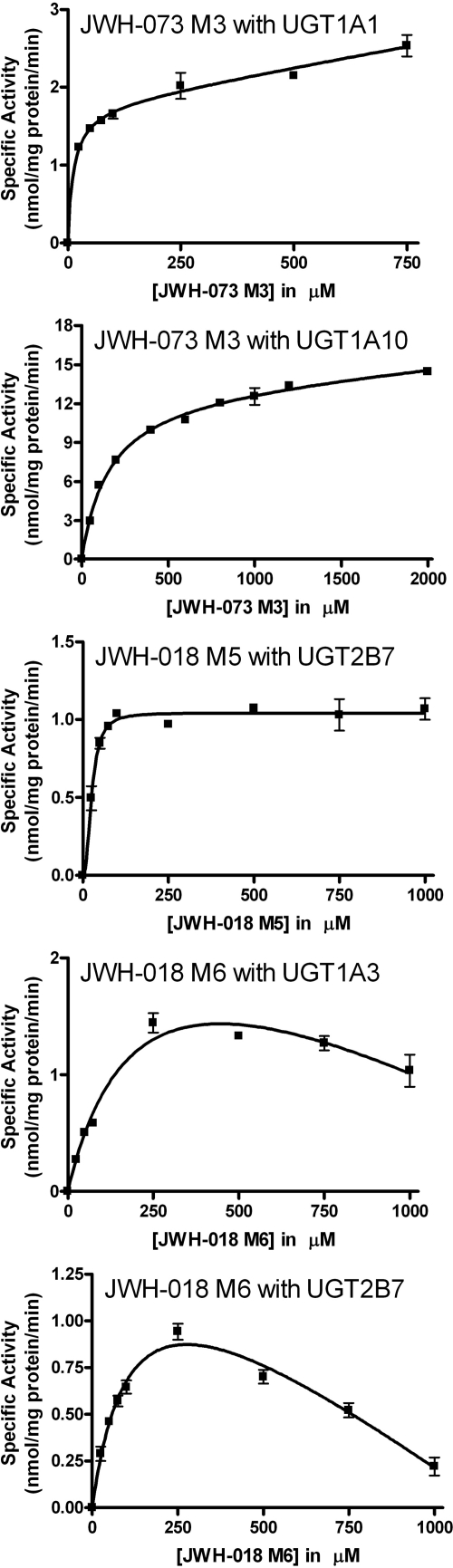
Some examples of atypical kinetics were also observed (Fig. 7). JWH-073 M3 showed biphasic kinetics with UGT1A1 and UGT1A10, which may indicate the presence of two separate active sites for this substrate within the enzyme (Zhou et al., 2010). JWH-018 M5 kinetics with UGT2B7 showed activation kinetics fitting the Hill equation (n = 2.3), which can indicate a two-site kinetic model with interactions between two identical binding sites (Uchaipichat et al., 2004). Kinetic profiles for JWH-018 M6 metabolism by UGTs 1A3 and 2B7 exhibited atypical substrate inhibition kinetics that did not fit the classic competitive/uncompetitive substrate inhibition models. Therefore, all values are approximated based on the data obtained. The reason for this inhibition is unknown and should be the subject of further study.
Fig. 7.
Kinetic curves for metabolites of JWH-018 and JWH-073 that display atypical kinetics with selected human recombinant UGTs. Kinetic constants for curves shown here are presented in Table 2.
Discussion
The pharmacological properties of several nonclassical cannabinoids, namely cyclohexylphenols and aminoalkylindoles, have been widely investigated, but these compounds have mostly been abandoned as new drug targets because of their increased potency and the retention of undesirable psychoactive properties (Iversen, 2008). Since approximately 2004, two AAIs named after John W. Huffman (JWH-018 and JWH-073) started appearing in a variety of herbal products being sold as “legal marijuana.” The emergence of these drugs is of serious concern to both clinicians and public health officials because the increased potency of JWH-018 and JWH-073 is expected to lead to a longer duration of action and an increased likelihood of adverse effects. Other than these few case reports detailing the symptoms of K2-AAI abuse (Every-Palmer, 2010; Muller et al., 2010; Vearrier and Osterhoudt, 2010), very little is known about the pharmacology or metabolism of JWH-018 or JWH-073 in man.
Analysis of human urine from individuals known to have ingested either JWH-018 or JWH-073 have identified several products of Phase I and II metabolism (Moller et al., 2010; Sobolevsky et al., 2010; Beuck et al., 2011; Grigoryev et al., 2011; Moran et al., 2011; Schneir et al., 2011). Many of these hydroxylated metabolites have been shown to be monohydroxy derivatives of the naphthalene, indole, or side-chain moieties (Sobolevsky et al., 2010) that are excreted in urine primarily as glucuronic acid conjugates (Sobolevsky et al., 2010; Wintermeyer et al., 2010; Moran et al., 2011), thus implicating the involvement of UGTs in this process.
The present studies were designed to identify and characterize the role of hepatic and extrahepatic UGTs in Phase II metabolism of K2-AAIs. We have performed detailed studies of the glucuronidation of several hydroxylated and carboxylated JWH-018 and JWH-073 metabolites by HLM, HIM, and 12 human recombinant UGTs. Our data demonstrate, for the first time, that the major isoforms involved in the glucuronidation of these compounds are predominantly hepatic UGT1A1, UGT1A9, and UGT2B7 and extrahepatic UGT1A10. Some activity was also seen with isoforms expressed in the lung (UGT1A7) and brain (UGT1A3 and UGT2B7). These findings are important because the lung and brain are the first two tissues exposed to K2 products when smoked. Our data also demonstrate that metabolites M5 and M6 of JWH-018 and JWH-073, which are the major hydroxylated metabolites excreted in human urine (Moran et al., 2011), are substrates primarily hepatic UGTs. Additional activity assays with other members of the UGT family will likely be needed to fully explain the levels of activity seen in the liver microsomes.
The kinetics of K2-AAI hydroxylated substrate glucuronidation by HLMs and recombinant UGTs were characterized extensively. The majority of glucuronidation reactions exhibited classical Michaelis-Menten kinetics; however, there were a few cases in which atypical kinetic profiles were found. There is growing evidence in the literature that UGT-catalyzed reactions, especially at high substrate concentrations, exhibit several different kinetic profiles (Iwuchukwu and Nagar, 2008; Hyland et al., 2009; Iwuchukwu et al., 2009; Aprile et al., 2010; Zhou et al., 2010), and our data are in agreement with these earlier reports. These atypical kinetic parameters indicate that, at higher concentrations, clearance by specific UGTs could be altered.
JWH-073 M3 showed biphasic kinetics with UGT1A1 and UGT1A10. Usually, biphasic kinetic fits are seen in microsomal enzyme preparations in which more than one type of enzyme is responsible for the formation of product. However, because these recombinant preparations contain only one isoform, these data indicate the presence of two separate active sites within the enzyme for this substrate. Multiple binding sites for UGTs have been observed previously (Zhou et al., 2010).
In addition, JWH-018 M5 glucuronidation kinetics with UGT2B7 showed activation. Autoactivation has been observed for several recombinant UGTs. This kinetic profile has been described previously for UGT2B7 and indicates a two-site kinetic model with interactions between two identical binding sites (Uchaipichat et al., 2004). It has been speculated elsewhere that these two binding sites could result from homo-dimerization (Lewis et al., 2007; Uchaipichat et al., 2008).
Our recently published data showing quantitative analysis of K2-AAI metabolites in three human urine samples demonstrated that M5 and M6 of JWH-018 and JWH-073 were the primary urinary metabolites excreted in these samples. Moreover, JWH-018 M5 and M6 were excreted at concentrations 2 to 5 times higher than any of the other metabolites measured and were specific for JWH-018 use (Moran et al., 2011). In addition, these samples were treated with β-glucuronidase to determine the percentage of these products that were excreted as glucuronides, and the results were consistent with previous reports (Sobolevsky et al., 2010) that the hydroxylated metabolites are excreted primarily as glucuronic acid conjugates. Comparison of our in vitro results with those obtained from the analysis of human urine samples points to the importance of human UGT2B7 in the metabolism of K2-AAIs. This isoform, which is predominantly expressed in liver and intestine, has been found to be involved in the biotransformation of a wide range of endogenous and exogenous compounds. Further study will be required to identify the potential for drug-drug interactions and adverse drug reactions.
In this work, we have identified, for the first time, specific human UGTs capable of processing hydroxylated derivatives of JWH-018 and JWH-073. It is evident from this work that several human tissues, including liver, intestine, brain, and lung, could be involved in the biotransformation of these compounds. Moreover, this information will lead to a better understanding of potential adverse drug reactions related to enzyme polymorphisms and drug-drug interactions. It is anticipated that these data may prompt other investigators to consider the importance of human UGTs in the metabolism and clearance of these compounds, especially the role of not only hepatic and intestinal biotransformation but also conjugation in the brain and lungs. The identification of relatively high activity of two human brain UGT isoforms, 1A3 and 2B7, is also of special interest because they may be able to control the concentrations of those compounds available for binding to the cannabinoid receptors located in this tissue.
This work was supported by the National Institutes of Health [Grant GM075893 (to A.R.-P.)]; and an Association of Public Health Laboratories Innovation Award (to J.H.M.).
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
doi:10.1124/dmd.111.040709.
- Δ9-THC
- Δ-9-tetrahydrocannabinol
- UGT
- uridine diphosphate-glucuronosyltransferase
- AAI
- amnioakylindole
- HLM
- human liver microsomes
- HIM
- human intestinal microsomes
- HPLC
- high-performance liquid chromatography
- UV/Vis
- UV-visible
- LC-MS/MS
- liquid chromatography/tandem mass spectrometry
- HEK
- human embryonic kidney
- JWH-018
- 1-naphthalenyl-1(1-pentyl-1H-indol-3-yl)-methanone
- JWH-073
- (1-butyl-1H-indol-3-yl)-1-naphthalenyl-methanone.
Authorship Contributions
Participated in research design: C.L. Moran, James, J.H. Moran, and Radominska-Pandya.
Conducted experiments: Chimalakonda, Bratton, Le, Yiew, and Dineva.
Performed data analysis: Chimalakonda, Bratton, J.H. Moran, and Radominska-Pandya.
Wrote or contributed to the writing of the manuscript: Chimalakonda, Bratton, J.H. Moran, and Radominska-Pandya.
References
- Aprile S, Del Grosso E, Grosa G. (2010) Identification of the human UDP-glucuronosyltransferases involved in the glucuronidation of combretastatin A-4. Drug Metab Dispos 38:1141–1146 [DOI] [PubMed] [Google Scholar]
- Beuck S, Moller I, Thomas A, Klose A, Schlorer N, Schanzer W, Thevis M. (2011) Structure characterisation of urinary metabolites of the cannabimimetic JWH-018 using chemically synthesised reference material for the support of LC-MS/MS-based drug testing. Anal Bioanal Chem doi:10.1007/s00216-00011-04931-00215 [DOI] [PubMed] [Google Scholar]
- Coffman BL, Rios GR, King CD, Tephly TR. (1997) Human UGT2B7 catalyzes morphine glucuronidation. Drug Metab Dispos 25:1–4 [PubMed] [Google Scholar]
- Doerge DR, Chang HC, Churchwell MI, Holder CL. (2000) Analysis of soy isoflavone conjugation in vitro and in human blood using liquid chromatography-mass spectrometry. Drug Metab Dispos 28:298–307 [PubMed] [Google Scholar]
- EMCDDA (2009) Understanding the “Spice” phenomenon, pp 1–25, Office for Official Publications of the European Communities, Lisbon [Google Scholar]
- Every-Palmer S. (2010) Warning: legal synthetic cannabinoid-receptor agonists such as JWH-018 may precipitate psychosis in vulnerable individuals. Addiction 105:1859–1860 [DOI] [PubMed] [Google Scholar]
- Gaoni YM, Mechoulam R. (1964) Isolation, structure and partial synthesis of an active constituent of hashish. J Am Chem Soc 86:1646–1647 [Google Scholar]
- Grigoryev A, Savchuk S, Melnik A, Moskaleva N, Dzhurko J, Ershov M, Nosyrev A, Vedenin A, Izotov B, Zabirova I, et al. (2011) Chromatography-mass spectrometry studies on the metabolism of synthetic cannabinoids JWH-018 and JWH-073, psychoactive components of smoking mixtures. J Chromatogr B Analyt Technol Biomed Life Sci 879:1126–1136 [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, Rice KC. (1990) Cannabinoid receptor localization in brain. Proc Natl Acad Sci USA 87:1932–1936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyland R, Osborne T, Payne A, Kempshall S, Logan YR, Ezzeddine K, Jones B. (2009) In vitro and in vivo glucuronidation of midazolam in humans. Br J Clin Pharmacol 67:445–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishac EJ, Jiang L, Lake KD, Varga K, Abood ME, Kunos G. (1996) Inhibition of exocytotic noradrenaline release by presynaptic cannabinoid CB1 receptors on peripheral sympathetic nerves. Br J Pharmacol 118:2023–2028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen LL. (2008) The Science of Marijuana, Oxford University Press, Inc., New York [Google Scholar]
- Iwuchukwu OF, Nagar S. (2008) Resveratrol (trans-resveratrol, 3,5,4′-trihydroxy-trans-stilbene) glucuronidation exhibits atypical enzyme kinetics in various protein sources. Drug Metab Dispos 36:322–330 [DOI] [PubMed] [Google Scholar]
- Iwuchukwu OF, Ajetunmobi J, Ung D, Nagar S. (2009) Characterizing the effects of common UDP glucuronosyltransferase (UGT) 1A6 and UGT1A1 polymorphisms on cis- and trans-resveratrol glucuronidation. Drug Metab Dispos 37:1726–1732 [DOI] [PubMed] [Google Scholar]
- Jude AR, Little JM, Bull AW, Podgorski I, Radominska-Pandya A. (2001) 13-Hydroxy- and 13-oxooctadecadienoic acids: novel substrates for human UDP-glucuronosyltransferases. Drug Metab Dispos 29:652–655 [PubMed] [Google Scholar]
- Kurkela M, García-Horsman JA, Luukkanen L, Mörsky S, Taskinen J, Baumann M, Kostiainen R, Hirvonen J, Finel M. (2003) Expression and characterization of recombinant human UDP-glucuronosyltransferases (UGTs). UGT1A9 is more resistant to detergent inhibition than other UGTs and was purified as an active dimeric enzyme. J Biol Chem 278:3536–3544 [DOI] [PubMed] [Google Scholar]
- Kuuranne T, Kurkela M, Thevis M, Schänzer W, Finel M, Kostiainen R. (2003) Glucuronidation of anabolic androgenic steroids by recombinant human UDP-glucuronosyltransferases. Drug Metab Dispos 31:1117–1124 [DOI] [PubMed] [Google Scholar]
- Lee HK, Choi EB, Pak CS. (2009) The current status and future perspectives of studies of cannabinoid receptor 1 antagonists as anti-obesity agents. Curr Top Med Chem 9:482–503 [DOI] [PubMed] [Google Scholar]
- Lewis BC, Mackenzie PI, Elliot DJ, Burchell B, Bhasker CR, Miners JO. (2007) Amino terminal domains of human UDP-glucuronosyltransferases (UGT) 2B7 and 2B15 associated with substrate selectivity and autoactivation. Biochem Pharmacol 73:1463–1473 [DOI] [PubMed] [Google Scholar]
- Little JM, Kurkela M, Sonka J, Jäntti S, Ketola R, Bratton S, Finel M, Radominska-Pandya A. (2004) Glucuronidation of oxidized fatty acids and prostaglandins B1 and E2 by human hepatic and recombinant UDP-glucuronosyltransferases. J Lipid Res 45:1694–1703 [DOI] [PubMed] [Google Scholar]
- Moller I, Wintermeyer A, Bender K, Jubner M, Thomas A, Krug O, Schanzer W, Thevis M. (2010) Screening for the synthetic cannabinoid JWH-018 and its major metabolites in human doping controls. Drug Test Anal doi:10.1002/dta.158 [DOI] [PubMed] [Google Scholar]
- Moran CL, Le VH, Chimalakonda KC, Smedley AL, Lackey FD, Owen SN, Kennedy PD, Endres GW, Ciske FL, Kramer JB, et al. (2011) Quantitative measurement of JWH-018 and JWH-073 metabolites excreted in human urine. Anal Chem 83:4228–4236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller H, Sperling W, Köhrmann M, Huttner HB, Kornhuber J, Maler JM. (2010) The synthetic cannabinoid Spice as a trigger for an acute exacerbation of cannabis induced recurrent psychotic episodes. Schizophr Res 118:309–310 [DOI] [PubMed] [Google Scholar]
- Schneir AB, Cullen J, Ly BT. (2011) “Spice” girls: synthetic cannabinoid intoxication. J Emerg Med 40:296–299 [DOI] [PubMed] [Google Scholar]
- Sobolevsky T, Prasolov I, Rodchenkov G. (2010) Detection of JWH-018 metabolites in smoking mixture post-administration urine. Forensic Sci Int 200:141–147 [DOI] [PubMed] [Google Scholar]
- Tims FM, Dennis ML, Hamilton N, Buchan JB, Diamond G, Funk R, Brantley LB. (2002) Characteristics and problems of 600 adolescent cannabis abusers in outpatient treatment. Addiction 97 (Suppl 1):46–57 [DOI] [PubMed] [Google Scholar]
- Uchaipichat V, Mackenzie PI, Guo XH, Gardner-Stephen D, Galetin A, Houston JB, Miners JO. (2004) Human UDP-glucuronosyltransferases: isoform selectivity and kinetics of 4-methylumbelliferone and 1-naphthol glucuronidation, effects of organic solvents, and inhibition by diclofenac and probenecid. Drug Metab Dispos 32:413–423 [DOI] [PubMed] [Google Scholar]
- Uchaipichat V, Galetin A, Houston JB, Mackenzie PI, Williams JA, Miners JO. (2008) Kinetic modeling of the interactions between 4-methylumbelliferone, 1-naphthol, and zidovudine glucuronidation by UDP-glucuronosyltransferase 2B7 (UGT2B7) provides evidence for multiple substrate binding and effector sites. Mol Pharmacol 74:1152–1162 [DOI] [PubMed] [Google Scholar]
- Vardakou I, Pistos C, Spiliopoulou Ch. (2010) Spice drugs as a new trend: mode of action, identification and legislation. Toxicol Lett 197:157–162 [DOI] [PubMed] [Google Scholar]
- Vearrier D, Osterhoudt KC. (2010) A teenager with agitation: higher than she should have climbed. Pediatr Emerg Care 26:462–465 [DOI] [PubMed] [Google Scholar]
- Weiss JN. (1997) The Hill equation revisited: uses and misuses. FASEB J 11:835–841 [PubMed] [Google Scholar]
- Wintermeyer A, Möller I, Thevis M, Jübner M, Beike J, Rothschild MA, Bender K. (2010) In vitro phase I metabolism of the synthetic cannabimimetic JWH-018. Anal Bioanal Chem 398:2141–2153 [DOI] [PubMed] [Google Scholar]
- Zhang Q, Ma P, Cole RB, Wang G. (2006) Identification of in vitro metabolites of JWH-015, an aminoalkylindole agonist for the peripheral cannabinoid receptor (CB2) by HPLC-MS/MS. Anal Bioanal Chem 386:1345–1355 [DOI] [PubMed] [Google Scholar]
- Zhou J, Tracy TS, Remmel RP. (2010) Glucuronidation of dihydrotestosterone and trans-androsterone by recombinant UDP-glucuronosyltransferase (UGT) 1A4: evidence for multiple UGT1A4 aglycone binding sites. Drug Metab Dispos 38:431–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann US, Winkelmann PR, Pilhatsch M, Nees JA, Spanagel R, Schulz K. (2009) Withdrawal phenomena and dependence syndrome after the consumption of “spice gold.” Dtsch Arztebl Int 106:464–467 [DOI] [PMC free article] [PubMed] [Google Scholar]