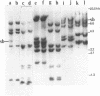
Abstract
Nuclei from a variety of human cell lines and tissues were digested with gradually increasing levels of DNase I. The DNA was then purified, treated with restriction enzymes and subjected to Southern blot hybridization using a cloned cDNA probe to 3-phosphoglycerate kinase (PGK) a housekeeping enzyme. At relatively high levels of DNase I, a specific, slightly sensitive site in chromatin sequences encoding PGK was observed in all of the cell types examined. This slightly sensitive site resides on the active X-chromosome since cell lines with increased numbers of inactive X-chromosomes do not show an increase in the region of chromatin which is sensitive. Except for this restricted region of enhanced sensitivity on the active X-chromosome, the data suggest that, for PGK encoding sequences, chromatin configurations on the active and inactive X-chromosomes are similar.
Full text
PDF






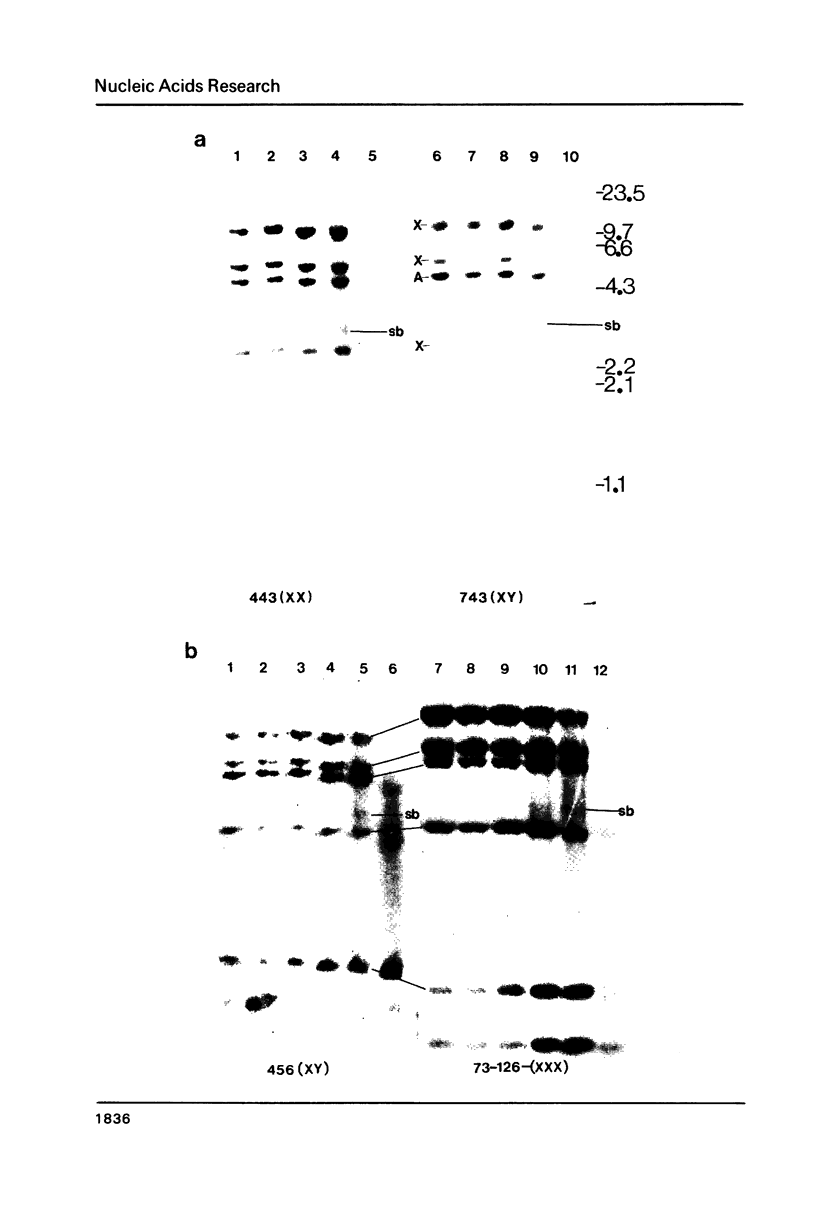

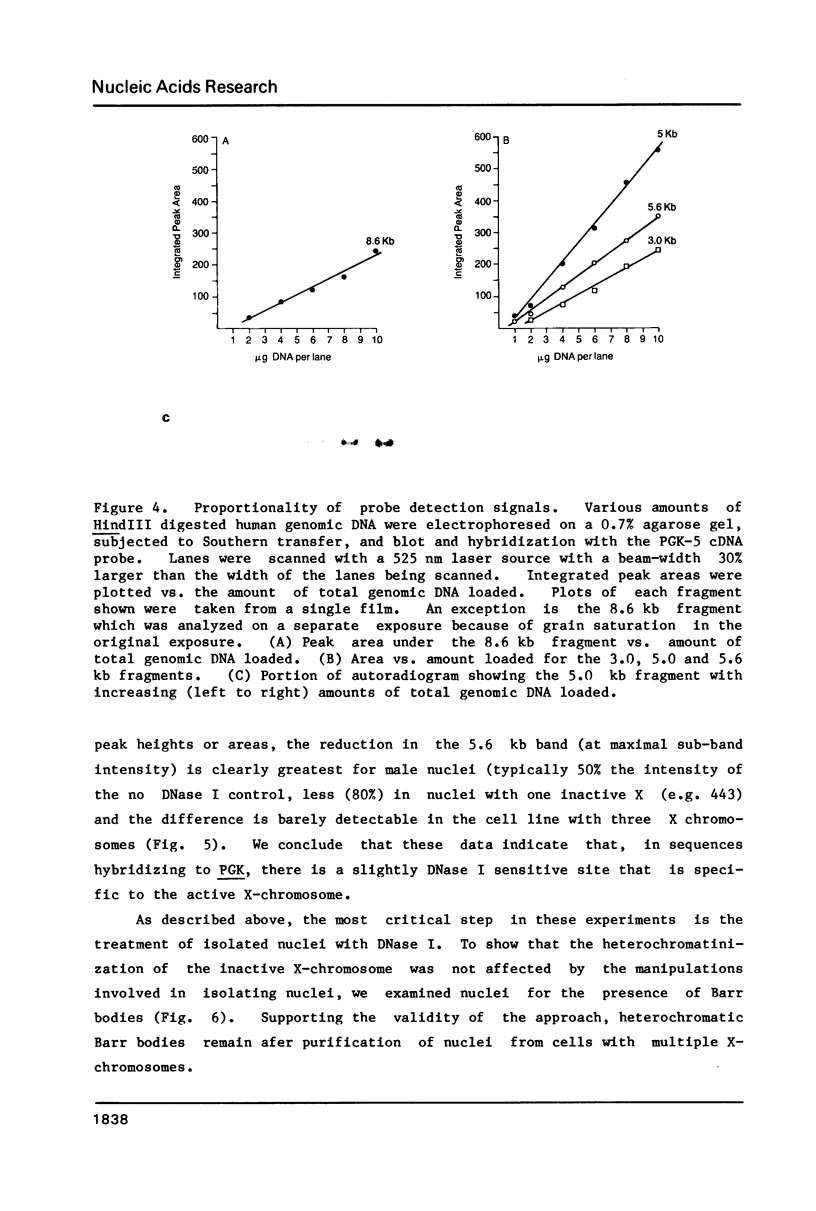







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carmon Y., Czosnek H., Nudel U., Shani M., Yaffe D. DNAase I sensitivity of genes expressed during myogenesis. Nucleic Acids Res. 1982 May 25;10(10):3085–3098. doi: 10.1093/nar/10.10.3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. H., Donahue R. P., Scott C. R. Characterization of phosphoglycerate kinase from human spermatozoa. Fertil Steril. 1976 Jun;27(6):699–701. [PubMed] [Google Scholar]
- Elgin S. C. DNAase I-hypersensitive sites of chromatin. Cell. 1981 Dec;27(3 Pt 2):413–415. doi: 10.1016/0092-8674(81)90381-0. [DOI] [PubMed] [Google Scholar]
- Garel A., Axel R. Selective digestion of transcriptionally active ovalbumin genes from oviduct nuclei. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3966–3970. doi: 10.1073/pnas.73.11.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garel A., Zolan M., Axel R. Genes transcribed at diverse rates have a similar conformation in chromatin. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4867–4871. doi: 10.1073/pnas.74.11.4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartler S. M., Andina R. J. Mammalian X-chromosome inactivation. Adv Hum Genet. 1976;7:99–140. doi: 10.1007/978-1-4757-0659-8_3. [DOI] [PubMed] [Google Scholar]
- Heilig R., Muraskowsky R., Mandel J. L. The ovalbumin gene family. The 5' end region of the X and Y genes. J Mol Biol. 1982 Mar 25;156(1):1–19. doi: 10.1016/0022-2836(82)90455-7. [DOI] [PubMed] [Google Scholar]
- Kerem B. S., Goitein R., Richler C., Marcus M., Cedar H. In situ nick-translation distinguishes between active and inactive X chromosomes. Nature. 1983 Jul 7;304(5921):88–90. doi: 10.1038/304088a0. [DOI] [PubMed] [Google Scholar]
- Lawson G. M., Tsai M. J., O'Malley B. W. Deoxyribonuclease I sensitivity of the nontranscribed sequences flanking the 5' and 3' ends of the ovomucoid gene and the ovalbumin and its related X and Y genes in hen oviduct nuclei. Biochemistry. 1980 Sep 16;19(19):4403–4441. doi: 10.1021/bi00560a004. [DOI] [PubMed] [Google Scholar]
- Lyon M. F. X-chromosome inactivation and developmental patterns in mammals. Biol Rev Camb Philos Soc. 1972 Jan;47(1):1–35. doi: 10.1111/j.1469-185x.1972.tb00969.x. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D., Mulvihill E. R., McKnight G. S., Senear A. W. Regulation of gene expression in the chick oviduct by steroid hormones. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):639–647. doi: 10.1101/sqb.1978.042.01.066. [DOI] [PubMed] [Google Scholar]
- Pegoraro B., Ansari A. A., Lee C. Y., Erickson R. P. Immunological relatedness of two isozymes of 3-phosphoglycerate kinase from the mouse. FEBS Lett. 1978 Nov 15;95(2):371–374. doi: 10.1016/0014-5793(78)81032-1. [DOI] [PubMed] [Google Scholar]
- Pegoraro B., Lee C. Y. Purification and characterization of two isozymes of 3-phosphoglycerate kinase from the mouse. Biochim Biophys Acta. 1978 Feb 10;522(2):423–433. doi: 10.1016/0005-2744(78)90075-x. [DOI] [PubMed] [Google Scholar]
- Riley D. E. Deoxyribonuclease I generates single-stranded gaps in chromatin deoxyribonucleic acid. Biochemistry. 1980 Jun 24;19(13):2977–2992. doi: 10.1021/bi00554a024. [DOI] [PubMed] [Google Scholar]
- Royston I., Smith R. W., Buell D. N., Huang E. S., Pagano J. S. Autologous human B and T lymphoblastoid cell lines. Nature. 1974 Oct 25;251(5477):745–746. doi: 10.1038/251745a0. [DOI] [PubMed] [Google Scholar]
- Samal B., Worcel A., Louis C., Schedl P. Chromatin structure of the histone genes of D. melanogaster. Cell. 1981 Feb;23(2):401–409. doi: 10.1016/0092-8674(81)90135-5. [DOI] [PubMed] [Google Scholar]
- Singer-Sam J., Simmer R. L., Keith D. H., Shively L., Teplitz M., Itakura K., Gartler S. M., Riggs A. D. Isolation of a cDNA clone for human X-linked 3-phosphoglycerate kinase by use of a mixture of synthetic oligodeoxyribonucleotides as a detection probe. Proc Natl Acad Sci U S A. 1983 Feb;80(3):802–806. doi: 10.1073/pnas.80.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stalder J., Groudine M., Dodgson J. B., Engel J. D., Weintraub H. Hb switching in chickens. Cell. 1980 Apr;19(4):973–980. doi: 10.1016/0092-8674(80)90088-4. [DOI] [PubMed] [Google Scholar]
- Stalder J., Larsen A., Engel J. D., Dolan M., Groudine M., Weintraub H. Tissue-specific DNA cleavages in the globin chromatin domain introduced by DNAase I. Cell. 1980 Jun;20(2):451–460. doi: 10.1016/0092-8674(80)90631-5. [DOI] [PubMed] [Google Scholar]
- VandeBerg J. L., Cooper D. W., Close P. J. Mammalian testis phosphoglycerate kinase. Nat New Biol. 1973 May 9;243(123):48–50. [PubMed] [Google Scholar]
- Venolia L., Gartler S. M. Comparison of transformation efficiency of human active and inactive X-chromosomal DNA. Nature. 1983 Mar 3;302(5903):82–83. doi: 10.1038/302082a0. [DOI] [PubMed] [Google Scholar]
- Venolia L., Gartler S. M. Comparison of transformation efficiency of human active and inactive X-chromosomal DNA. Nature. 1983 Mar 3;302(5903):82–83. doi: 10.1038/302082a0. [DOI] [PubMed] [Google Scholar]
- Venolia L., Gartler S. M., Wassman E. R., Yen P., Mohandas T., Shapiro L. J. Transformation with DNA from 5-azacytidine-reactivated X chromosomes. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2352–2354. doi: 10.1073/pnas.79.7.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H., Groudine M. Chromosomal subunits in active genes have an altered conformation. Science. 1976 Sep 3;193(4256):848–856. doi: 10.1126/science.948749. [DOI] [PubMed] [Google Scholar]
- Wickens M. P., Buell G. N., Schimke R. T. Synthesis of double-stranded DNA complementary to lysozyme, ovomucoid, and ovalbumin mRNAs. Optimization for full length second strand synthesis by Escherichia coli DNA polymerase I. J Biol Chem. 1978 Apr 10;253(7):2483–2495. [PubMed] [Google Scholar]
- Wu C., Bingham P. M., Livak K. J., Holmgren R., Elgin S. C. The chromatin structure of specific genes: I. Evidence for higher order domains of defined DNA sequence. Cell. 1979 Apr;16(4):797–806. doi: 10.1016/0092-8674(79)90095-3. [DOI] [PubMed] [Google Scholar]
- Wu C. The 5' ends of Drosophila heat shock genes in chromatin are hypersensitive to DNase I. Nature. 1980 Aug 28;286(5776):854–860. doi: 10.1038/286854a0. [DOI] [PubMed] [Google Scholar]