Abstract
Copper levels are elevated in a variety of liver fibrosis conditions. Lowering copper to a certain level protects against fibrosis. However, whether severe copper deficiency is protective against liver fibrosis is not known. The purpose of the present study is to evaluate this question by inducing severe copper deficiency using the copper chelator, tetrathiomolybdate (TM), in a bile duct ligation (BDL) rat model. Male Sprague-Dawley rats were divided into four groups: sham, sham plus TM, BDL, and BDL plus TM. TM was given in a daily dose of 10 mg/kg by body weight by means of intragastric gavage, beginning 5 days after BDL. All animals were killed 2 weeks after surgery. Severe copper deficiency was induced by TM overdose in either sham or BDL rats, as shown by decreased plasma ceruloplasmin activity. Liver injury and fibrosis were exacerbated in BDL rats with TM treatment, as illustrated by robustly increased plasma aspartate aminotransferase and hepatic collagen accumulation. Iron stores, as measured by plasma ferritin, were significantly increased in copper-deficient BDL rats. Moreover, hepatic heme oxygenase-1 expression was markedly down-regulated by copper deficiency in BDL rats. In addition, hepatic gene expression involving mitochondrial biogenesis and β-oxidation was significantly up-regulated in BDL rats, and this increase was abolished by copper deficiency. In summary, severe copper deficiency exacerbates BDL-induced liver injury and liver fibrosis, probably caused by increased iron overload and decreased antioxidant defenses and mitochondrial dysfunction.
Introduction
Copper is an essential trace element for many biological processes, including mitochondrial respiration, iron metabolism, detoxification of free radicals, and cross-linking of connective tissue (Bonham et al., 2002). Disruption of copper homeostasis is associated with many human diseases, particularly liver diseases. Excessive copper accumulation in the liver secondary to cholestasis has been well documented in patients with primary biliary cirrhosis (Deering et al., 1977), as well as in alcoholic cirrhotics (Rodríguez-Moreno et al., 1997) and experimental fibrosis (Schaff et al., 1991). Conversely, copper is decreased in the early stage of some liver diseases, such as alcoholic steatosis (Uhlikova et al., 2008), nonalcoholic fatty liver disease (Aigner et al., 2008, 2010), and acute experimental liver injury (Domitrović et al., 2008). Therefore, maintaining normal copper homeostasis could be an important therapeutic target for liver diseases.
It is well established that the fibrotic pathway is modulated by copper (Brewer et al., 2004). Anticopper therapy has been shown to be effective in protecting against bleomycin-induced pulmonary fibrosis (Brewer et al., 2003) and carbon tetrachloride-induced liver cirrhosis in mice (Askari et al., 2004), at least in part, by inhibition of transforming growth factor-β. In addition, lowering copper was demonstrated to protect against both concanavalin A-induced (Askari et al., 2004) and acetaminophen-induced (Ma et al., 2004) liver injury through inhibition of the inflammatory cytokines, tumor necrosis factor-α and interleukin-1β. However, early studies showed that severe copper deficiency exacerbated acute carbon tetrachloride-induced hepatotoxicity (DiSilvestro and Carlson, 1991), whereas hepatic copper preloading protected against carbon tetrachloride-induced liver injury (Barrow and Tanner, 1989). The underlying mechanisms remain to be elucidated.
Copper deficiency is associated with decreased antioxidant defenses, including copper/zinc superoxide dismutase (SOD1), cytochrome c oxidase, glutathione peroxidase (Prohaska, 1991; Johnson and DeMars, 2004) and ceruloplasmin. Copper deficiency has been observed in patients with nonalcoholic fatty liver disease and was correlated with alterations in iron metabolism (Aigner et al., 2008, 2010). Moreover, dietary fructose interacted with copper deficiency and markedly enhanced the metabolic complications of copper deficiency (Fields et al., 1984), suggesting that copper deficiency might be an important component in the “two-hit” model of nonalcoholic steatohepatitis. The mechanisms for these interactions are not well defined.
Our previous work has shown that lowering copper to 30% of baseline protected against bile duct ligation (BDL)-induced liver injury and fibrosis (Song et al., 2008). However, it is not clear whether severe copper deficiency is protective against liver fibrosis. The present study was designed to investigate the effect of severe copper deficiency on BDL-induced liver fibrosis and explore the potential underlying mechanisms.
Materials and Methods
Animal Surgery and Experimental Protocol.
Seven-week-old male Sprague-Dawley rats (200–220 g) were obtained from Harlan (Indianapolis, IN). Rats were housed in the animal facilities of University of Louisville Research Resources Center on a 12-h light/dark cycle and fed food and water ad libitum for 1 week before beginning the experiments. All studies were approved by the University of Louisville Institutional Animal Care and Use Committee, which is certified by the American Association of Accreditation of Laboratory Animal Care. The animals were randomly divided into four groups: sham, sham plus tetrathiomolybdate (TM), BDL, and BDL plus TM. TM [(NH4)2MoS4; PubChem Substance ID 24859366], as an ammonium salt (kindly provided by Dr. George Brewer, University of Michigan, Ann Arbor, MI) was dissolved in deionized water. In TM-treated animals, it was given in a daily dose of 10 mg/kg by body weight by means of intragastric gavage, beginning 5 days after BDL.
Bile duct ligation was performed using a standard technique. In brief, rats were anesthetized with ketamine and xylazine. After midline laparotomy, the common bile duct was exposed and twice ligated with 1-0 silk suture. Sham operation was performed by gently touching the bile duct. All of the animals were killed 2 weeks after surgery, and blood and liver samples were harvested.
Assessment of Copper and Iron Status.
Plasma ceruloplasmin was measured on the basis of its oxidase activity (Schosinsky et al., 1974). Copper and iron content in the liver tissue was measured by inductively coupled plasma mass spectroscopy after predigestion of the tissues with trace metal-grade nitric acid (Thermo Fisher Scientific, Waltham, MA). Plasma ferritin was determined by using a commercially available kit (ALPCO Diagnostics, Salem, NH).
Liver Enzyme Assay.
Plasma alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), and total bilirubin assays were performed with commercially available kits (Infinity; Thermo Fisher Scientific) based on a colorimetric method.
Histology and Immunohistochemistry.
Formalin-fixed, paraffin-embedded liver sections were cut at a 3-μm thickness. Liver injury was determined by staining with hematoxylin and eosin (H&E). Extracellular matrix accumulation in liver sections was determined by staining with Masson's trichrome and Sirius red-fast green. The area in the liver section of positive Sirius red staining was quantified using MetaMorph software (Molecular Devices, Sunnyvale, CA). Specifically, a Molecular Devices Image-1/AT image acquisition and analysis system incorporating an Axioskop 50 microscope (Carl Zeiss Inc., Thornwood, NY) was used to capture and analyze eight nonoverlapping fields per section at 400× magnification. Data from each section were pooled to determined means. Image analysis was performed using techniques described previously (Bergheim et al., 2006).
For immunohistochemical analysis, sections were incubated with anti-α-smooth muscle actin (α-SMA) (DAKO North America, Inc., Carpenteria, CA) or anti-4-hydroxynonenal (4-HNE) (Alpha Diagnostic International Inc., San Antonio, TX), for 30 min. Staining was visualized using the horseradish peroxidase-conjugated DAKO staining system (InVision; DAKO North America, Inc.).
Isolation of RNA and Real-Time RT-PCR.
Total RNA was extracted from liver tissues using TRIzol (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. For real-time RT-PCR, the first-strand cDNA was synthesized using TaqMan Reverse transcription reagents (Applied Biosystems, Foster City, CA). The reverse transcription was carried out using 1× TaqMan RT buffer, 5.5 mM MgCl2, 500 mM of each dNTP, 2.5 mM random hexamer, 8 U of RNase inhibitor, and 25 U of Multiscribe Reverse Transcriptase with 200 ng of total RNA. The RT conditions were 10 min at 25°C, 30 min at 48°C, and 5 min at 95°C. Real-time PCR was performed with an ABI prism 7500 sequence detection system (Applied Biosystems), and reactions were prepared using SYBR green master mix (Applied Biosystems). Primers were designed and synthesized by SABiosciences (Frederick, MD) (see Table 1 for Genbank accession numbers). The parameter Ct (threshold cycle) was defined as the fraction cycle number at which the fluorescence passed the threshold. The relative gene expression was analyzed using the 2−ΔΔCt method (Livak and Schmittgen, 2001) by normalizing with 18S rRNA gene expression in all the experiments.
TABLE 1.
Genbank accession numbers for primers used
Western Blot.
Hepatic nuclear and cytosol segments were extracted by using a commercial available kit (Active Motif Inc., Carlsbad, CA). Equal amounts of protein were loaded and resolved on 10% SDS-polyacrylamide gels and transferred to polyvinylidene difluoride membrane (Millipore Corporation, Billerica, MA). The membrane was blocked and probed with primary antibody for copper chaperone for SOD1 (CCS) (dilution 1:500), SOD1 (dilution 1:1000), manganese superoxide dismutase (SOD2) (dilution 1:1000), nuclear factor erythroid-2-related factor 2 (Nrf2) (dilution 1:500), heme oxygenase-1 (HO-1) (dilution 1:500) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), or histone H3 (dilution 1:5000) (Abcam Inc., Cambridge, MA) overnight at 4°C, and then was incubated with the corresponding horseradish peroxidase-conjugated secondary antibody. Protein signals were visualized using the enhanced chemiluminescence system (GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK). Band intensities were quantified using ImageJ software (http://rsb.info.nih.gov/ij/).
Hepatic Reduced Glutathione/Oxidized Glutathione, and S-Adenosylmethionine Assay.
Reduced glutathione (GSH), oxidized glutathione (GSSG), and S-adenosylmethionine (SAM) were determined by high-performance liquid chromatography.
Statistical Analysis.
Results are expressed as mean ± S.D. Statistical analysis was performed using one-way analysis of variance followed by Newman-Keuls' multiple comparison test. P < 0.05 was considered statistically significant.
Results
Effects of Copper Deficiency and BDL on Body Weight, Liver Weight, and Blood Metabolites.
After 2-week BDL, body weight gain was significantly decreased in BDL rats compared with sham-operated rats. Both body weight and body weight gain were significantly decreased by TM-induced copper deficiency in BDL rats; however, they were not significantly affected by TM treatment in sham-operated rats. Liver weight and liver/body weight ratio were significantly increased after BDL, and they were further increased by copper deficiency. Likewise, plasma cholesterol was significantly higher in BDL rats compared with sham-operated rats, and it was further increased by TM-induced copper deficiency, which is a typical sign associated with copper deficiency (Table 2).
TABLE 2.
Effects of TM and BDL on body weight, liver weight, and plasma cholesterol
Seven-week-old male Sprague-Dawley rats were given either BDL or sham operation. TM was given in a daily dose of 10 mg/kg/day by means of intragastric gavage beginning 5 days after surgery. All animals were killed 2 weeks after surgery. Data are means ± S.D. (n = 5–6).
| Variable | Sham | Sham + TM | BDL | BDL + TM |
|---|---|---|---|---|
| Body weight, g | 284.6 ± 6.0 | 277.6 ± 14.7 | 268.5 ± 14.2 | 237.2 ± 16.9*† |
| Body weight gain, g | 65.20 ± 4.55 | 52.60 ± 12.26 | 42.00 ± 15.54* | 11.33 ± 13.44*† |
| Liver weight, g | 10.42 ± 0.69 | 9.46 ± 0.52 | 12.46 ± 1.49* | 15.28 ± 0.77*† |
| Liver/body weight ratio, % | 3.659 ± 0.169 | 3.410 ± 0.139 | 4.664 ± 0.718* | 6.469 ± 0.521*† |
| Cholesterol, mg/dl | 94.3 ± 14.2 | 85.3 ± 4.6 | 119.6 ± 17.4* | 171.7 ± 28.3*† |
, significantly different from sham group.
, significantly different from BDL group.
Copper and Iron Status.
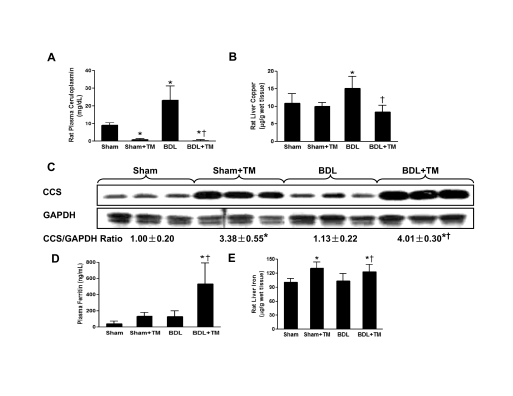
As expected, the plasma ceruloplasmin level was significantly elevated (approximately 2.5-fold) in the rats after 2 weeks of BDL. Conversely, the plasma ceruloplasmin was significantly decreased in both sham and BDL animals treated with TM compared with animals with sham operation only (approximately 8 and 3% of baseline, respectively) (Fig. 1A). Liver copper was significantly increased in BDL rats compared with sham animals, and it was significantly decreased in BDL plus TM rats compared with BDL animals (Fig. 1B). As an indicator of copper status (Harvey and McArdle, 2008), hepatic CCS expression was up-regulated by copper deficiency and was negatively related to the liver copper and plasma ceruloplasmin level, as shown by Western blot (Fig. 1C). All of the data suggested that biologically available copper was severely deficient in TM-treated animals. The plasma ferritin, a marker of total body iron stores, was robustly increased (approximately 13-fold) in BDL rats treated with TM compared with controls (Fig. 1D). Liver iron was also significantly increased in both sham and BDL rats treated with TM compared with control (Fig. 1E).
Fig. 1.
Effect of TM on copper and iron status 2 weeks after bile duct ligation. BDL or sham operation was performed in male Sprague-Dawley rats as described under Materials and Methods. TM was given in a daily dose of 10 mg/kg/day by means of intragastric gavage beginning 5 days after surgery until 2 weeks later. A, B, D, and E, plasma ceruloplasmin (A), liver copper (B), plasma ferritin (D), and liver iron (E) were determined as described under Materials and Methods. Data represent means ± S.D. (n = 5–6). *, significantly different from sham group; †, significantly different from BDL group. C, hepatic CCS expression was examined by Western blot analysis using whole liver cytosol extract, and optical density of band was quantified by ImageJ software. The ratio to GAPDH was calculated by assigning the value from sham controls as one. Data represent means ± S.D. (n = 3).
Copper Deficiency Exacerbated BDL-Induced Cholestatic Liver Injury.
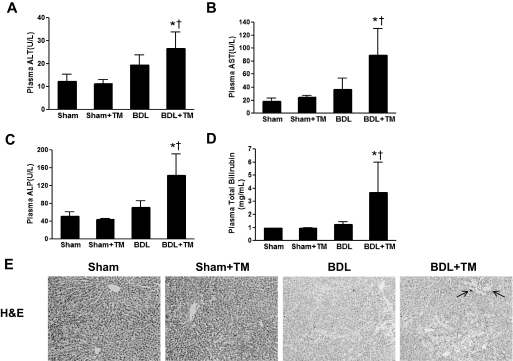
Liver injury was assessed by plasma liver enzymes (ALT, AST, and ALP), total bilirubin, and histology. The plasma ALT, AST, and ALP levels were not significantly changed by TM-induced copper deficiency in sham-operated rats compared with controls. After 2 weeks of BDL, plasma ALT, AST, and ALP were slightly elevated (differences did not reach statistical significance). However, TM-induced copper deficiency led to a significant increase in plasma ALT level by 2-fold in BDL rats compared with sham-operated animals (Fig. 2A). The plasma AST level was robustly increased by approximately 4- and 2-fold, respectively in BDL plus TM rats compared with sham and BDL animals (Fig. 2B). Likewise, the plasma ALP and total bilirubin were also significantly elevated in copper-deficient BDL rats compared with controls (Fig. 2, C and D), suggesting exacerbated cholestatic liver injury caused by copper deficiency in BDL animals.
Fig. 2.
Effect of copper deficiency and BDL on plasma liver enzymes, total bilirubin, and liver histology. The animals were subjected to the same treatment protocol as described in Fig. 1. A to D, ALT (A), AST (B), ALP (C), and total bilirubin (D) were determined in plasma samples by colorimetric assay. Data represent means ± S.D. (n = 5–6). *, significantly different from sham group; †, significantly different from BDL group. E, representative photomicrographs (100×) of liver sections with H&E staining depicting liver injury as shown by bile infarcts and neutrophil infiltration (arrows).
Bile infarcts are the typical features of cholestatic liver injury caused by bile acid-induced hepatocyte necrosis. Consistent with the biochemical findings, H&E staining showed extensive bile infarcts, along with distinct neutrophil infiltration in the liver sections of BDL plus TM rats; by contrast, very few bile infarcts were seen in the livers of rats with 2 weeks of BDL alone. Short-term copper deficiency induced by TM did not cause significant pathological changes in the livers of sham-operated animals compared with controls (Fig. 2E). Collectively, these data suggest that copper deficiency exacerbated BDL-induced cholestatic liver injury.
TM-Induced Copper Deficiency Aggravated Hepatic Fibrosis Induced by BDL.
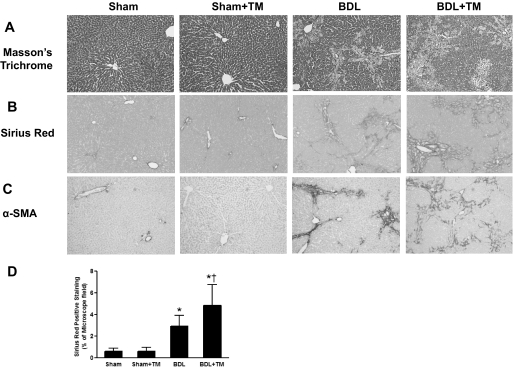
Hepatic fibrosis was evaluated by Masson's trichrome and Sirius red staining of liver sections. Collagen content was assessed by morphometrical analysis of Sirius red staining. In sham-operated rat livers, only normal staining around vessels and no fibrosis was observed. TM-induced copper deficiency did not cause overt histological changes in sham-operated rats. After 2 weeks of BDL, collagen accumulation was obvious in both the portal triad and interstitial areas, with bridging fibrosis formation, and this was significantly increased by TM-induced copper deficiency as shown by Masson's trichrome and Sirius red staining (Fig. 3, A and B). Quantification of Sirius red staining by image analysis showed that collagen content was robustly increased (5-fold) in the livers of BDL rats, and it was further significantly increased (8-fold) with TM-induced copper deficiency compared with controls (Fig. 3D).
Fig. 3.
Effect of copper deficiency on BDL-induced liver fibrosis. The animals and treatment were the same as described in Fig. 1. Collagen deposition was evaluated by Masson's trichrome and Sirius red staining. Hepatic fibrogenesis was assessed by α-SMA, a marker of hepatic stellate cell activation. A, representative photomicrographs (100×) of liver sections with Masson's trichrome staining. B, representative photomicrographs (100×) of liver sections with Sirius red staining. C, representative photomicrographs (100×) of immunohistochemistry staining for liver α-SMA. D, quantification of Sirius red-positive staining by image analysis. Data represent means ± S.D. (n = 5–6). *, significantly different from sham group; †, significantly different from BDL group.
We further evaluated fibrogenesis by immunohistochemistry staining for α-SMA, a marker of hepatic stellate cell activation. Compared with controls, α-SMA expression was markedly increased in the livers of BDL rats, and it was further enhanced by TM-induced copper deficiency, suggesting enhanced fibrogenesis (Fig. 3C). Taken together, our data clearly showed that copper deficiency aggravated BDL-induced liver fibrosis, which paralleled the severity of liver injury.
TM-Induced Copper Deficiency Inhibited Hepatic Gene Expression Involved in Mitochondrial Biogenesis and Fatty Acid β-Oxidation.
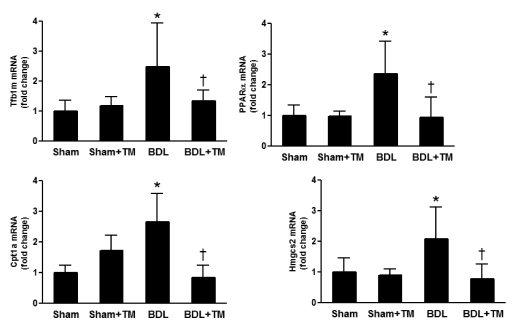
Data from liver enzyme assays suggested that mitochondrial impairment is a likely potential mechanism involved in copper deficiency-associated liver injury. To further evaluate mitochondrial function, the gene expression implicated in mitochondria biogenesis and fatty acid β-oxidation was determined by real-time RT-PCR (Fig. 4). Our data showed that the mRNA expression of the mitochondrial transcription factor B1 (Tfb1m), a transcription factor that is necessary for basal transcription of mammalian mitochondrial DNA (Falkenberg et al., 2002), was significantly up-regulated more than 2-fold in the livers of BDL rats, and this was abrogated in copper-deficient BDL rats. The mRNA expression of the key genes regulating fatty acid oxidation, such as peroxisome proliferator-activated receptor α (Ppar-α), carnitine palmitoyltransferase 1a, liver (Cpt1a), and 3-hydroxy-3-methylglutaryl-coenzyme A synthase 2 (mitochondrial) (Hmgcs2), was significantly increased in BDL rat liver, whereas this increase was blocked in copper-deficient BDL rats.
Fig. 4.
Effect of copper deficiency and BDL on the gene expression involving mitochondrial biogenesis and fatty acid β-oxidation. Real-time RT-PCR was performed as described under Materials and Methods to determine hepatic Tfb1m, Pparα, Cpt1a, and Hmgcs2 mRNA expression. The expression was normalized as a ratio using 18S ribosomal RNA (18SrRNA) as housekeeping gene. A value of 1 for this ratio was arbitrarily assigned to the data obtained from sham-operated rat. Data represent means ± S.D. (n = 5–6). *, significantly different from sham group; †, significantly different from BDL group.
Increased Oxidative Stress and Decreased Antioxidant Defense by TM-Induced Copper Deficiency in BDL Rats.
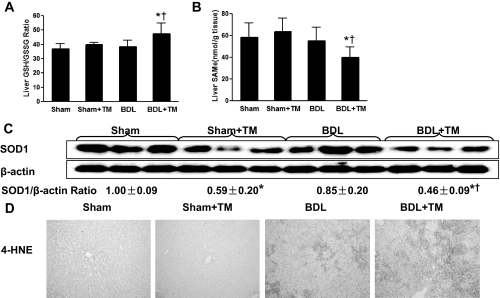
To evaluate the possible effects of BDL and copper deficiency on oxidative stress, GSH/GSSG ratio, SAM, and 4-HNE were determined. A significant increase in GSH/GSSG ratio (Fig. 5A) was observed, suggesting a response to more oxidative stress in copper-deficient BDL rats. Hepatic SAM, a precursor of GSH synthesis, was also significantly decreased in copper-deficient BDL rats (Fig. 5B). SODs are important antioxidant defenses against oxidative stress. SOD1, a cuproenzyme, is distributed mainly in the cytosol and the intermembrane space of mitochondria (Okado-Matsumoto and Fridovich, 2001). SOD2 is located in the mitochondria. Hepatic SOD1 expression was significantly down-regulated in both sham and BDL rats in response to TM-induced copper deficiency, as shown by Western blot (Fig. 5C). However, hepatic SOD2 expression was not significantly different in all groups (data not show). 4-HNE is the end product of lipid peroxidation, and its adduction to proteins serves as a marker of lipid peroxidation. As shown in Fig. 5D, immunoreactivity of 4-HNE in the liver was increased by BDL, and it was further enhanced by copper deficiency in BDL rats.
Fig. 5.
Effect of copper deficiency and BDL on hepatic oxidative stress and antioxidant defense system. A and B, hepatic GSH/GSSG ratio and SAM were measured by high-performance liquid chromatography. Data represent means ± S.D. (n = 5–6). *, significantly different from sham group; †, significantly different from BDL group. C, hepatic SOD1 expression was examined by Western blot analysis using whole liver cytosol extract (except nuclear), and optical density of band was quantified by ImageJ software. The ratio to β-actin was calculated by assigning the value from sham controls as 1. Data represent means ± S.D. (n = 3). D, representative photomicrographs of the immunohistochemistry staining for 4-HNE in liver section (100×).
Hepatic Heme Oxygenase-1 Expression Was Significantly Inhibited by BDL and Copper Deficiency Interaction.
HO-1 is a stress-related Nrf2 target gene and an antioxidant defense enzyme. As shown in Fig. 6A, HO-1 expression was significantly down-regulated by copper deficiency in BDL rat liver. Nrf2 is a transcription factor that regulates the expression of antioxidative and other cytoprotective genes, including HO-1.To further understand the molecular mechanism by which copper deficiency and BDL induced inhibition of antioxidant defense, Nrf2 expression was evaluated by Western blot. Hepatic nuclear Nrf2 expression was slightly up-regulated in response to copper deficiency in sham-operated rat liver, whereas it was slightly down-regulated after 2-week BDL. However, the differences did not reach statistical significance (data not show).
Fig. 6.
Effect of copper deficiency and BDL on hepatic HO-1 expression. Hepatic HO-1 expression was determined by Western blots. Optical density of band was quantified by ImageJ software. The ratio to GAPDH was calculated by assigning the value from sham controls as 1. Data represent means ± S.D. (n = 3). *, significantly different from sham group; †, significantly different from BDL group.
Discussion
Our previous data have demonstrated that lowering copper to 30% of basal level protected against BDL-induced liver fibrosis (Song et al., 2008). However, when copper is severely deficient (<8% of baseline in terms of ceruloplasmin) (Fig. 1A), it exacerbated BDL-induced liver injury and liver fibrosis. Sprague-Dawley rats are known to be a sensitive model in response to copper deficiency, and low-dose TM (10 mg/kg/day) depleted copper to a greater extent in the Sprague-Dawley rat than it did in our previous mouse model (Song et al., 2008). It seemed that the severity of liver fibrosis paralleled the severity of liver injury and hepatic stellate cell activation in rats subjected to BDL and severe copper deficiency, suggesting that liver injury is the primary event in the liver fibrosis induced by copper deficiency and BDL. Moreover, liver injury in BDL rats resulting from copper deficiency is characterized by robustly increased plasma AST (Fig. 2B), suggesting potential mitochondrial impairment (Panteghini, 1990). In fact, previous work has shown that both acute and chronic severe copper deficiency led to abnormal mitochondria (Gallagher et al., 1973). However, the underlying mechanisms are not fully understood.
One of the principal cuproenzymes, cytochrome c oxidase, is the terminal enzyme of the mitochondrial respiratory chain. Another cuproenzyme, SOD1, is an important antioxidant defense against oxidative stress. The distribution of SOD1 is mainly in the cytosol, and it has also been found in the intermembrane space of mitochondria (Okado-Matsumoto and Fridovich, 2001). It is well documented that activity of both cytochrome c oxidase and SOD1 were decreased by copper deficiency (Prohaska, 1991; Johnson and DeMars, 2004). Consistent with previous studies, our data also showed that hepatic SOD1 expression was significantly decreased in response to copper deficiency (Fig. 5C). Increased iron stores secondary to copper deficiency may be another important factor for the mitochondrial damage. Iron export from nonintestinal cells requires ceruloplasmin, the principal copper carrying protein with ferroxidase activity. Ferrous (Fe2+) ion must be converted to ferric (Fe3+) ion by ferroxidase before being exported out of the cells for transport in the plasma. Ceruloplasmin deficiency prevents iron release from cells and iron accumulates in the liver in macrophages, hepatocytes, and cells from several other organs (Harris et al., 1999). Our data clearly showed extremely low plasma ceruloplasmin activity in both sham and BDL animals induced by TM (Fig. 1A).
Iron plays an essential role in the maintenance of mitochondria, through its two major functional forms, heme and iron-sulfur clusters. Most of the iron within cells is routed to the mitochondria for heme biosynthesis and maturation of Fe-S clusters (Atamna et al., 2002). Heme degradation is controlled predominantly by heme oxygenase (HO). Humans and rodents have two HO isoenzymes, namely HO-1 and HO-2, with HO-1 being the only inducible form in response to oxidative stress (Gozzelino et al., 2010). Mice lacking HO-1 displayed hepatic iron overload and liver injury (Poss and Tonegawa, 1997), whereas induction of HO-1 suppressed inflammation and oxidative stress and protected against steatohepatitis (Yu et al., 2010) and a variety of other types of liver injury (Lin et al., 2010; Yun et al., 2010). In the present study, hepatic HO-1 expression was significantly suppressed by copper deficiency in BDL mice, and this may be another contributor to increased iron stores and liver injury. Moreover, in addition to copper, heme is an important component of the cytochrome c oxidase subunit (Fontanesi et al., 2008). Thus, it is plausible that disturbing heme homeostasis may also contribute to decreased cytochrome c oxidase activity. Collectively, copper deficiency-associated ceruloplasmin activity inhibition coupled with HO-1 suppression led to iron accumulation in mitochondria, which in turn, led to increased reactive oxygen species via the Fenton reaction, mitochondria dysfunction, and ultimately, cell death. It seemed that increased iron stores paralleled the severity of liver injury and fibrosis, suggesting it may be an important mechanism underlying exacerbated liver injury by copper deficiency in BDL rats.
Maintaining mitochondrial homeostasis involves biogenesis and replacement. Therefore, we further evaluated mitochondrial biogenesis by determining the related gene expression. We found that the mRNA expression of Tfb1m, a transcription factor that is necessary for basal transcription of mammalian mitochondrial DNA (Falkenberg et al., 2002), was significantly up-regulated more than 2-fold in the livers of BDL rats, and this was abrogated in copper-deficient BDL rats. Likewise, the mRNA expression of the key genes regulating fatty acid β-oxidation, such as PPAR-α (Shalev et al., 1996), Cpt1a (Akkaoui et al., 2009), and Hmgcs2 (Kostiuk et al., 2008), were significantly increased in BDL rat livers, whereas this increase was blocked in copper-deficient BDL rats. A study showed that mitochondrial biogenesis may be regulated by HO-1 in heart (Piantadosi et al., 2008), which may also explain our results. Taken together, it seems that disruption of mitochondrial biogenesis is another likely mechanism by which copper deficiency exacerbates liver injury in BDL rats.
Previous work showed that the activity of hepatic HO-1 in rats is elevated in response to 5 weeks of copper deficiency (Johnson and DeMars, 2004). Our data showed that hepatic HO-1 expression did not significantly increase in response to copper deficiency in sham-operated rats, probably because of the short term of copper deficiency (10 days). Conversely, HO-1 expression was significantly inhibited in BDL rats in response to copper deficiency, suggesting other factors, such as accumulated bile acids, may play a role. The mechanism responsible for the decreased HO-1 expression is poorly understood. Nrf2 is a transcription factor that regulates the expression of antioxidative and other cytoprotective genes, including HO-1 (Yao et al., 2007; Yeligar et al., 2010). However, hepatic nuclear Nrf2 expression in BDL rats was not significantly changed in response to copper deficiency (data not shown), suggesting either a disturbance of Nrf2 DNA binding or other transcription factors might be involved.
In summary, our data demonstrated that copper deficiency may lead to iron overload, possibly by inhibition of ceruloplasmin activity and HO-1 expression, which, in turn, cause mitochondria dysfunction. Severe copper deficiency synchronized with BDL to exacerbate liver injury and fibrosis. Our data provide further insights into the understanding of the role of copper homeostasis in liver injury and fibrosis.
Acknowledgments
We thank Dr. Gavin E. Arteel for the help with image quantification; staff of the Center for Regulatory and Environmental Analytical Metabolomics Mass Spectrometry Facility at the University of Louisville for support; and Dr. Richard M. Higashi and Dr. Teresa W.-M. Fan for help with measuring trace metals.
This study was supported by the National Institutes of Health National Institute on Alcohol Abuse and Alcoholism [Grants PO1-AA017103, P30-AA019360, RO1-AA015970, RO1-AA018016, RO1-AA018869, R37-AA010762, RC2-AA019385, RO1-AA014623, RO1-AA016013 (to C.J.M.), RO1-AA018844 (to Z.Z.)]; National Institute of Diabetes and Digestive and Kidney Diseases [Grant RO1-DK071765] (to C.J.M.); Louisville VA Medical Center [Grant 5I01BX000350] (to C.J.M.); and the National Science Foundation Experimental Program to Stimulate Competitive Research [Grant EPS-0447479].
This article was presented previously as a poster presentation: Song M, Zhou Z, Chen T, Zhang J, and McClain CJ (2010) Copper deficiency exacerbates bile duct ligation-induced liver injury and fibrosis in Rats, at the 15th International Society for Hepatic Sinusoidal Research Symposium; 2010 Aug 28-Sept 1; Pasadena, CA. International Society for Hepatic Sinusoidal Research.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.111.184325.
- SOD1
- copper/zinc superoxide dismutase
- CCS
- copper chaperone for SOD1
- SOD2
- manganese superoxide dismutase
- TM
- tetrathiomolybdate
- BDL
- bile duct ligation
- ALT
- alanine aminotransferase
- AST
- aspartate aminotransferase
- ALP
- alkaline phosphatase
- H&E
- hematoxylin and eosin
- α-SMA
- α-smooth muscle actin
- Nrf2
- nuclear factor erythroid-2-related factor 2
- HO
- heme oxygenase
- GSH
- reduced glutathione
- GSSG
- oxidized glutathione
- Tfb1m
- mitochondrial transcription factor B1
- SAM
- S-adenosylmethionine
- 4-HNE
- hydroxynonenal
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- PPARα
- peroxisome proliferator-activated receptor α
- Cpt1a
- carnitine palmitoyltransferase 1a, liver
- Hmgcs2
- 3-hydroxy-3-methylglutaryl-coenzyme A synthase 2 (mitochondrial)
- 18SrRNA
- 18S ribosomal RNA
- RT-PCR
- reverse transcription-polymerase chain reaction.
Authorship Contributions
Participated in research design: Song and McClain.
Conducted experiments: Song, Zhou, and Zhang.
Performed data analysis: Song, Chen, and McClain.
Wrote or contributed to the writing of the manuscript: Song and McClain.
References
- Aigner E, Strasser M, Haufe H, Sonnweber T, Hohla F, Stadlmayr A, Solioz M, Tilg H, Patsch W, Weiss G, et al. (2010) A role for low hepatic copper concentrations in nonalcoholic fatty liver disease. Am J Gastroenterol 105:1978–1985 [DOI] [PubMed] [Google Scholar]
- Aigner E, Theurl I, Haufe H, Seifert M, Hohla F, Scharinger L, Stickel F, Mourlane F, Weiss G, Datz C. (2008) Copper availability contributes to iron perturbations in human nonalcoholic fatty liver disease. Gastroenterology 135:680–688 [DOI] [PubMed] [Google Scholar]
- Akkaoui M, Cohen I, Esnous C, Lenoir V, Sournac M, Girard J, Prip-Buus C. (2009) Modulation of the hepatic malonyl-CoA-carnitine palmitoyltransferase 1A partnership creates a metabolic switch allowing oxidation of de novo fatty acids. Biochem J 420:429–438 [DOI] [PubMed] [Google Scholar]
- Askari FK, Dick R, Mao M, Brewer GJ. (2004) Tetrathiomolybdate therapy protects against concanavalin A and carbon tetrachloride hepatic damage in mice. Exp Biol Med (Maywood) 229:857–863 [DOI] [PubMed] [Google Scholar]
- Atamna H, Walter PB, Ames BN. (2002) The role of heme and iron-sulfur clusters in mitochondrial biogenesis, maintenance, and decay with age. Arch Biochem Biophys 397:345–353 [DOI] [PubMed] [Google Scholar]
- Barrow L, Tanner MS. (1989) The effect of carbon tetrachloride on the copper-laden rat liver. Br J Exp Pathol 70:9–19 [PMC free article] [PubMed] [Google Scholar]
- Bergheim I, Guo L, Davis MA, Duveau I, Arteel GE. (2006) Critical role of plasminogen activator inhibitor-1 in cholestatic liver injury and fibrosis. J Pharmacol Exp Ther 316:592–600 [DOI] [PubMed] [Google Scholar]
- Brewer GJ, Dick R, Ullenbruch MR, Jin H, Phan SH. (2004) Inhibition of key cytokines by tetrathiomolybdate in the bleomycin model of pulmonary fibrosis. J Inorg Biochem 98:2160–2167 [DOI] [PubMed] [Google Scholar]
- Brewer GJ, Ullenbruch MR, Dick R, Olivarez L, Phan SH. (2003) Tetrathiomolybdate therapy protects against bleomycin-induced pulmonary fibrosis in mice. J Lab Clin Med 141:210–216 [DOI] [PubMed] [Google Scholar]
- Bonham M, O'Connor JM, Hannigan BM, Strain JJ. (2002) The immune system as a physiological indicator of marginal copper status? Br J Nutr 87:393–403 [DOI] [PubMed] [Google Scholar]
- Deering TB, Dickson ER, Fleming CR, Geall MG, McCall JT, Baggenstoss AH. (1977) Effect of d-penicillamine on copper retention in patients with primary billiary cirrhosis. Gastroenterology 72:1208–1212 [PubMed] [Google Scholar]
- DiSilvestro RA, Carlson GP. (1991) Effects of moderate copper deficiency on carbon tetrachloride-induced hepatotoxicity in rats. Proc Soc Exp Biol Med 197:32–35 [DOI] [PubMed] [Google Scholar]
- Domitrović R, Jakovac H, Grebić D, Milin C, Radosević-Stasić B. (2008) Dose- and time-dependent effects of luteolin on liver metallothioneins and metals in carbon tetrachloride-induced hepatotoxicity in mice. Biol Trace Elem Res 126:176–185 [DOI] [PubMed] [Google Scholar]
- Falkenberg M, Gaspari M, Rantanen A, Trifunovic A, Larsson NG, Gustafsson CM. (2002) Mitochondrial transcription factors B1 and B2 activate transcription of human mtDNA. Nat Genet 31:289–294 [DOI] [PubMed] [Google Scholar]
- Fields M, Ferretti RJ, Smith JC, Jr, Reiser S. (1984) The interaction of type of dietary carbohydrates with copper deficiency. Am J Clin Nutr 39:289–295 [DOI] [PubMed] [Google Scholar]
- Fontanesi F, Soto IC, Barrientos A. (2008) Cytochrome c oxidase biogenesis: new levels of regulation. IUBMB Life 60:557–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher CH, Reeve VE, Wright R. (1973) Copper deficiency in the rat. Effect on the ultrastructure of hepatocytes. Aust J Exp Biol Med Sci 51:181–189 [DOI] [PubMed] [Google Scholar]
- Gozzelino R, Jeney V, Soares MP. (2010) Mechanisms of cell protection by heme oxygenase-1. Annu Rev Pharmacol Toxicol 50:323–354 [DOI] [PubMed] [Google Scholar]
- Harris ZL, Durley AP, Man TK, Gitlin JD. (1999) Targeted gene disruption reveals an essential role for ceruloplasmin in cellular iron efflux. Proc Natl Acad Sci U S A 96:10812–10817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey LJ, McArdle HJ. (2008) Biomarkers of copper status: a brief update. Br J Nutr 99:S10–S13 [DOI] [PubMed] [Google Scholar]
- Johnson WT, DeMars LC. (2004) Increased heme oxygenase-1 expression during copper deficiency in rats results from increased mitochondrial generation of hydrogen peroxide. J Nutr 134:1328–1333 [DOI] [PubMed] [Google Scholar]
- Kostiuk MA, Corvi MM, Keller BO, Plummer G, Prescher JA, Hangauer MJ, Bertozzi CR, Rajaiah G, Falck JR, Berthiaume LG. (2008) Identification of palmitoylated mitochondrial proteins using a bio-orthogonal azido-palmitate analogue. FASEB J 22:721–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Yu CH, Jen CY, Cheng CF, Chou Y, Chang CC, Juan SH. (2010) Adiponectin-mediated heme oxygenase-1 induction protects against iron-induced liver injury via a PPARα dependent mechanism. Am J Pathol 177:1697–1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. (2001) Analysis of relative gene expression data using real-time quantitative PCR and 2−ΔΔCt method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- Ma S, Hou G, Dick RD, Brewer GJ. (2004) Tetrathiomolybdate protects against liver injury from acetaminophen in mice. J Appl Res Clin Exp Ther 4:419–426 [Google Scholar]
- Okado-Matsumoto A, Fridovich I. (2001) Subcellular distribution of superoxide dismutases (SOD) in rat liver: Cu,Zn-SOD in mitochondria. J Biol Chem 276:38388–38393 [DOI] [PubMed] [Google Scholar]
- Panteghini M. (1990) Aspartate aminotransferase isoenzymes. Clin Biochem 23:311–319 [DOI] [PubMed] [Google Scholar]
- Piantadosi CA, Carraway MS, Babiker A, Suliman HB. (2008) Heme oxygenase-1 regulates cardiac mitochondrial biogenesis via Nrf2-mediated transcriptional control of nuclear respiratory factor-1. Circ Res 103:1232–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poss KD, Tonegawa S. (1997) Heme oxygenase 1 is required for mammalian iron reutilization. Proc Natl Acad Sci U S A 94:10919–10924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prohaska JR. (1991) Changes in Cu,Zn-superoxide dismutase, cytochrome c oxidase, glutathione peroxidase and glutathione transferase activities in copper-deficient mice and rats. J Nutr 121:355–363 [DOI] [PubMed] [Google Scholar]
- Rodríguez-Moreno F, González-Reimers E, Santolaria-Fernández F, Galindo-Martín L, Hernandez-Torres O, Batista-López N, Molina-Perez M. (1997) Zinc, copper, manganese, and iron in chronic alcoholic liver disease. Alcohol 14:39–44 [DOI] [PubMed] [Google Scholar]
- Schaff Z, Lapis K, Szende B, Jeney A, Gergely P, Simon K, Divald A, Timár F, Major J. (1991) The effect of d-penicillamine on CCl4-induced experimental liver cirrhosis. Exp Pathol 43:111–120 [DOI] [PubMed] [Google Scholar]
- Schosinsky KH, Lehmann HP, Beeler MF. (1974) Measurement of ceruloplasmin from its oxidase activity in serum by use of o-dianisidine dihydrochloride. Clin Chem 20:1556–1563 [PubMed] [Google Scholar]
- Shalev A, Siegrist-Kaiser CA, Yen PM, Wahli W, Burger AG, Chin WW, Meier CA. (1996) The peroxisome proliferator-activated receptor α is a phosphoprotein: regulation by insulin. Endocrinology 137:4499–4502 [DOI] [PubMed] [Google Scholar]
- Song M, Song Z, Barve S, Zhang J, Chen T, Liu M, Arteel GE, Brewer GJ, McClain CJ. (2008) Tetrathiomolybdate protects against bile duct ligation-induced cholestatic liver injury and fibrosis. J Pharmacol Exp Ther 325:409–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlikova E, Kupcova V, Szantova M, Turecky L. (2008) Plasma copper and ceruloplasmin in patients with alcoholic liver steatosis. Bratisl Lek Listy 109:431–433 [PubMed] [Google Scholar]
- Yao P, Nussler A, Liu L, Hao L, Song F, Schirmeier A, Nussler N. (2007) Quercetin protects human hepatocytes from ethanol-derived oxidative stress by inducing heme oxygenase-1 via the MAPK/Nrf2 pathways. J Hepatol 47:253–261 [DOI] [PubMed] [Google Scholar]
- Yeligar SM, Machida K, Kalra VK. (2010) Ethanol-induced HO-1 and NQO1 are differentially regulated by HIF-1α and Nrf2 to attenuate inflammatory cytokine expression. J Biol Chem 285:35359–35373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Chu ES, Wang R, Wang S, Wu CW, Wong VW, Chan HL, Farrell GC, Sung JJ. (2010) Heme oxygenase-1 protects against steatohepatitis in both cultured hepatocytes and mice. Gastroenterology 138:694–704 [DOI] [PubMed] [Google Scholar]
- Yun N, Eum HA, Lee SM. (2010) Protective role of heme oxygenase-1 against liver damage caused by hepatic ischemia and reperfusion in rats. Antioxid Redox Signal 13:1503–1512 [DOI] [PubMed] [Google Scholar]