Abstract
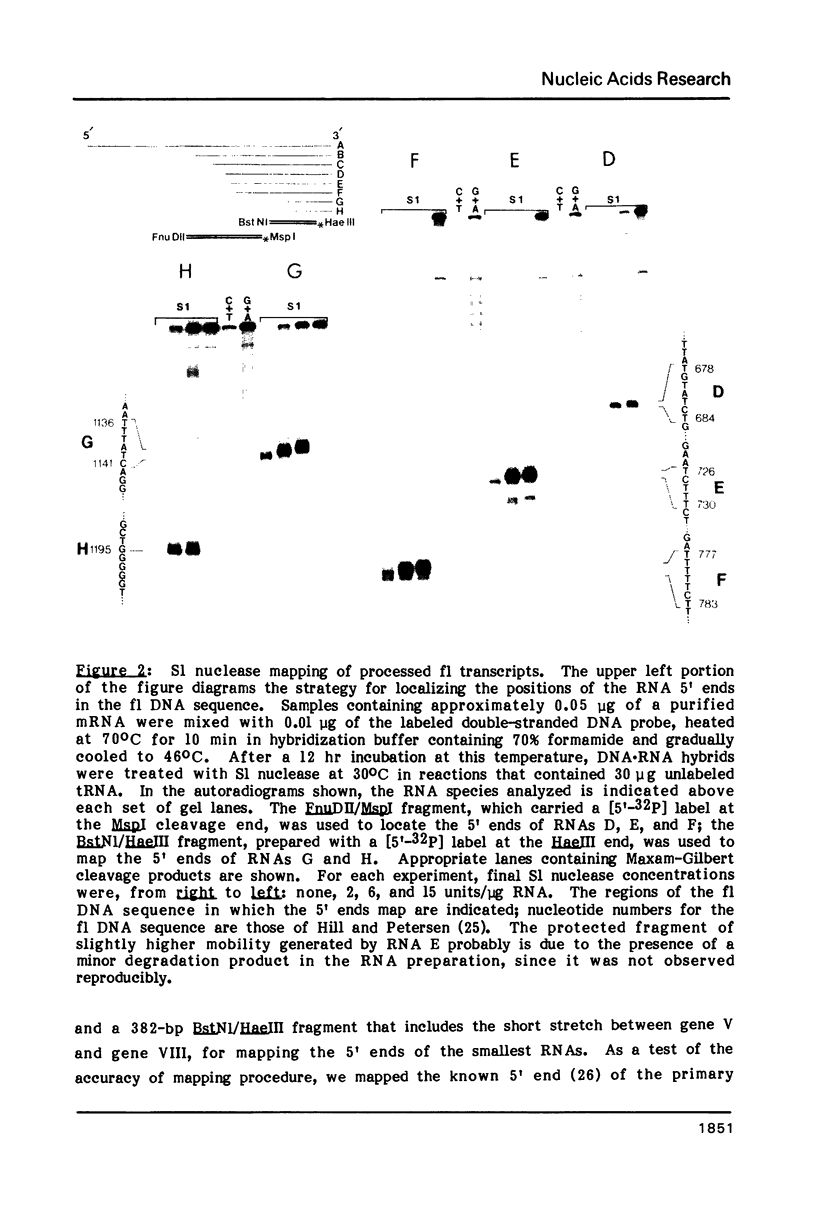
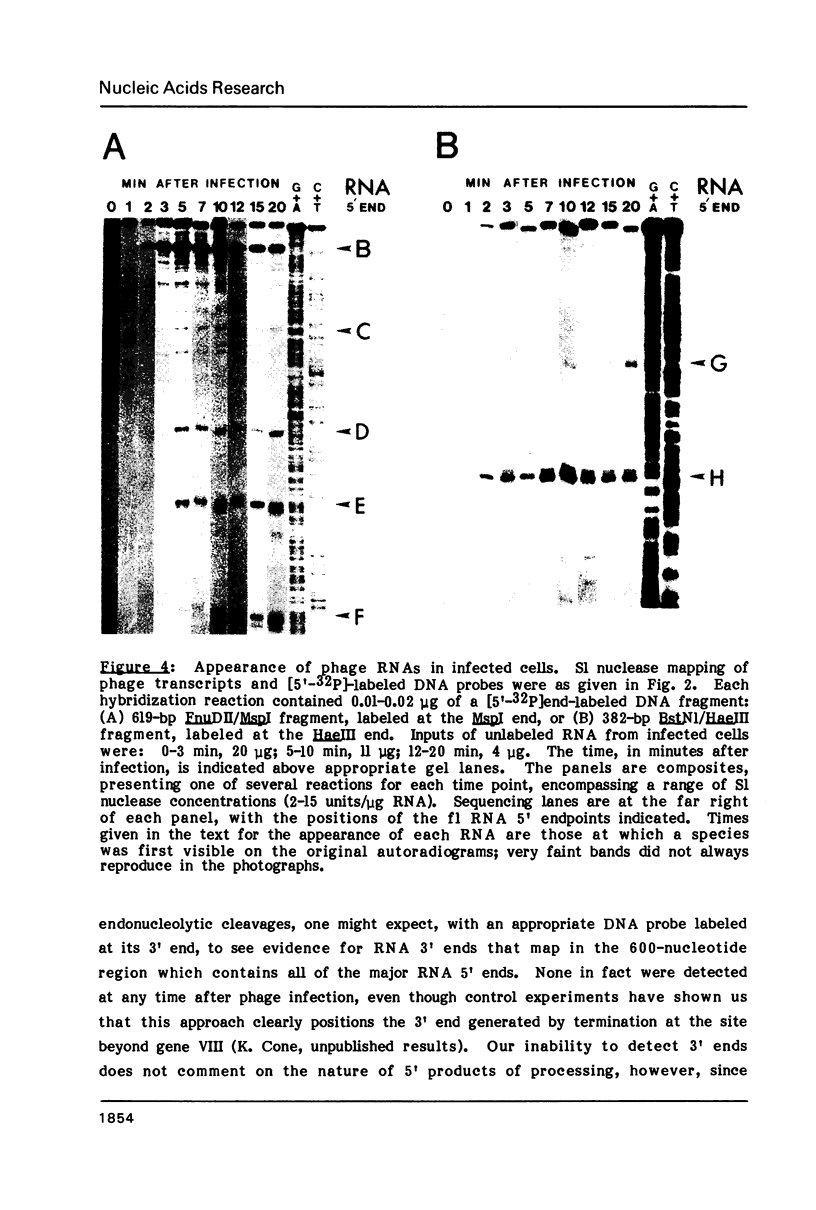
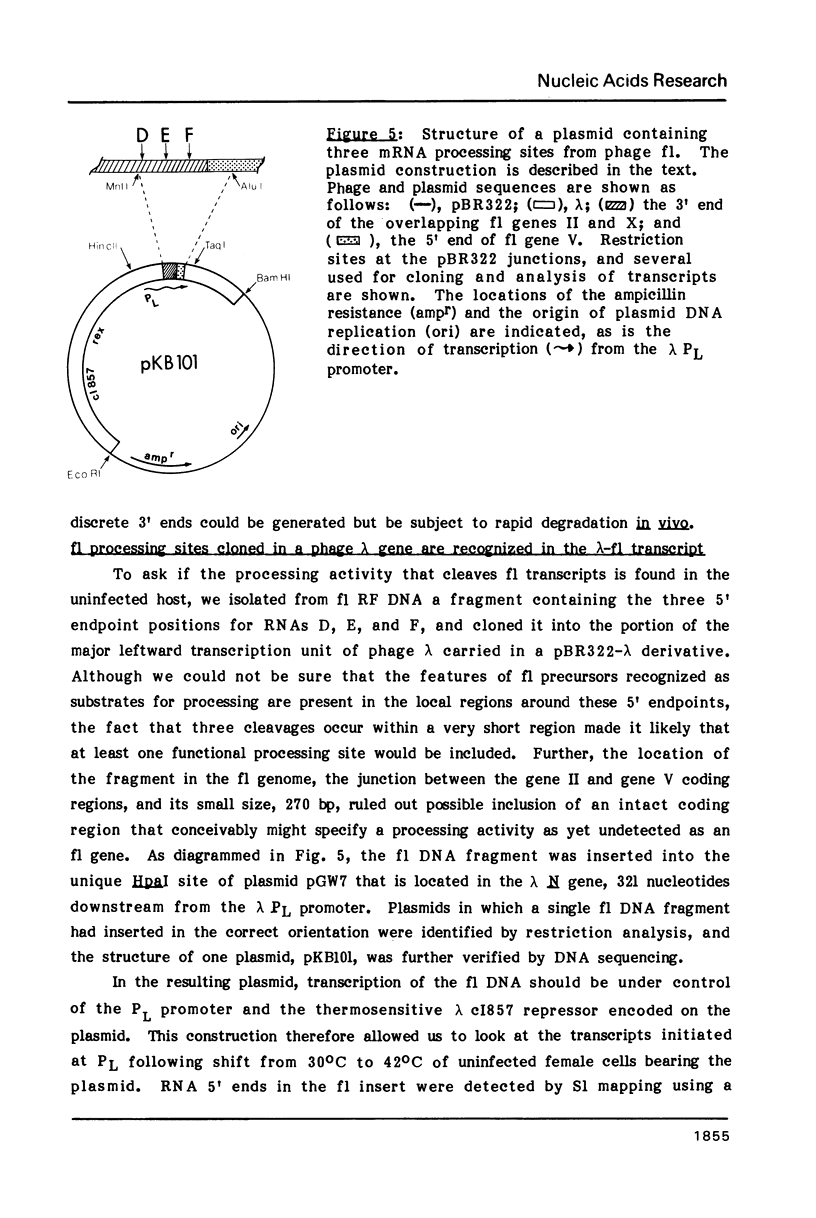
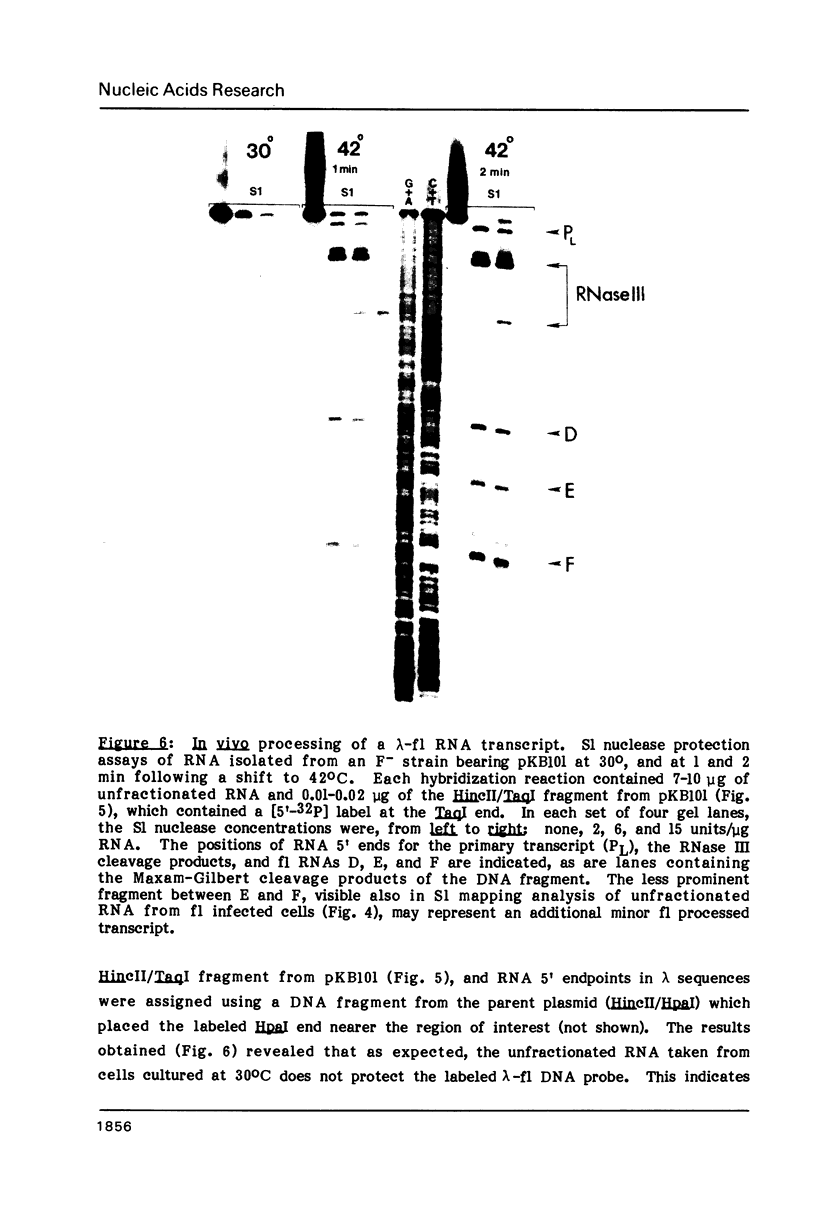
To examine the regions of the male-specific filamentous bacteriophage f1 genome that include signals for mRNA processing, the 5' endpoints of the major in vivo phage mRNAs have been located in the f1 DNA sequence by S1 nuclease mapping. The 5' ends of the purified mRNAs and additional phage-specific RNAs transiently visible early after infection occur in clusters of T-rich residues within genes that code for three phage proteins. When a 270-nucleotide region encompassing the 5' endpoints of three processed RNAs is transcribed as part of the bacteriophage lambda N mRNA in uninfected female cells, RNA 5' ends identical to ends of the three f1 RNAs are generated from the lambda-f1 precursor. This finding indicates that the mRNA processing activity is encoded by the bacterial host, and that its recognition sites are present in the local regions near the 5' ends which result from RNA cleavage. Several characteristics of f1 mRNA processing events have implications for the differential regulation of adjacent phage genes constrained in the same transcription unit, and may be representative of similar processing events occurring in the bacterial cell.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abelson J. RNA processing and the intervening sequence problem. Annu Rev Biochem. 1979;48:1035–1069. doi: 10.1146/annurev.bi.48.070179.005131. [DOI] [PubMed] [Google Scholar]
- Alberts B., Frey L., Delius H. Isolation and characterization of gene 5 protein of filamentous bacterial viruses. J Mol Biol. 1972 Jul 14;68(1):139–152. doi: 10.1016/0022-2836(72)90269-0. [DOI] [PubMed] [Google Scholar]
- Burton Z. F., Gross C. A., Watanabe K. K., Burgess R. R. The operon that encodes the sigma subunit of RNA polymerase also encodes ribosomal protein S21 and DNA primase in E. coli K12. Cell. 1983 Feb;32(2):335–349. doi: 10.1016/0092-8674(83)90453-1. [DOI] [PubMed] [Google Scholar]
- Cashman J. S., Webster R. E. Bacteriophage f1 infection of Escherichia coli: identification and possible processing of f1-specific mRNAs in vivo. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1169–1173. doi: 10.1073/pnas.76.3.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashman J. S., Webster R. E., Steege D. A. Transcription of bacteriophage fl. The major in vivo RNAs. J Biol Chem. 1980 Mar 25;255(6):2554–2562. [PubMed] [Google Scholar]
- Cone K. C., Sellitti M. A., Steege D. A. Lac repressor mRNA transcription terminates in vivo in the lac control region. J Biol Chem. 1983 Sep 25;258(18):11296–11304. [PubMed] [Google Scholar]
- Fukada K., Abelson J. DNA sequence of a T4 transfer RNA gene cluster. J Mol Biol. 1980 May 25;139(3):377–391. doi: 10.1016/0022-2836(80)90136-9. [DOI] [PubMed] [Google Scholar]
- Gegenheimer P., Apirion D. Processing of procaryotic ribonucleic acid. Microbiol Rev. 1981 Dec;45(4):502–541. doi: 10.1128/mr.45.4.502-541.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry T. J., Pratt D. The proteins of bacteriophage M13. Proc Natl Acad Sci U S A. 1969 Mar;62(3):800–807. doi: 10.1073/pnas.62.3.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill D. F., Petersen G. B. Nucleotide sequence of bacteriophage f1 DNA. J Virol. 1982 Oct;44(1):32–46. doi: 10.1128/jvi.44.1.32-46.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. A., Walseth T. F. The enzymatic preparation of [alpha-32P]ATP, [alpha-32P]GTP, [32P]cAMP, and [32P]cGMP, and their use in the assay of adenylate and guanylate cyclases and cyclic nucleotide phosphodiesterases. Adv Cyclic Nucleotide Res. 1979;10:135–167. [PubMed] [Google Scholar]
- La Farina M., Model P. Transcription in bacteriophage f1-infected Escherichia coli. Messenger populations in the infected cell. J Mol Biol. 1983 Mar 5;164(3):377–393. doi: 10.1016/0022-2836(83)90057-8. [DOI] [PubMed] [Google Scholar]
- Lin T. C., Webster R. E., Konigsberg W. Isolation and characterization of the C and D proteins coded by gene IX and gene VI in the filamentous bacteriophage fl and fd. J Biol Chem. 1980 Nov 10;255(21):10331–10337. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Mazzara G. P., Plunkett G., 3rd, McClain W. H. DNA sequence of the transfer RNA region of bacteriophage T4: implications for transfer RNA synthesis. Proc Natl Acad Sci U S A. 1981 Feb;78(2):889–892. doi: 10.1073/pnas.78.2.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T. F., Geider K. Bacteriophage fd gene II-protein. I. Purification, involvement in RF replication, and the expression of gene II. J Biol Chem. 1979 Dec 25;254(24):12636–12641. [PubMed] [Google Scholar]
- Meyer T. F., Geider K. Bacteriophage fd gene II-protein. II. Specific cleavage and relaxation of supercoiled RF from filamentous phages. J Biol Chem. 1979 Dec 25;254(24):12642–12646. [PubMed] [Google Scholar]
- Modrich P., Zabel D. EcoRI endonuclease. Physical and catalytic properties of the homogenous enzyme. J Biol Chem. 1976 Oct 10;251(19):5866–5874. [PubMed] [Google Scholar]
- Panganiban A. T., Whiteley H. R. Bacillus subtilis RNAase III cleavage sites in phage SP82 early mRNA. Cell. 1983 Jul;33(3):907–913. doi: 10.1016/0092-8674(83)90033-8. [DOI] [PubMed] [Google Scholar]
- Panganiban A. T., Whiteley H. R. Purification and properties of a new bacillus subtilis RNA processing enzyme. Cleavage of phage SP82 mRNA and Bacillus subtilis precursor rRNA. J Biol Chem. 1983 Oct 25;258(20):12487–12493. [PubMed] [Google Scholar]
- Ravetch J., Horiuchi K., Model P. Mapping of bacteriophage f1 ribosome binding sites to their cognate genes. Virology. 1977 Sep;81(2):341–351. doi: 10.1016/0042-6822(77)90150-7. [DOI] [PubMed] [Google Scholar]
- Robertson H. D. Escherichia coli ribonuclease III cleavage sites. Cell. 1982 Oct;30(3):669–672. doi: 10.1016/0092-8674(82)90270-7. [DOI] [PubMed] [Google Scholar]
- Rowen L., Kornberg A. Primase, the dnaG protein of Escherichia coli. An enzyme which starts DNA chains. J Biol Chem. 1978 Feb 10;253(3):758–764. [PubMed] [Google Scholar]
- Sekiya T., Contreras R., Takeya T., Khorana H. G. Total synthesis of a tyrosine suppressor transfer RNA gene. XVII. Transcription, in vitro, of the synthetic gene and processing of the primary transcript to transfer RNA. J Biol Chem. 1979 Jul 10;254(13):5802–5816. [PubMed] [Google Scholar]
- Simons G. F., Konings R. N., Schoenmakers J. G. Genes VI, VII, and IX of phage M13 code for minor capsid proteins of the virion. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4194–4198. doi: 10.1073/pnas.78.7.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B., Apirion D. Primary and secondary structure in a precursor of 5 S rRNA. Biochim Biophys Acta. 1982 Sep 27;698(3):252–259. doi: 10.1016/0167-4781(82)90155-5. [DOI] [PubMed] [Google Scholar]
- Smits M. A., Schoenmakers J. G., Konings R. N. Expression of bacteriophage M13 DNA in vivo. Isolation, identification and characterization of phage-specific mRNA species. Eur J Biochem. 1980 Nov;112(2):309–321. doi: 10.1111/j.1432-1033.1980.tb07206.x. [DOI] [PubMed] [Google Scholar]
- Smits M. A., Simons G., Konings R. N., Schoenmakers J. G. Expression of bacteriophage M13 dna in vivo. I. Synthesis of phage-specific RNA and protein in minicells. Biochim Biophys Acta. 1978 Nov 21;521(1):27–44. doi: 10.1016/0005-2787(78)90246-0. [DOI] [PubMed] [Google Scholar]
- Steege D. A., Graves M. C., Spremulli L. L. Euglena gracilis chloroplast small subunit rRNA. Sequence and base pairing potential of the 3' terminus, cleavage by colicin E3. J Biol Chem. 1982 Sep 10;257(17):10430–10439. [PubMed] [Google Scholar]
- Sugimoto K., Sugisaki H., Okamoto T., Takanami M. Studies on bacteriophage fd DNA. IV. The sequence of messenger RNA for the major coat protein gene. J Mol Biol. 1977 Apr 25;111(4):487–507. doi: 10.1016/s0022-2836(77)80065-x. [DOI] [PubMed] [Google Scholar]
- Thomas M., White R. L., Davis R. W. Hybridization of RNA to double-stranded DNA: formation of R-loops. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2294–2298. doi: 10.1073/pnas.73.7.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen T. S., Webster R. E. Bacteriophage f1 gene II and X proteins. Isolation and characterization of the products of two overlapping genes. J Biol Chem. 1981 Nov 10;256(21):11259–11265. [PubMed] [Google Scholar]
- Yen T. S., Webster R. E. Translational control of bacteriophage f1 gene II and gene X proteins by gene V protein. Cell. 1982 Jun;29(2):337–345. doi: 10.1016/0092-8674(82)90150-7. [DOI] [PubMed] [Google Scholar]
- van Wezenbeek P. M., Hulsebos T. J., Schoenmakers J. G. Nucleotide sequence of the filamentous bacteriophage M13 DNA genome: comparison with phage fd. Gene. 1980 Oct;11(1-2):129–148. doi: 10.1016/0378-1119(80)90093-1. [DOI] [PubMed] [Google Scholar]