Abstract
Dysregulation of the 5-HT2A receptor is implicated in both the etiology and treatment of schizophrenia. Although the essential role of 5-HT2A receptors in atypical antipsychotic drug actions is widely accepted, the contribution of 5-HT2A down-regulation to their efficacy is not known. We hypothesized that down-regulation of cortical 5-HT2A receptors contributes to the therapeutic action of atypical antipsychotic drugs. To test this hypothesis, we assessed the effect of chronically administered antipsychotics (clozapine, olanzapine, and haloperidol) and several 5-HT2A antagonists [ketanserin, altanserin, α-(2,3-dimethoxyphenyl)-1-[2-(4-fluorophenylethyl)]-4-piperidinemethanol (M100907), α-phenyl-1-(2-phenylethyl)-4-piperidinemethano (M11939), 4-[(2Z)-3-{[2-(dimethylamino)ethoxy]amino}-3-(2-fluorophenyl)prop-2-en-1-ylidene]cyclohexa-2,5-dien-1-one (SR46349B), and pimavanserin], on the phencyclidine (PCP)-induced hyperlocomotor response and cortical 5-HT2A receptor levels in C57BL/6J mice. Clozapine and olanzapine, but not haloperidol, induced receptor down-regulation and attenuated PCP-induced locomotor responses. Of the selective 5-HT2A antagonists tested, only ketanserin caused significant receptor protein down-regulation, whereas SR46349B up-regulated 5-HT2A receptors and potentiated PCP-hyperlocomotion; the other 5-HT2A receptor antagonists were without effect. The significance of these findings with respect to atypical antipsychotic drug action is discussed.
Introduction
According to classic concepts of pharmacology, competitive receptor antagonists occupy the ligand binding site and “antagonize” receptor activity by precluding agonist binding: “In competitive antagonism, agonist and antagonist, simultaneously present in solution, are each considered to compete for receptors to the exclusion of the other….” (Stephenson, 1956). This notion of blockade of G protein-coupled receptor activation by “steric hindrance” has been supported by recent crystallographic studies of the β-adrenergic receptor, showing that agonists and antagonists occupy overlapping (but not identical) binding sites (Rosenbaum et al., 2007, 2011; Warne et al., 2011). The notion that some G protein-coupled receptor antagonists can also stabilize the inactive state of the receptor and thereby function as inverse agonists was first put forth by Costa and Herz (1989) in studies of opioid receptors. It is now well accepted that so-called antagonists could have negative intrinsic activity and thus function as inverse agonists or lack activity and thus function as neutral antagonists. However, by definition an “antagonist” is devoid of efficacy.
Contradicting these fundamental notions of pharmacology have been observations dating to the late 1970s that some so-called antagonists can display various patterns of efficacy. Thus, Bergstrom and Kellar (1979) and Peroutka and Snyder (1980) showed that chronic administration of antidepressants with potent 5-HT2A antagonist activity induces a down-regulation of 5-HT2A receptor levels. This property was subsequently demonstrated to be shared by a number of drugs with potent 5-HT2A antagonist activity, including typical and atypical antipsychotic drugs (Andree et al., 1986), typical and atypical antidepressants (Blackshear and Sanders-Bush, 1982; Brunello et al., 1982), and even relatively selective 5-HT2A antagonists (Leysen et al., 1986). There have also been reports that some 5-HT2A antagonists can up-regulate 5-HT2A receptors after chronic administration (Rinaldi-Carmona et al., 1993) or have no effect (Gray and Roth, 2001). Antagonist-induced 5-HT2A down-regulation might have therapeutic relevance (Elphick et al., 2004; Harvey et al., 2004; O'Connor and Roth, 2005). We now know that antagonist-induced receptor down-regulation can be readily explained by the concept of functional selectivity (Urban et al., 2007) or biased agonism (Roth and Chuang, 1987; Galandrin et al., 2007).
The mechanism(s) responsible for the paradoxical antagonist-induced receptor “down-regulation” are still unclear. Several studies have demonstrated that 5-HT2A antagonists can induce receptor internalization in vitro (Berry et al., 1996; Willins et al., 1999; Bhatnagar et al., 2001) and in vivo (Willins et al., 1998, 1999). In addition, receptor down-regulation is apparently independent of changes in receptor gene transcription (Roth and Ciaranello, 1991). Two independent studies have indicated that antagonist-induced internalization occurs via clathrin-dependent processes in vitro (Bhatnagar et al., 2001; Hanley and Hensler, 2002).
In addition, although there have been a large number of reports of antagonist-induced down-regulation of 5-HT2A receptors, all prior studies have relied on radioligand binding to quantify changes in total receptor protein. There are a number of possible explanations for alterations in radioligand binding after drug administration that could account for changes in receptor protein levels independent of receptor down-regulation (e.g., residual drug, pseudo-irreversible binding, and receptor inactivation). Ideally, one would like to be able to quantify 5-HT2A receptor protein via a conventional biochemical technique (for instance, Western blotting) to begin to definitively address the question of antagonist functional selectivity.
It has long been known that available 5-HT2A antibodies are neither sufficiently specific nor sensitive for biochemical studies in vivo (Xia et al., 2003). In recent studies, however, we identified and validated a 5-HT2A receptor antibody in wild-type and 5-HT2A knockout mice and showed that it was suitable for immunochemical and biochemical studies (Magalhaes et al., 2010; Yadav et al., 2011). Similar results were reported by another group (Weber and Andrade, 2010). Using this new reagent, we are now able to report that 5-HT2A antagonists differentially regulate 5-HT2A receptor protein levels in vivo. In addition, we are able to identify three classes of 5-HT2A antagonists: those that induce receptor down-regulation, those that induce receptor up-regulation, and those that have no effect on receptor protein levels.
Materials and Methods
Mice.
Male C57BL/6J mice were either obtained from The Jackson Laboratory (Bar Harbor, ME) or bred for two to three generations in-house to obtain a sufficient number of male mice (24–30) between 8 and 10 weeks of age to test drugs. All experiments were approved by the Institutional Animal Care and Use Committee at the University of North Carolina, Chapel Hill. Mice were housed under standard conditions: 12-h light/dark cycle and food and water ad libitum.
Drugs.
Clozapine [8-chloro-11-(4-methyl-1-piperazinyl)-5H-dibenzo[b,e][1,4]diazepin] and olanzapine [2-methyl-4-(4-methyl-1-piperazinyl)-10H-thieno[2,3-b][1,5]benzodiazepine] were purchased from Sigma-Aldrich (St. Louis, MO), and haloperidol [(4-[4-(4-chlorophenyl)-4-hydroxy-1-piperidinyl]-1-(4-fluorophenyl)-1-butanone hydrochloride)] was obtained from Sigma/RBI (Natick, MA). Ketanserin [3-[2-[4-(4-fluorobenzoyl)-1-piperidinyl]ethyl]-2,4[1H,3H]-quinazolinedione tartrate], altanserin [3-[2-[4-(4-fluorobenzoyl)-1-piperidinyl]ethyl]-2,3-dihydro-2-thioxo-4(1H)-quinazolinone hydrochloride], and α-phenyl-1-(2-phenylethyl)-4-piperidinemethano (M11939) were purchased from Tocris Bioscience (Ellisville, MO). α-(2,3-Dimethoxyphenyl)-1-[2-(4-fluorophenylethyl)]-4-piperidine methanol (M100907) and 4-[(2Z)-3-{[2-(dimethylamino)ethoxy]amino}-3-(2-fluorophenyl)prop-2-en-1-ylidene]cyclohexa-2,5-dien-1-one (SR46349B)was obtained from sanofi-aventis (Bridgewater, NJ). Pimavanserin was kindly provided by Dr. Herb Meltzer (Department of Psychiatry, Vanderbilt University, Nashville, TN).
Chronic Drug Treatment and Behavior Testing Regimen.
C57/BL6 mice (8–10 weeks old) were first tested for short-term vehicle (0.9% NaCl solution)- or phencyclidine (PCP) (6.0 mg/kg i.p.)-induced locomotor response. After 8 to 10 days of washout, the same cohort of mice were used for long-term drug treatments (one injection/day between 2:00 and 4:00 PM for 14 days) either with vehicle (5 ml/kg solution containing 0.9% NaCl, 50 mM tartaric acid and1% dimethyl sulfoxide) or with M100907 (5.0 mg/kg) or M11939 (5.0 mg/kg) or SR46349B (5.0 mg/kg) or ketanserin (10.0 mg/kg), altanserin (10.0 mg/kg), or pimavanserin (5.0 mg/kg) or clozapine (10.0 mg/kg) or olanzapine (10.0 mg/kg) or haloperidol (0.5 mg/kg). The dosage of each drug used in this study was decided on the basis of literature doses to induce a regulatory adaptation of 5-HT2A receptors (Gray and Roth, 2001) or the maximal tolerated doses that are in excess of those shown previously to induce antipsychotic-like activity. Thus, the doses of M100907 and M11939 chosen (5 mg/kg) exceed the maximal effective doses used in behavioral studies (0.1 mg/kg for M100907 and M11939) (Costall and Naylor, 1995; Martin et al., 1997). Likewise, for ketanserin, the dose chosen (10 mg/kg) exceeds the effective dose for normalization of MK801-induced hyperactivity (0.12 mg/kg) (O'Neill et al., 1998) and was the maximal tolerated dose for this drug. For altanserin, we chose a dose of 10 mg/kg, which was the maximal tolerated dose and in excess of that previously used for behavioral studies of 5-HT2A receptors (0.5 mg/kg) (Stoessl et al., 1990). For pimavanserin, the dose chosen (5 mg/kg) was the maximal tolerated dose and again exceeded the dose used previously in behavioral studies (3 mg/kg) (Horiguchi et al., 2011). To determine the chronic effects of drug on PCP-induced locomotor response, 12 to 14 h after the last drug administration, the same cohort of mice were challenged with PCP (6.0 mg/kg i.p.), and locomotor responses were measured. Furthermore, mice were euthanized right after locomoter testing, and cortical tissue were isolated for the measurement of 5-HT2A receptor levels.
Radioligand Binding Assays.
For saturation binding assays, brain regions were rapidly microdissected, frozen on dry ice, and then stored at −80°C. A Tissue-Tearor (BioSpec Products, Bartlesville, OK) was used to homogenize tissue (15 s, 15,000 rpm) in ice-cold standard binding buffer (SBB) (50 mM Tris-HCl, pH 7.4, 10 mM MgCl2, and 0.1 mM EDTA). Homogenized tissue was centrifuged for 20 min at 27,000g (4°C); crude membrane pellets were collected and washed two more times in a total of 20 ml of ice-cold SBB. After the last wash, the membrane pellet was either used immediately for binding or stored at −80°C until use. Saturation binding assays were performed with the homogenized brain tissue and [3H]ketanserin and then incubated in SBB for 1.5 h as detailed (Abbas et al., 2009). Nonspecific binding was determined by incubating the reactions with 10 μM ritanserin. Reactions were harvested by vacuum filtration through glass filters (3× ice-cold 50 mM Tris, pH 7.4; pH 6.9 at room temperature) and measured by liquid scintillation using a Tri-Carb 2800TR scintillation counter (PerkinElmer Life and Analytical Sciences, Waltham, MA). Nonlinear saturation analysis was done with GraphPad Prism 4.01 (GraphPad Software Inc., San Diego, CA) to obtain Bmax values, and Bradford protein assays (Bio-Rad Laboratories, Hercules, CA) were performed to normalize Bmax determinations to the amount of protein in each assay.
Western Blotting.
For 5-HT2A receptor immunoblotting, frontal cortex crude membrane preparations were resuspended in cold lysis buffer (50 mM Tris-Cl, pH 7.4, 150 mM NaCl, 10% glycerol, 1% NP-40, 0.5% sodium deoxycholate, and 0.1% SDS, plus protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN) and incubated on ice for 1 h. Detergent-soluble proteins were collected after 20 min of centrifugation at 4°C and 12,500g, and 500 μg (1.0 mg/ml) of protein was incubated with 25 to 30 μl (packed volume) of wheat germ agglutinin (WGA)-conjugated agarose beads (Vector Laboratories, Inc., Burlingame, CA) for a minimum of 2 h at 4°C on a rotary mixer. After three washes with 500 μl of lysis buffer, WGA-bound proteins were eluted with 40 μl of 1× SDS sample buffer, and whole eluates were resolved by SDS-polyacrylamide gel electrophoresis, followed by electrotransfer of protein from gel to polyvinylidene difluoride membrane. Membranes were blocked for 1 h using buffer containing 3% nonfat dry milk, 1% bovine serum albumin, and 5% glycerol in Tris-buffered saline-Tween 20. Rabbit polyclonal anti-5-HT2A receptor antibody (Neuromics, Edina, MN) was used at a 1:500 dilution for 12 to 18 h at 4°C for measurement of 5-HT2A receptor immunoreactivity. For a loading control, the same membrane was stripped and reprobed with anti-transferrin receptor mouse monoclonal antibody (Invitrogen, Carlsbad, CA). We have previously verified the specificity of the anti-5-HT2A receptor antibody using 5-HT2A wild-type and knockout mouse cortex tissue by immunoblotting (Magalhaes et al., 2010).
Locomotor and Stereotypic Activity.
Locomotor activity was assessed in photocell-based activity chambers under standardized environmental conditions, using an AccuScan activity monitor (AccuScan Instruments, Columbus, OH) with a 25.8 × 25.8 cm Plexiglas chamber and a beam spacing of 1.52 cm as described (Abbas et al., 2009). Mice received intraperitoneal injections of vehicle (0.9% NaCl solution) or PCP (6 mg/kg, dissolved in saline solution) after 20 to 30 min of habituation in activity chambers followed by measurement of horizontal activity for 60 to 90 min. Horizontal activity was measured as the total distance covered in centimeters as the total of all vectored X-Y coordinate changes.
Statistical Analysis.
For quantitation of immunoblots, comparison of Bmax data, and other two-group comparisons, two-tailed unpaired t tests were used to ascertain statistical significance. All behavioral data were analyzed by two-way analysis of variance followed by Bonferroni post-tests for comparing multiple groups. Comparisons were considered significant if p < 0.05.
Results
Olanzapine and Clozapine but Not Haloperidol Down-Regulate 5-HT2A Receptors.
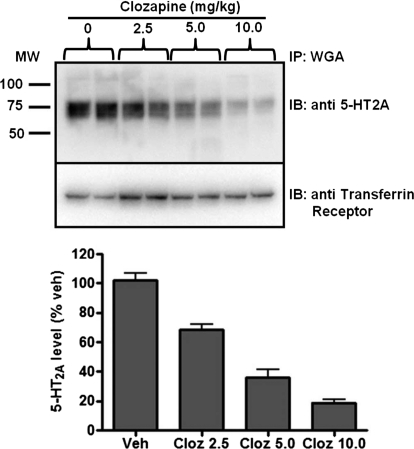
In initial studies, we performed dose-response experiments to investigate the ability of the atypical antipsychotic drug clozapine to induce cortical 5-HT2A receptor down-regulation in C57BL/6J mice. To quantify 5-HT2A receptor protein levels, we used our recently validated Western blot procedure (Magalhaes et al., 2010; Yadav et al., 2011) along with parallel radioligand binding studies with [3H]ketanserin. As can be seen in dose-response studies (Fig. 1), 10.0 mg/kg clozapine induced maximal down-regulation of 5-HT2A receptor protein, and thus this dose was chosen for further studies.
Fig. 1.
Dose-dependent down-regulation of 5-HT2A receptor by clozapine. Top, C57BL/6J mice were treated with vehicle (Veh) or clozapine (Cloz) (2.5–10.0 mg/kg i.p.) for 14 days, and 5-HT2A receptor levels were determined by immunoblotting (IB) of WGA immunoprecipitates (IP) from frontal cortex membrane lysates (top panel). The same blots were stripped and probed for transferrin receptor as loading controls (bottom panel). Bottom, quantitation of immunoblots by densitometry of 5-HT2A immunoreactivity (n = 4 mice/treatment group). MW, molecular weight.
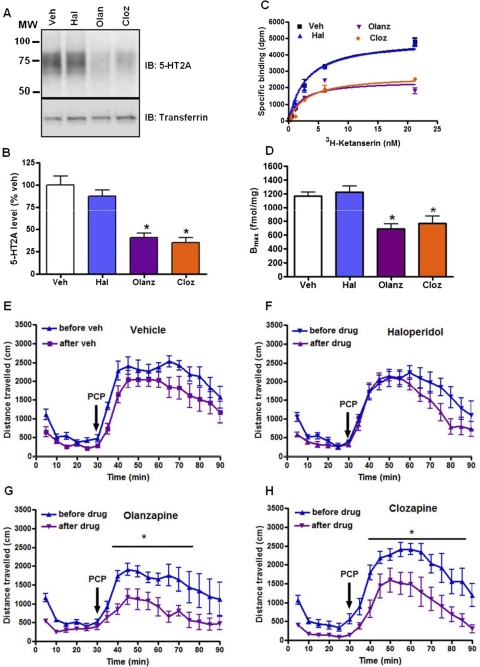
We next evaluated three drugs we had previously shown to induce a differential pattern of receptor internalization in vitro and in vivo: clozapine, olanzapine, and haloperidol (Willins et al., 1999). As can be seen, chronic treatment with clozapine and olanzapine, but not haloperidol, induced a down-regulation of 5-HT2A receptor protein as assessed by both Western blot (Fig. 2, A and B) and radioligand binding (Fig. 2, C and D) studies. As is seen in Fig. 2, E to H, chronic olanzapine and clozapine, but not haloperidol or vehicle, was associated with an attenuation of the PCP-induced locomotor response.
Fig. 2.
Chronic clozapine and olanzapine but not haloperidol down-regulate 5-HT2A receptor and attenuate PCP-induced hyperlocomotor responses in C57BL/6J mice. A, immunoblot (IB) of immunoprecipitates from cortex membrane lysates. The same blots were stripped and probed for transferrin receptor as loading controls. B, densitometry of immunoblots (n = 6 mice/treatment group; *, p < 0.05 by unpaired t test). C, representative saturation isotherms obtained by binding of [3H]ketanserin with equal amounts of cortical membrane proteins (25 μg) from vehicle (Veh) or clozapine (Cloz) or olanzapine (Olanz) or haloperidol (Hal) treatment groups. D, Bmax estimates were obtained by performing [3H]ketanserin saturation binding on cortical membrane homogenates. Data are presented as mean ± S.E.M. (n = 6/group; *, p < 0.05 unpaired t test). E–H, chronic treatments with clozapine or olanzapine (10.0 mg/kg, 14 days) significantly attenuated PCP (6.0 mg/kg)-induced hyperactivity, but haloperidol (0.5 mg/kg) had no significant effect in C57BL/6J mice (n = 8/group). Data are expressed as mean total horizontal distance traveled in 5-min bins over 60 min after PCP administration (±S.E.M.). *, p < 0.05, two-way analysis of variance, followed by Bonferroni post-tests for multiple comparisons. MW, molecular weight.
Selective and Preferential 5-HT2A Antagonists Differentially Modulate 5-HT2A Receptor Protein Levels after Chronic Administration.
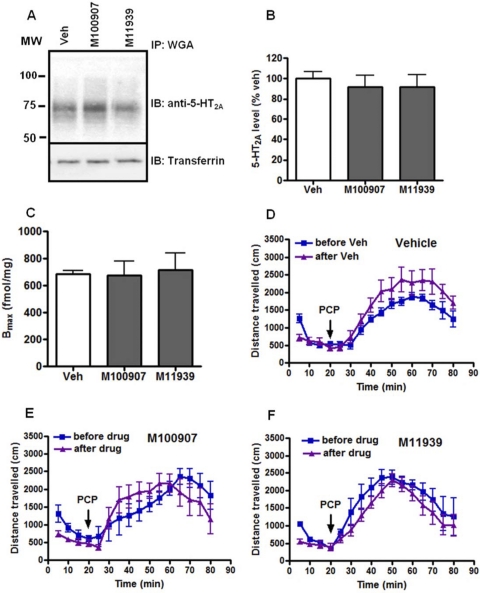
We next evaluated a series of selective 5-HT2A antagonists for their ability to induce 5-HT2A receptor down-regulation. As shown in Fig. 3, A to C, chronic treatment with M100907 and M11939 had no effect on either 5-HT2A receptor protein or radioligand binding. In addition, no effect on PCP-induced locomotor responses was seen (Fig. 3, D–F).
Fig. 3.
Chronic administration of 5-HT2A selective antagonists M100907 or M11939 did not modulate 5-HT2A receptor level and PCP-induced hyperlocomotor response in C57BL/6J mice. A, immunoblot (IB) of immunoprecipitates (IP) from cortex membrane lysates. The same blots were stripped and probed for transferrin receptor as loading controls. B, densitometry of immunoblots of immunoprecipitates from n = 4 mice/treatment group. C, Bmax estimates were obtained by performing [3H]ketanserin saturation binding on cortical membrane homogenates. Data are presented as mean ± S.E.M. (n = 6/group). D–F, chronic treatments with vehicle (Veh) or M100907 or M11939 (5.0 mg/kg, 14 days) had no significant effect on PCP (6.0 mg/kg)-induced hyperactivity in C57BL/6J mice (n = 7/group). Data are expressed as mean total horizontal distance traveled in 5-min bins over 60 min after PCP administration (±S.E.M.). MW, molecular weight.
In contrast, the prototypical 5-HT2A antagonist ketanserin, but not the closely related compound altanserin, induced 5-HT2A receptor protein down-regulation (Fig. 4, A–C). Chronic treatment with these compounds had no significant effect on PCP-induced locomotor responses (Fig. 4, D–F).
Fig. 4.
Chronic administration of the 5-HT2A antagonist ketanserin (Ket) but not altanserin (Alt) down-regulates the cortical 5-HT2A receptor level in C57BL/6J mice. A, measurement of 5-HT2A receptor level in frontal cortex membrane lysates by immunoblotting (IB) of immunoprecipitates (IP). The same blots were reprobed for transferrin receptor as loading controls. B, densitometry of immunoblots of immunoprecipitates from n = 4 mice/treatment group. *, p < 0.05, unpaired t test. C, Bmax estimates were obtained by performing [3H]ketanserin saturation binding on cortical membrane homogenates. Data are presented as mean ± S.E.M. (n = 6/group). D–F, chronic treatment with vehicle or ketanserin or altanserin (10.0 mg/kg, 14 days) had no significant effect on PCP (6.0 mg/kg)-induced hyperactivity in C57BL/6J mice (n = 8/group). Data are expressed as mean total horizontal distance traveled in 5-min bins over 90 min after PCP administration (±S.E.M.). MW, molecular weight; Veh, vehicle.
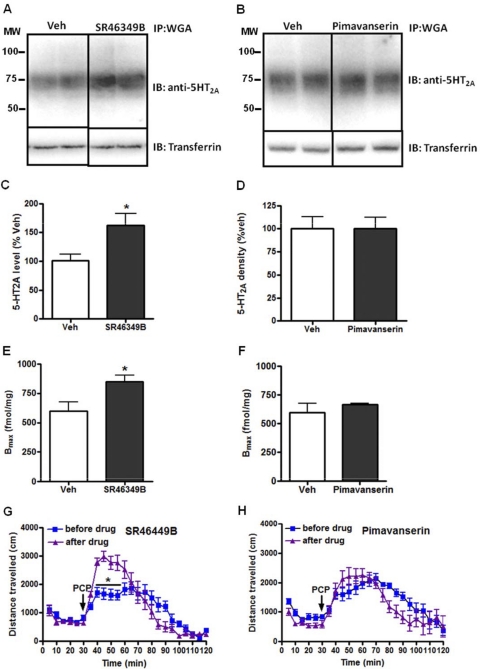
We next tested two 5-HT2A antagonists that have been shown in small-scale trials to have efficacy in treating psychosis, SR46349B (Meltzer et al., 2004) and pimavanserin (Meltzer et al., 2010). In the case of SR46349B, an up-regulation of 5-HT2A receptor protein was noted, whereas chronic pimavanserin treatment had no effect (Fig. 5, A–F). These results indicate that reasonably selective 5-HT2A antagonists may display strikingly different effects on receptor protein levels: up-regulation (SR46349B), down-regulation (ketanserin), or no effect (M100907, altanserin, pimavanserin, and M11939). SR46349B-mediated up-regulation was associated with a potentiation of PCP-induced locomotor responses (Fig. 5G), whereas chronic pimavanserin treatment had no effect (Fig. 5H).
Fig. 5.
Chronic administration of the 5-HT2A selective inverse agonist SR46349b, but not pimavanserin, up-regulates 5-HT2A receptor level but had no significant effect on PCP-induced hyperlocomotor response in C57BL/6J mice, A and B, immunoblot (IB) of immunoprecipitates (IP) from cortex membrane lysates. The same blots were reprobed for transferrin receptor as loading controls. C and D, densitometry of immunoblots of immunoprecipitates from n = 4 mice/treatment group. *, p < 0.05, unpaired t test. E and F, Bmax estimates were obtained by performing [3H]ketanserin saturation binding on cortical membrane homogenates. Data are presented as mean ± S.E.M. (n = 6/group), *, p < 0.05, unpaired t test. G and H, chronic treatment with vehicle or SR46349b or pimavanserin (5.0 mg/kg, 14 days) had no significant effect on PCP (6.0 mg/kg)-induced hyperactivity in C57BL/6J mice (n = 6–8/group). Data are expressed as mean total horizontal distance traveled in 5-min bins over 90 min after PCP administration (±S.E.M.). *, p < 0.05, unpaired t test. MW, molecular weight; Veh, vehicle.
Discussion
The main finding of these studies is that 5-HT2A receptor antagonists can be classified into three major categories on the basis of their differing abilities to regulate 5-HT2A receptor protein expression: 1) up-regulating antagonists (SR46349B), 2) down-regulating antagonists (ketanserin, clozapine, and olanzapine), and 3) antagonists that do not alter receptor expression in male C57BL/6J mice at the doses used in this study (M100907, M11939, pimavanserin, and altanserin). We also report that the SR46349B-induced receptor up-regulation is associated with an apparently augmented PCP-induced locomotor response. Although it has been widely reported that 5-HT2A antagonists display differential abilities to induce alterations in 5-HT2A receptor radioligand binding, this is the first study demonstrating an actual change in 5-HT2A receptor protein levels after chronic drug administration.
For many years, it has been suggested that 5-HT2A down-regulation might be associated with the therapeutic actions of many drugs (Gray and Roth, 2001; Elphick et al., 2004; O'Connor and Roth, 2005). Indeed, down-regulation of 5-HT2A receptors is probably part of the mechanism by which certain 5-HT2A antagonists apparently protect against JC virus infections (Elphick et al., 2004; O'Connor and Roth, 2005). It has also been suggested that 5-HT2A receptor down-regulation itself might contribute to atypical antipsychotic drug actions. Indeed, as this article clearly shows, chronic treatment with clozapine and olanzapine, two prototypical atypical antipsychotic drugs, induces substantial down-regulation of 5-HT2A receptor protein. This down-regulation was associated with an attenuated ability of PCP to induce a locomotor response. In contrast, chronic treatment with the typical antipsychotic drug haloperidol was without effect on either PCP-induced locomotion or 5-HT2A receptor levels.
Of interest, chronic treatment with three candidate antipsychotic drugs (M100907, SR46349B, and pimavanserin) either had no effect (M100907 and pimavanserin) or up-regulated 5-HT2A receptors. It is interesting in this regard that not only did none of these drugs attenuate the hyperlocomotive ability of PCP but also none of these drugs displayed efficacy in large-scale phase III clinical trials. Taken together, these findings are consistent with the notion that down-regulation of 5-HT2A receptors may be important for certain therapeutic actions of atypical antipsychotic drugs. However, atypical antipsychotics clozapine and olanzapine have marked polypharmacology, with affinities for dopamine, serotonin, muscarinic, and adrenergic receptors (Roth et al., 2004). Thus, it is quite complicated and the subject of considerable controversy to explain unique salutary effects of clozapine and other related atypical antipsychotics (Allen and Roth, 2011). The most important implication of these findings is the clear-cut evidence in favor of the hypothesis that antagonists display unique patterns of functional selectivity. The precise molecular mechanisms responsible for the paradoxical down-regulation of 5-HT2A receptors in vivo are currently unknown and are the topic of intensive research at present (P. M. Yadev and B. L. Roth, manuscript in preparation).
This work was supported by the National Institutes of Health National Institute of Mental Health [Grants U19-MH82441, R01-MH61887] (to B.L.R.) and the Michael Hooker Chair.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.111.183780.
- M11939
- α-phenyl-1-(2-phenylethyl)-4-piperidinemethano
- M100907
- α-(2,3-dimethoxyphenyl)-1-[2-(4-fluorophenylethyl)]-4-piperidine methanol
- SR46349B
- 4-[(2Z)-3-{[2-(dimethylamino)ethoxy]amino}-3-(2-fluorophenyl)prop-2-en-1-ylidene]cyclohexa-2,5-dien-1-one
- PCP
- phencyclidine
- SBB
- standard binding buffer
- WGA
- wheat germ agglutinin.
Authorship Contributions
Participated in research design: Yadav and Roth.
Conducted experiments: Yadav, Kroeze, and Farrell.
Performed data analysis: Yadav and Roth.
Wrote or contributed to the writing of the manuscript: Yadav, Kroeze, and Roth.
References
- Abbas AI, Yadav PN, Yao WD, Arbuckle MI, Grant SG, Caron MG, Roth BL. (2009) PSD-95 is essential for hallucinogen and atypical antipsychotic drug actions at serotonin receptors. J Neurosci 29:7124–7136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JA, Roth BL. (2011) Strategies to discover unexpected targets for drugs active at G protein-coupled receptors. Annu Rev Pharmacol Toxicol 51:117–144 [DOI] [PubMed] [Google Scholar]
- Andree TH, Mikuni M, Tong CY, Koenig JI, Meltzer HY. (1986) Differential effect of subchronic treatment with various neuroleptic agents on serotonin2 receptors in rat cerebral cortex. J Neurochem 46:191–197 [DOI] [PubMed] [Google Scholar]
- Bergstrom DA, Kellar KJ. (1979) Adrenergic and serotonergic receptor binding in rat brain after chronic desmethylimipramine treatment. J Pharmacol Exp Ther 209:256–261 [PubMed] [Google Scholar]
- Berry SA, Shah MC, Khan N, Roth BL. (1996) Rapid agonist-induced internalization of the 5-hydroxytryptamine2A receptor occurs via the endosome pathway in vitro. Mol Pharmacol 50:306–313 [PubMed] [Google Scholar]
- Bhatnagar A, Willins DL, Gray JA, Woods J, Benovic JL, Roth BL. (2001) The dynamin-dependent, arrestin-independent internalization of 5-hydroxytryptamine 2A (5-HT2A) serotonin receptors reveals differential sorting of arrestins and 5-HT2A receptors during endocytosis. J Biol Chem 276:8269–8277 [DOI] [PubMed] [Google Scholar]
- Blackshear MA, Sanders-Bush E. (1982) Serotonin receptor sensitivity after acute and chronic treatment with mianserin. J Pharmacol Exp Ther 221:303–308 [PubMed] [Google Scholar]
- Brunello N, Chuang DM, Costa E. (1982) Different synaptic location of mianserin and imipramine binding sites. Science 215:1112–1115 [DOI] [PubMed] [Google Scholar]
- Costa T, Herz A. (1989) Antagonists with negative intrinsic activity at delta opioid receptors coupled to GTP-binding proteins. Proc Natl Acad Sci USA 86:7321–7325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costall B, Naylor RJ. (1995) Behavioural interactions between 5-hydroxytryptophan, neuroleptic agents and 5-HT receptor antagonists in modifying rodent responding to aversive situations. Br J Pharmacol 116:2989–2999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elphick GF, Querbes W, Jordan JA, Gee GV, Eash S, Manley K, Dugan A, Stanifer M, Bhatnagar A, Kroeze WK, et al. (2004) The human polyomavirus, JCV, uses serotonin receptors to infect cells. Science 306:1380–1383 [DOI] [PubMed] [Google Scholar]
- Galandrin S, Oligny-Longpré G, Bouvier M. (2007) The evasive nature of drug efficacy: implications for drug discovery. Trends Pharmacol Sci 28:423–430 [DOI] [PubMed] [Google Scholar]
- Gray JA, Roth BL. (2001) Paradoxical trafficking and regulation of 5-HT2A receptors by agonists and antagonists. Brain Res Bull 56:441–451 [DOI] [PubMed] [Google Scholar]
- Hanley NR, Hensler JG. (2002) Mechanisms of ligand-induced desensitization of the 5-hydroxytryptamine2A receptor. J Pharmacol Exp Ther 300:468–477 [DOI] [PubMed] [Google Scholar]
- Harvey JA, Quinn JL, Liu R, Aloyo VJ, Romano AG. (2004) Selective remodeling of rabbit frontal cortex: relationship between 5-HT2A receptor density and associative learning. Psychopharmacology (Berl) 172:435–442 [DOI] [PubMed] [Google Scholar]
- Horiguchi M, Huang M, Meltzer HY. (2011) The role of 5-hydroxytryptamine 7 receptors in the phencyclidine-induced novel object recognition deficit in rats. J Pharmacol Exp Ther 338:605–614 [DOI] [PubMed] [Google Scholar]
- Leysen JE, Van Gompel P, Gommeren W, Woestenborghs R, Janssen PA. (1986) Down regulation of serotonin-S2 receptor sites in rat brain by chronic treatment with the serotonin-S2 antagonists: ritanserin and setoperone. Psychopharmacology (Berl) 88:434–444 [DOI] [PubMed] [Google Scholar]
- Magalhaes AC, Holmes KD, Dale LB, Comps-Agrar L, Lee D, Yadav PN, Drysdale L, Poulter MO, Roth BL, Pin JP, et al. (2010) CRF receptor 1 regulates anxiety behavior via sensitization of 5-HT2 receptor signaling. Nat Neurosci 13:622–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P, Waters N, Waters S, Carlsson A, Carlsson ML. (1997) MK-801-induced hyperlocomotion: differential effects of M100907, SDZ PSD 958 and raclopride. Eur J Pharmacol 335:107–116 [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Arvanitis L, Bauer D, Rein W, and Meta-Trial Study Group (2004) Placebo-controlled evaluation of four novel compounds for the treatment of schizophrenia and schizoaffective disorder. Am J Psychiatry 161:975–984 [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Mills R, Revell S, Williams H, Johnson A, Bahr D, Friedman JH. (2010) Pimavanserin, a serotonin2A receptor inverse agonist, for the treatment of Parkinson's disease psychosis. Neuropsychopharmacology 35:881–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor KA, Roth BL. (2005) Finding new tricks for old drugs: an efficient route for public-sector drug discovery. Nat Rev Drug Discov 4:1005–1014 [DOI] [PubMed] [Google Scholar]
- O'Neill MF, Hicks CA, Shaw G, Parameswaran T, Cardwell GP, O'Neill MJ. (1998) Effects of 5-hydroxytryptamine2 receptor antagonism on the behavioral activation and immediate early gene expression induced by dizocilpine. J Pharmacol Exp Ther 287:839–846 [PubMed] [Google Scholar]
- Peroutka SJ, Snyder SH. (1980) Long-term antidepressant treatment decreases spiroperidol-labeled serotonin receptor binding. Science 210:88–90 [DOI] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Congy C, Simiand J, Oury-Donat F, Soubrie P, Breliere JC, Le Fur G. (1993) Repeated administration of SR 46349B, a selective 5-hydroxytryptamine2 antagonist, up-regulates 5-hydroxytryptamine2 receptors in mouse brain. Mol Pharmacol 43:84–89 [PubMed] [Google Scholar]
- Rosenbaum DM, Cherezov V, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, Choi HJ, Yao XJ, Weis WI, Stevens RC, et al. (2007) GPCR engineering yields high-resolution structural insights into β2-adrenergic receptor function. Science 318:1266–1273 [DOI] [PubMed] [Google Scholar]
- Rosenbaum DM, Zhang C, Lyons JA, Holl R, Aragao D, Arlow DH, Rasmussen SG, Choi HJ, Devree BT, Sunahara RK, et al. (2011) Structure and function of an irreversible agonist-β2 adrenoceptor complex. Nature 469:236–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth BL, Ciaranello RD. (1991) Chronic mianserin treatment decreases 5-HT2 receptor binding without altering 5-HT2 receptor mRNA levels. Eur J Pharmacol 207:169–172 [DOI] [PubMed] [Google Scholar]
- Roth BL, Chuang DM. (1987) Multiple mechanisms of serotonergic signal transduction. Life Sci 41:1051–1064 [DOI] [PubMed] [Google Scholar]
- Roth BL, Sheffler DJ, Kroeze WK. (2004) Magic shotguns versus magic bullets: selectively non-selective drugs for mood disorders and schizophrenia. Nat Rev Drug Discov 3:353–359 [DOI] [PubMed] [Google Scholar]
- Stephenson RP. (1956) A modification of receptor theory. Br J Pharmacol Chemother 11:379–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoessl AJ, Dourish CT, Iversen SD. (1990) Pharmacological characterization of the behavioural syndrome induced by the NK-3 tachykinin agonist senktide in rodents: evidence for mediation by endogenous 5-HT. Brain Res 517:111–116 [DOI] [PubMed] [Google Scholar]
- Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, Javitch JA, Roth BL, Christopoulos A, Sexton PM, et al. (2007) Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther 320:1–13 [DOI] [PubMed] [Google Scholar]
- Warne T, Moukhametzianov R, Baker JG, Nehmé R, Edwards PC, Leslie AG, Schertler GF, Tate CG. (2011) The structural basis for agonist and partial agonist action on a β1-adrenergic receptor. Nature 469:241–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber ET, Andrade R. (2010) Htr2a gene and 5-HT2A receptor expression in the cerebral cortex studied using genetically modified mice. Front Neurosci 4:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willins D, Berry S, Alsayegh L, Backstrom J, Sanders-Bush E, Roth B. (1999) Clozapine and other 5-hydroxytryptamine2A receptor antagonists alter the subcellular distribution of 5-hydroxytryptamine2A receptors in vitro and in vivo. Neuroscience 91:599–606 [DOI] [PubMed] [Google Scholar]
- Willins DL, Alsayegh L, Berry SA, Backstrom JR, Sanders-Bush E, Friedman L, Khan N, Roth BL. (1998) Serotonergic antagonist effects on trafficking of serotonin 5-HT2A receptors in vitro and in vivo. Ann NY Acad Sci 861:121–127 [DOI] [PubMed] [Google Scholar]
- Xia Z, Hufeisen SJ, Gray JA, Roth BL. (2003) The PDZ-binding domain is essential for the dendritic targeting of 5-HT2A serotonin receptors in cortical pyramidal neurons in vitro. Neuroscience 122:907–920 [DOI] [PubMed] [Google Scholar]
- Yadav PN, Abbas AI, Farrell MS, Setola V, Sciaky N, Huang XP, Kroeze WK, Crawford LK, Piel DA, Keiser MJ, et al. (2011) The presynaptic component of the serotonergic system is required for clozapine's efficacy. Neuropsychopharmacology 36:638–651 [DOI] [PMC free article] [PubMed] [Google Scholar]