Abstract
In anesthetized normotensive rats, activation of brainstem hemeoxygenase (HO) elicits sympathoinhibition and hypotension. Accordingly, we tested the hypothesis that attenuated basal or induced HO activity in the rostral ventrolateral medulla (RVLM) contributes to hypertension in the spontaneously hypertensive rat (SHR). We measured basal RVLM HO expression and catalytic activity and investigated the effects of intra-RVLM HO activation (hemin) or selective HO isoform 1 (HO-1) inhibition [zinc protoporphyrin IX (ZnPPIX)] on mean arterial pressure (MAP), heart rate, and RVLM neuronal norepinephrine (NE) level (index of sympathetic activity) in conscious SHRs and Wistar Kyoto rats. Basal RVLM HO catalytic activity (bilirubin level) and HO-1 expression were significantly higher in the SHR. These neurochemical findings were corroborated by the significantly greater decreases (hemin) and increases (ZnPPIX) in RVLM NE and MAP in the SHR. By contrast, HO-independent CO release in the RVLM (CO-releasing molecule 3) elicited similar MAP reductions in both rat strains. Furthermore, pretreatment with ZnPPIX or the selective neuronal nitric-oxide synthase (nNOS) inhibitor N-propyl-l-arginine abrogated the neurochemical (RVLM cGMP) and hypotensive responses caused by hemin. In addition to demonstrating, for the first time, higher basal RVLM HO catalytic activity and HO-1 expression in the SHR, the findings suggest: 1) the exaggerated hypotension elicited by intra-RVLM HO activation in the SHR is nNOS-dependent, and 2) in the SHR, the enhanced RVLM HO-nNOS signaling compensates for the reduced expression/activity of the downstream target, soluble guanylyl cyclase. Together, the findings suggest a protective role for the RVLM HO-nNOS pathway against further increases in MAP in the SHR.
Introduction
Mounting evidence implicates carbon monoxide (CO) in blood pressure regulation both centrally and peripherally (Lo et al., 2006; Lee and Yen, 2009; Peterson et al., 2009). Whereas CO could be generated through non-HO pathways, the majority of endogenous CO is generated by HO, which catalyzes heme degradation to CO and biliverdin (Lee and Yen, 2009). There are two HO isozymes, shared between humans and other species, the inducible (HO-1) and constitutive (HO-2) isoforms (Pflueger et al., 2005; Lee and Yen, 2009; Peterson et al., 2009). Similar to NO, CO mediates its biological effects by activating sGC and generation of cGMP in neuronal and cardiovascular tissues (Lee and Yen, 2009; Peterson et al., 2009).
Few studies have directly shown that central HO-CO signaling in the nucleus tractus solitarius (NTS) exerts a tonic restraining influence on sympathetic activity and blood pressure (Johnson et al., 1997; Lo et al., 2006). Although the NTS modulates sympathetic activity and blood pressure, at least partly, via projections to the RVLM (Guyenet et al., 1990), there are no reports on the role of the RVLM HO in blood pressure regulation. The RVLM contains the premotor (presympathetic) neurons (Lipski et al., 1995; Dampney et al., 2003), which express HO (Mazza et al., 2001). Equally important, it is not known whether alteration in RVLM HO activity contributes to, or guards against, exacerbation of, hypertension. In the few studies on HO-CO signaling in hypertensive animals, systemic HO substrates elicited greater hypotension in hypertensive than in normotensive rats (Johnson et al., 1996; Ndisang et al., 2010) despite hypofunctional sGC-cGMP signaling in SHR vasculature (Ruetten et al., 1999; Ndisang et al., 2003). Earlier attempts to investigate the role of RVLM sGC in blood pressure regulation were hampered by the inability to measure its product cGMP in RVLM neurons (Powers-Martin et al., 2008). Nonetheless, more sensitive methodologies have not been exploited for the measurement of cGMP, particularly after localized activation or inhibition of HO in the RVLM of conscious rats.
The purpose of the present study was to elucidate the role of the RVLM HO-CO-sGC pathway in the control of blood pressure in the SHR. We showed that basal HO catalytic activity was higher in the RVLM of SHRs compared with Wistar Kyoto (WKY) rats. Because this new finding is discordant with the higher mean arterial pressure (MAP) in the SHR, the studies were extended to: 1) determine whether the higher RVLM HO activity guards against exacerbation of hypertension in the SHR, and 2) elucidate the role of the RVLM sGC-cGMP pathway in the exaggerated hypotensive response elicited by HO activation (hemin) in the RVLM of the SHR. We also investigated the effect of HO-independent CO release [CO-releasing molecule 3 (CORM-3)] in the RVLM on blood pressure to determine whether the HO products biliverdin/bilirubin contribute to the exaggerated hypotensive response elicited by HO activation in the RVLM of the SHR. Finally, given that the HO products biliverdin/bilirubin preserve/activate nNOS and the ample distribution of nNOS in the brainstem (Gao et al., 2008; Xia et al., 2008), we investigated the role of RVLM nNOS in exaggerated hemin-evoked hypotension in the SHR. In these studies, we measured RVLM nitrite/nitrate concentration (NOx) levels (index of NO) and phosphorylated nNOS (p-nNOS) in control and hemin-treated SHRs and WKY rats and investigated the impact of pharmacological inhibition of nNOS on hypotension and the associated increases in RVLM cGMP and NOx levels elicited by intra-RVLM hemin. The integrative studies were conducted in conscious rats to circumvent potential confounding effects of anesthesia on the measured variables.
Materials and Methods
Animals
Male SHRs and WKY rats (12–13 weeks old) used in the study were originally obtained from Charles River (Raleigh, NC) and bred in our animal facility. All rats were maintained in an Association for Assessment and Accreditation of Laboratory Animal Care-accredited animal housing facility with controlled environment at a constant temperature of 23 ± 1°C, humidity of 50 ± 10%, and a 12-h light/dark cycle. Food (Prolab RMH300; Granville Milling, Creedmoor, NC) and water were available ad libitum. Surgical procedures and postoperative care were performed in accordance with and approved by the Institutional Animal Care and Use Committee and in accordance with the Institute of Laboratory Animal Resources.
Intracranial Cannulation and Intravascular Catheterization
Animals were anesthetized with ketamine (90 mg/kg i.p.) and xylazine (10 mg/kg i.p.) supplemented as needed. Intravascular cannulation was performed as described in our previous studies (Nassar and Abdel-Rahman, 2009). After vascular catheterization, the animals were placed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA) for insertion of the RVLM cannula as reported previously (Mao et al., 2003). Each rat received intramuscular buprenorphine (30 μg/kg; Rickitt and Colman, Richmond, VA) and subcutaneous penicillin G benzathine and penicillin G procaine in an aqueous suspension (100,000 U/kg; Durapen; Vedco, Inc., Overland Park, KS) and was housed in a separate cage.
Blood Pressure and RVLM NE Measurement
Experiments were conducted 5 days after cannulation and catheterization surgeries. Blood pressure was computed using a Grass polygraph (model 7D; Grass Instruments, Quincy, MA) or PowerLab (ADInstruments, Colorado Springs, CO), interfaced with a computer. Measurement of real-time changes in RVLM NE was performed by a computer-controlled electrochemical instrument (IVEC-10; Medical Systems Corporation, Greenvale, NY). In brief, this methodology permitted measurements of increases or decreases in neuronal NE level from baseline, which was set at 0 after a 90-min stabilization period after insertion of the carbon fiber electrode as detailed in our previous studies (Li et al., 2005; Li and Abdel-Rahman, 2009).
Western Blot
For the determination of RVLM HO-1 and HO-2, p-nNOS as well as GC-S-α-1 and GC-S-β-1, protein levels micropunches were taken out of the RVLM as reported previously (Bardgett et al., 2010). Sample protein was quantified (Bio-Rad protein assay system; Bio-Rad Laboratories, Hercules, CA). Protein extracts for HO-1 and HO-2 (5 μg per lane) and GC-S-α-1 and GC-S-β-1 (50 μg per lane) were run on a 4 to 12% SDS-polyacrylamide gel electrophoresis (Invitrogen, Carlsbad, CA) and electroblotted to nitrocellulose membranes. Blots were then incubated overnight at 4°C with rabbit antibodies to HO-1 and HO-2 (1:500; Abcam Inc., Cambridge, MA) or rabbit antibodies to GC-S-α-1 or GC-S-β-1 1:250 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), polyclonal anti-p-nNOS antibody (1:200) and anti-mouse antibody to β-actin 1:1500 (Abcam Inc.) or monoclonal anti-nNOS (1:200; BD) in Odyssey blocking buffer (LI-COR Biosciences, Lincoln, NE). After three washes with phosphate-buffered saline/Tween buffer, the blots were incubated for 60 min at room temperature with goat-anti-mouse secondary antibody IRDye 800CW (LI-COR Biosciences). After three washes with PBS/Tween buffer, the blots were incubated for 60 min at room temperature with goat-anti-rabbit and -mouse secondary antibody IRDye 700CW and 800CW, respectively (LI-COR Biosciences). The blots were detected by the Odyssey Infrared Scanning System (LI-COR Biosciences) and expressed in relation to β-actin; p-nNOS was expressed in relation to total nNOS.
Heme Oxygenase Activity Measurement
Attempts to use the conventional spectrophotometric method for detection of HO activity (Kim et al., 2006) were not successful because of the low protein yield in micropunched tissue from the RVLM. Therefore, we adapted a widely used immunological “dot blot” method for the measurement of enzyme activity (Siu et al., 2004) and measurement of the HO product bilirubin. In brief, HO activity was determined by adding 5 μg of protein in 30 μl of reaction mixture (2 mg of liver cytosol, source of biliverdin reductase), 20 mM hemin, 0.8 mM NADPH, 2 mM glucose 6-phosphate, and 0.0016 U/μl of glucose 6-phosphate dehydrogenase) (Carraway et al., 1998; Kim et al., 2006). A NADPH-free reaction mixture served as a negative control. After a 4-h reaction at 37°C, the produced bilirubin was determined by dot blot using nitrocellulose membrane from a 96-well sample template of a Microfiltration blotting device (Bio-Rad Laboratories). After 120 min of blocking with Odyssey blocking buffer, the blots were incubated with the primary antibody (antibilirubin/albumin antibody 24G7; 1:500; Shino-Test Corporation, Tokyo, Japan) overnight at 4°C; after three washes with rinse buffer, the blots were incubated for 60 min at room temperature with goat-anti-mouse secondary antibody IRDye 800CW (LI-COR Biosciences). The Odyssey Infrared Scanning System was used for the quantification. Serial concentrations of bilirubin + albumin were used to construct the bilirubin calibration curve; albumin alone served as background “control” for the bilirubin standard. HO activity was expressed as bilirubin nmol/mg protein/h.
RVLM Nitrite/Nitrate Level
NOx (index of NO) was measured by using the Cayman Chemical (Ann Arbor, MI) NOx Assay Kit.
Measurement of cGMP in the RVLM
cGMP level in RVLM micropunches was measured using an enzyme immunoassay kit (Enzo Life Sciences, Plymouth Meeting, PA) according to the manufacturer's instructions and normalized to protein content as determined by the Bradford assay.
Protocols and Experimental Groups
Effect of Intra-RVLM Hemin or ZnPPIX on MAP and RVLM NE.
On the day of the experiment, the arterial catheter was connected to a pressure transducer for recording blood pressure and heart rate and the probe that permitted microinjection of drugs/aCSF into the RVLM; continuous monitoring of RVLM NE was made via the preimplanted guide cannula as in our reported studies (Li et al., 2005; Li and Abdel-Rahman, 2009). Each rat was allowed to stabilize for 90 min after insertion of the RVLM probe; the data collected during the last 30 min of the stabilization period represented baseline values. Presympathetic neurons in the RVLM exhibit adrenergic phenotype (Lipski et al., 1995). Furthermore, we previously demonstrated that continuously monitored RVLM NE reflects sympathetic neuronal activity (Mao et al., 2003). Microinjections containing the drug or aCSF (80 nl) were made unilaterally into the RVLM in accordance with the following treatment regimen.
In the first experiment, the HO substrate hemin (1 nmol) was microinjected into the RVLM of conscious SHRs or WKY rats (n = 5 each); the dose was based on reported intra-NTS hemin in normotensive rats (Lo et al., 2006). As a control for hemin and other treatments (see below), equal volume of aCSF (123 mM NaCl, 0.86 mM CaCl2, 3 mM KCl, 0.89 mM MgCl2, 25 mM NaHCO3, 0.5 mM NaH2PO4, and 0.25 mM Na2HPO4, pH 7.4) was microinjected into the RVLM of control groups (n = 5 each). The blood pressure, heart rate, and RVLM NE were monitored for 60 min after hemin or aCSF microinjection.
In the second experiment, we investigated the consequences of inhibiting RVLM HO-1 on blood pressure and RVLM NE as well as on the reductions in both variables caused by intra-RVLM hemin (HO activation). To this end, ZnPPIX (1 nmol; dissolved in 35% dimethyl sulfoxide in aCSF), in a dose used to inhibit HO-1 in the NTS in conscious normotensive rats (Lo et al., 2006), was microinjected into the RVLM of conscious SHRs or WKY rats 30 min before hemin. It is noteworthy that compared with aCSF microinjection of a similar concentration of dimethyl sulfoxide in aCSF into rat brainstem had no impact on baseline blood pressure or its response to subsequent pharmacologic interventions (Tsukamoto et al., 2002).
Effect of Intra-RVLM CORM or NPLA on MAP.
In the third set of experiments, CORM-3, which releases CO independently of HO (Chatterjee, 2004; Foresti et al., 2004; Guo et al., 2004) was microinjected into the RVLM of conscious SHRs or WKY rats (n = 5 each). The CORM-3 dose (2 nmol/80 nl) was based on doses used in reported studies (Foresti et al., 2004). Finally, the effect of inhibition of neuronal NOS [N-propyl-l-arginine (NPLA) 12.5 μg/kg i.v.] was investigated in three SHRs and three WKY rat groups (n = 5 each) that received the inhibitor or its vehicle (saline) 30 min before intra-RVLM hemin or its vehicle (aCSF). The dose and route of NPLA administration was based on our previous studies (El-Mas et al., 2009). For each strain, the rats received: 1) NPLA followed by intra-RVLM hemin, 2) NPLA vehicle (saline) followed by intra-RVLM hemin, or 3) NPLA followed by intra-RVLM aCSF. Measurement of blood pressure and heart rate continued for 60 min after hemin or its vehicle.
Levels and Catalytic Activity of HO, sGC (cGMP), and NOx Level in the RVLM of SHR versus WKY Rats.
At the conclusion of the integrative studies, the brains were removed and stored at −80°C. The RVLM punches obtained from the control (aCSF) SHRs or WKY rats were used for measurements of basal HO catalytic activity and cGMP as well as the level of HO-1, HO-2, and the two subunits of sGC. It is noteworthy that, for obtaining the micropunches, the landmarks for the RVLM were the nucleus ambiguous (rostral) and the inferior olives (lateral) (Bardgett et al., 2010). The molecular studies were extended to investigate the impact of the pharmacological interventions used in the study on the catalytic activities of HO and its downstream effector sGC as well as NOx level in the RVLM of SHRs and WKY rats as detailed above. For each strain of rats, the pooled RVLM cGMP data from intra-RVLM vehicle and intra-RVLM hemin represented the basal cGMP and HO-evoked cGMP generation, respectively.
Drugs
Hemin was from Sigma-Aldrich (St. Louis, MO), and ZnPPIX and NPLA were from Tocris Biosciences (Ellisville, MO). Ketamine, xylazine, and pentobarbital sodium were provided by the East Carolina University Department of Comparative Medicine (Webster Veterinary Supplies, Devens, MA; Vortech Pharmaceutical Ltd., Dearborn, MI). CORM-3 was generously synthesized by Dr. Shouquan Huo at the Department of Chemistry at East Carolina University.
Statistical Analysis
MAP was calculated as: diastolic pressure + one-third (systolic pressure − diastolic pressure). The time-course data were analyzed by repeated-measures ANOVA using the SPSS 16.0 statistical package for Windows (SPSS Inc., Chicago, IL), for differences in time and treatment trends followed by a one-way ANOVA to assess individual differences at different time points among different groups. The t test and the ANOVA error terms were used to compare treatment or strain effect. P < 0.05 was considered significant.
Results
Baseline MAP and HR before drug treatment (hemin, ZnPPIX, CORM-3, or NPLA) or vehicle were similar among the groups of each strain of rats, but baseline (pretreatment) MAP was significantly (P < 0.05) higher in SHRs than in WKY rats (Table 1).
TABLE 1.
Baseline (pretreatment) blood pressure and heart rate values for SHRs and WKY rats (n = 5–6; each group)
| Strain/Treatment | MAP | Heart Rate |
|---|---|---|
| mm Hg | beats/min | |
| SHR | ||
| aCSF | 166 ± 10 | 365 ± 33 |
| Hemin | 170 ± 4 | 405 ± 25 |
| ZnPPIX | 169 ± 8 | 385 ± 30 |
| NPLA | 171 ± 5 | 349 ± 11 |
| Saline | 176 ± 4 | 381 ± 20 |
| CORM-3 | 172 ± 3 | 389 ± 12 |
| WKY | ||
| aCSF | 113 ± 7 | 343 ± 23 |
| Hemin | 119 ± 5 | 360 ± 29 |
| ZnPPIX | 116 ± 3 | 375 ± 20 |
| NPLA | 128 ± 8 | 355 ± 15 |
| Saline | 126 ± 5 | 314 ± 16 |
| CORM-3 | 124 ± 6 | 322 ± 18 |
Effect of Intra-RVLM Hemin or ZnPPIX on MAP and RVLM NE.
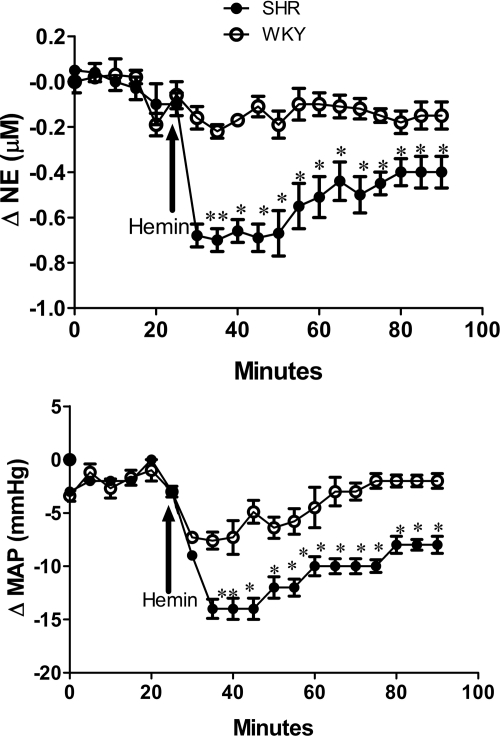
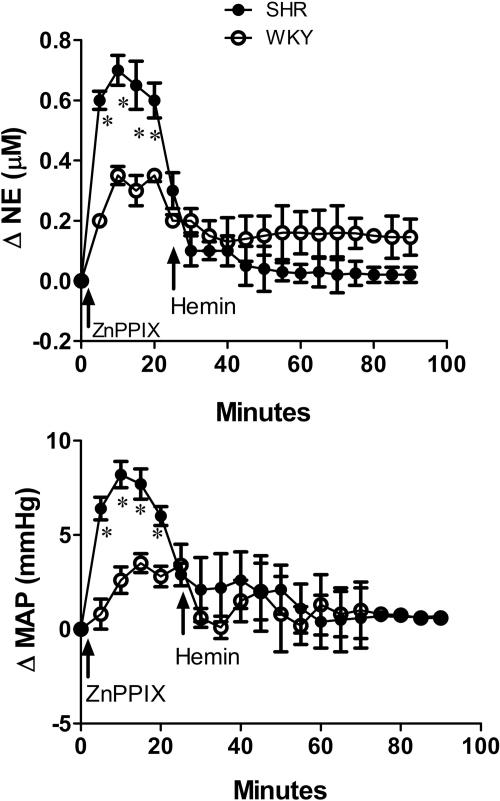
Intra-RVLM hemin (1 nmol) in conscious, freely moving rats reduced MAP and RVLM NE in both rat strains, but the responses were greater (P < 0.05) in SHRs (Fig. 1). Localized inhibition of HO-1 in the RVLM by the selective HO-1 inhibitor ZnPPIX (1 nmol) significantly (P < 0.05) increased RVLM NE and MAP (Fig. 2), but these responses were at least 2-fold greater (P < 0.05) in SHRs compared with WKY rats (Fig. 2). Furthermore, pretreatment with ZnPPIX virtually abolished the reductions in RVLM NE and MAP caused by intra-RVLM hemin in both strains of rats (Fig. 2). Both hemin and ZnPPIX elicited inconsistent changes in heart rate, which were not statistically different when interstrain and intrastrain comparisons were made (data not shown).
Fig. 1.
Time-course changes in RVLM NE (top) and MAP (bottom) in response to intra-RVLM hemin (1 nmol) microinjection in conscious SHRs and WKY rats. Values are means ± S.E.M. (n = 5 per group). *, P < 0.05 compared with corresponding values from WKY rats.
Fig. 2.
Time-course changes in RVLM NE (top) and MAP (bottom) after intra-RVLM microinjection of the selective HO-1 inhibitor ZnPPIX (1 nmol) and subsequent intra-RVLM hemin (1 nmol) microinjection in conscious SHRs and WKY rats. Values are means ± S.E.M. (n = 5 per group). *, P < 0.05 compared with corresponding values from WKY rats.
Effect of Intra-RVLM Hemin or ZnPPIX on HO Activity and cGMP Level.
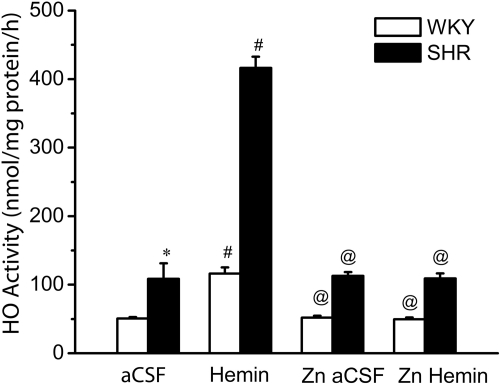
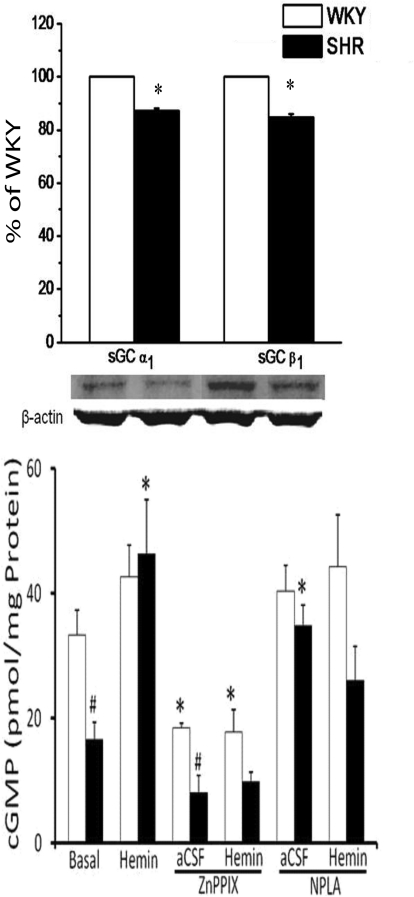
In this experiment, we investigated the effects of intra-RVLM microinjection of the HO substrate hemin, the selective HO-1 inhibitor ZnPPIX, or their combination (discussed above) on RVLM HO activity and cGMP level. In aCSF-treated (control) rats, basal HO catalytic activity was significantly (P < 0.05) higher and cGMP level was significantly (P < 0.05) lower in the RVLM of SHRs compared with WKY rats (Figs. 3 and 4). Western blot analyses showed no strain differences in basal RVLM HO-1 or HO-2 levels (data not shown). On the other hand, the RVLM of the SHRs exhibited significantly (P < 0.05) lower expression of the two subunits of sGC, GC-S-α-1 or GC-S-β-1 (Fig. 4). Intra-RVLM hemin elicited significantly (p < 0.05) greater increase in HO activity (bilirubin level) and cGMP generation in the RVLM of SHRs (Figs. 3 and 4), which paralleled the significantly greater reductions in MAP and RVLM NE in SHRs discussed above (Fig. 1). The selective HO-1 inhibitor ZnPPIX fully abrogated the increases in RVLM HO activity and cGMP level caused by intra-RVLM hemin (Figs. 3 and 4). These neurochemical findings paralleled ZnPPIX abrogation of hemin-evoked reductions in blood pressure and RVLM NE (Fig. 2).
Fig. 3.
Bar graph depicting HO activity in control/treatment groups of SHRs and WKY rats. Values are means ± S.E.M (n = 5 per group). Basal RVLM HO activity is significantly (*, P < 0.05) higher in SHRs, and intra-RVLM hemin increased HO activity 4-fold in SHRs versus 2-fold in WKY rats compared with their respective aCSF levels (#, P < 0.05). The selective HO-1 inhibitor ZnPPIX abrogated (@, P < 0.05) the increases in HO activity caused by intra-RVLM hemin in both strains of rats.
Fig. 4.
Top, bar graph depicting the basal expression of the two sGC subunits, α1 and β1 subunits. Bottom, basal RVLM cGMP and its response to intra-RVLM hemin (1 nmol) in the absence or presence of ZnPPIX (1 nmol) or NPLA (12.5 μg/kg i.v.) in SHRs and WKY rats is shown. Values are means ± S.E.M (n = 4–5 per group). *, P < 0.05, compared with respective basal level; #, P < 0.05, compared with WKY rats.
Effect of Intra-RVLM Hemin on HO-1 and HO-2 Expression and p-nNOS and NOx Levels in the RVLM.
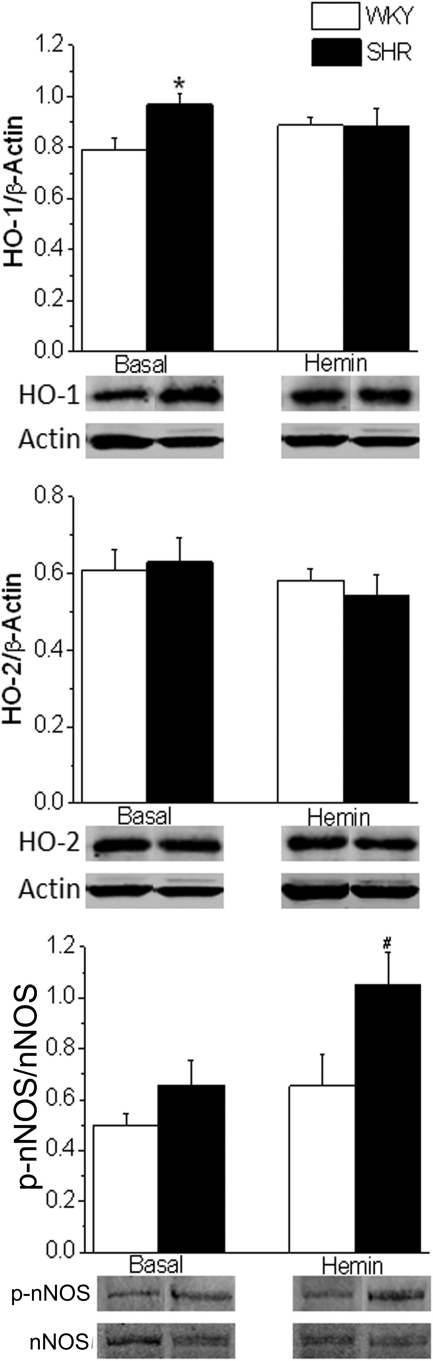
HO-1, but not HO-2, expression was significantly (P < 0.05) higher in the RVLM of the SHRs compared with WKY rats (Fig. 5). Intra-RVLM hemin had no effect on the expression of HO-1 or HO-2 in either rat strain (Fig. 5). As shown in Fig. 6, intra-RVLM hemin significantly (P < 0.05) increased the NOx level in the RVLM of SHRs but not WKY rats. Likewise, hemin induced expression RVLM p-nNOS in SHRs but not in WKY rats (Fig. 5).
Fig. 5.
Bar graphs showing expression of HO-1 (top), HO-2 (middle), and p-nNOS (bottom) in the RVLM of SHRs and WKY rats (n = 5 per group) in the absence (basal) or after intra-RVLM hemin (1 nmol). HO-1 and HO-2 were expressed in relation to actin, whereas p-nNOS was expressed in relation to total nNOS. *, P < 0.05, compared with corresponding value from WKY rats; #, P < 0.05, compared with untreated (basal) level.
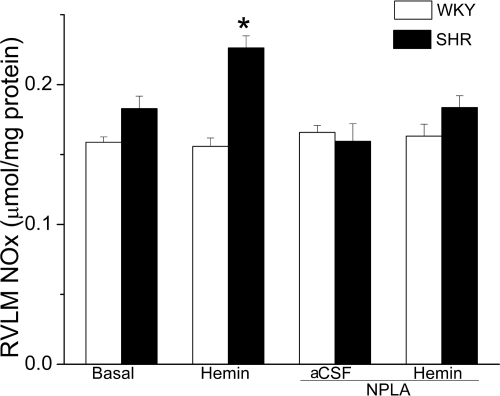
Fig. 6.
Bar graph depicting RVLM NOx levels in control/treatment groups of SHRs and WKY rats. Values are means ± S.E.M (n = 5 per group). Hemin significantly (*, P < 0.05) increased RVLM NOx in SHR, and this response was abrogated by pretreatment with the selective nNOS inhibitor NPLA.
Effect of nNOS Inhibition on Intra-RVLM and the Hypotension and Neurochemical Responses Elicited by Intra-RVLM Hemin.
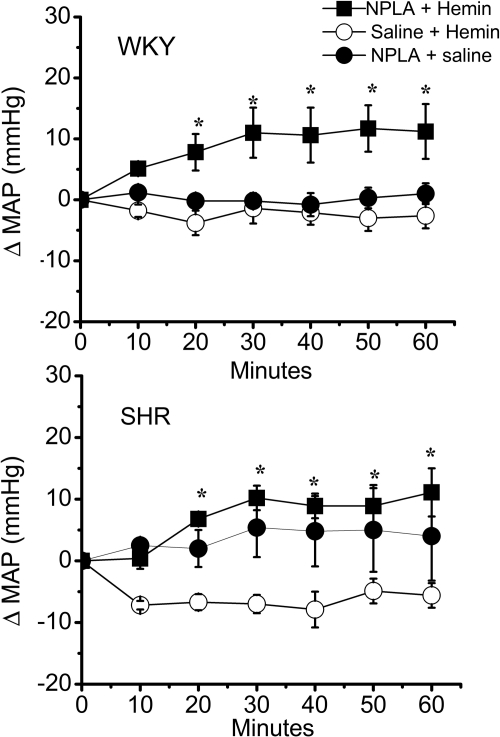
In this experiment, we investigated the role of nNOS in the significantly greater hypotensive and neurochemical response elicited by intra-RVLM hemin in SHRs. As shown in Fig. 7, pretreatment with the selective nNOS inhibitor NPLA abrogated the hypotensive response elicited by intra-RVLM hemin. Likewise, pretreatment with NPLA abrogated the increases in RVLM cGMP (Fig. 4) and NOx (Fig. 6) levels elicited by intra-RVLM hemin in the SHR.
Fig. 7.
Time-course changes in MAP in SHRs (bottom) and WKY rats (top) pretreated with NPLA (12.5 μg/kg i.v) or an equal volume of saline. Intra-RVLM hemin (1 nmol) or equal volume of aCSF was microinjected, 30 min after NPLA/saline, into the RVLM of conscious SHRs or WKY rats. Values are means ± S.E.M. (n = 5 per group). *, P < 0.05, compared with corresponding control values.
Effect of Intra-RVLM CORM-3 Administration on MAP.
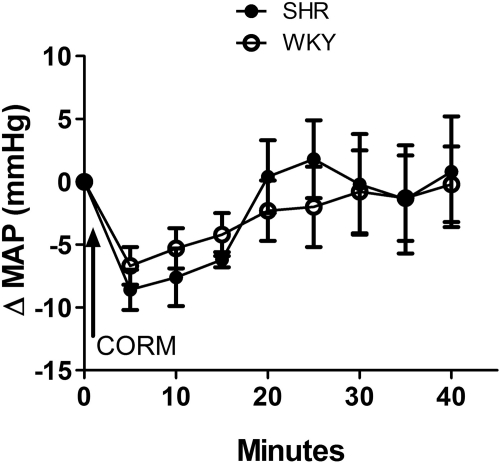
In this experiment, we investigated the effect of HO-independent CO release after intra-RVLM microinjection of the CO donor CORM-3 (2 nmol) on blood pressure in conscious SHRs and WKY rats. Intra-RVLM CORM-3 elicited similar short-lived reductions in MAP in both strains of rats (Fig. 8). Furthermore, there was no significant difference between cGMP levels in the RVLM of CORM-3-treated SHRs and WKY rats (data not shown).
Fig. 8.
Time-course changes in MAP elicited by intra-RVLM microinjection of CORM-3 in conscious SHRs and WKY rats. Values are means ± S.E.M. (n = 5 per group).
Discussion
Systemic HO substrates lowered blood pressure more in hypertensive than normotensive rats (Johnson et al., 1996; Ndisang et al., 2010). The current investigation, which is the first to deal with HO-CO-cGMP signaling in the RVLM of normotensive and hypertensive rats, presents the following important findings: 1) SHRs exhibit significantly higher basal RVLM HO catalytic activity (measured as bilirubin level); 2) HO-1, but not HO-2, expression is significantly higher in the RVLM of SHRs; 3) these are functionally relevant because localized HO inhibition (ZnPPIX) and activation (hemin) elicited significantly larger increases and decreases, respectively, in RVLM neuronal activity and blood pressure in the SHR; by contrast, HO-independent release of CO in the RVLM (CORM-3) elicited similar hypotensive response in SHRs and WKY rats; 4) the basal expression and the catalytic activity of RVLM sGC are significantly lower in the SHR; and 5) pharmacological and neurochemical findings support a major role for RVLM nNOS-derived NO in the exaggerated hypotensive response caused by HO activation in the RVLM of the SHR after intra-RVLM hemin. Collectively, the data support a protective role for the hyperactive RVLM HO against exacerbation of hypertension in SHRs. However, such a protective role seems to be hampered by the hypoactivity of the downstream effector sGC in the RVLM of this genetic model of hypertension.
Results of the present study are the first to directly elucidate the role of HO catalytic activity in the RVLM in blood pressure control. Reported studies have indirectly linked CO-mediated hypotension to sympathoinhibition in normotensive rats (Johnson et al., 1995, 1996, 1997, 2002; Lo et al., 2000, 2004, 2006; Hirakawa and Hayashida, 2006). We focused on the RVLM because it contains the presympathetic neurons (Burke et al., 2008). Furthermore, we used electrochemically measured neuronal RVLM NE as a surrogate of RVLM (sympathetic) neuronal activity (Li et al., 2005; Burke et al., 2008). Together, the findings of our integrative studies in conscious rats present the first direct evidence that locally released HO products (CO and/or biliverdin/bilirubin) in the RVLM lower blood pressure by inhibiting presympathetic neuronal activity.
The findings that pharmacological activation of peripheral (Johnson et al., 1996) or RVLM (this study) HO lowers blood pressure substantially more in SHRs than in WKY rats raise important questions about the role of basal HO activity in genetic hypertension and inferred higher HO level and/or activity in the SHR. However, ours is the first to report a significantly higher basal RVLM HO catalytic activity (Fig. 3) along with significantly higher HO-1, but not HO-2, expression in the RVLM of the SHR (Fig. 5). These neurochemical findings fully agree with similar findings in the hippocampus of the SHR and support the view that HO-1 activation serves a neuroprotective role in the SHR (Huang et al., 2006). Therefore, we focused on the role of RVLM HO-1 in hypertension in the present study.
We show that selective HO-1 inhibition in the RVLM (Zn-PPIX) leads to increases in RVLM neuronal activity and MAP (Fig. 2). The pressor response fully agrees with a similar response elicited by nonselective HO inhibition in the NTS of normotensive rats (Johnson et al., 1997). However, the present findings show, for the first time, that HO inhibition in the RVLM causes substantially larger increases in MAP and RVLM (sympathetic) neuronal activity in SHRs compared with WKY rats (Fig. 2). Furthermore, the pharmacological findings with ZnPPIX, complemented with the neurochemical findings, have highlighted a pivotal role for RVLM HO-1 in restraining the sympathetic activity and blood pressure, particularly in the SHR. We acknowledge two possibilities that might explain the lower magnitude and the shorter pressor response elicited by HO inhibition in our study compared with the pressor response elicited by intra-NTS HO inhibition (Johnson et al., 1997). First, in addition to targeting different neuroanatomical areas, we used ZnPPIX, a selective HO-1 inhibitor (Lo et al., 2006), whereas ZnDPBG, used in the reported study (Johnson et al., 1997), is a nonselective HO-1/HO-2 inhibitor. Second, a possible compensatory generation of CO via the available HO-2- and/or HO-independent mechanisms (Lamon et al., 2009) might counterbalance the pressor and sympathoexcitatory responses elicited by selective HO-1 inhibition (ZnPPIX) in the present study. Nonetheless, it is imperative to note that intra-RVLM ZnPPIX virtually abolished the hypotension and sympathoinhibition elicited by subsequent HO activation (Fig. 2). Collectively, the latter finding, along with the higher RVLM HO catalytic activity, the greater HO-1 expression in the SHR RVLM (Fig. 5), and the greater pressor response elicited by selective HO-1 inhibition (ZnPPIX) in the SHR, supports an important role for RVLM HO-1 in blood pressure control in hypertension. It is imperative to note that hemin enhancement of RVLM HO activity, which was more evident in the SHR (Fig. 3), occurred in the absence of any increase in HO-1 or HO-2 expression (Fig. 5). The mechanism of this interesting finding remains to be investigated. Nonetheless, as discussed below, we demonstrated a clear link between HO activation by hemin and nNOS activation in the RVLM of the SHR.
Similar to findings in SHR vasculature (Ndisang and Wang, 2003), we report a significantly lower sGC basal catalytic activity in the RVLM of the SHR. Furthermore, we present the first evidence that the lower sGC activity in the SHR RVLM is caused, at least in part, by lower protein expression of two sGC subunits, GC-S-α-1 and GC-S-β-1 (Fig. 4). This new finding might explain the lower basal cGMP, which is the downstream cellular product of sGC (Peterson et al., 2009), in the SHR RVLM (Fig. 4). It is noteworthy that we demonstrated that RVLM HO activation (hemin) elicited significantly greater neurochemical and hypotensive response in the SHR. We considered the possibility that, in addition to CO, the other HO product biliverdin and its degradation product bilirubin might have contributed to the larger responses in the SHR. We focused on the potential contribution of nNOS-derived NO to the hemin-evoked generation of cGMP because the neuroprotection conferred by HO-1 in the RVLM depends on nNOS signaling (Dai et al., 2010) and nNOS is protected by the catalytic HO product biliverdin and its subsequent product bilirubin (De Backer and Lefebvre, 2008). Accordingly, the higher generation of bilirubin (measured as the index of HO activity in the present study) after RVLM HO activation (hemin) in the SHR is expected to elicit greater activation of sGC via nNOS in this rat strain. Ultimately, these neurochemical events would explain the higher RVLM cGMP level and the larger hypotensive response elicited by intra-RVLM hemin in the SHR (Figs. 1 and 4). This notion is bolstered by the following novel findings. First, intra-RVLM hemin enhanced nNOS activation (phosphorylation) and increased the NOx level in the RVLM of the SHR (Figs. 5 and 6); these neurochemical findings are functionally relevant because they paralleled the exaggerated hemin-evoked hypotension in the SHR. Second, nNOS inhibition (NPLA) abrogated the hypotension and the elevation in RVLM cGMP and NOx elicited by intra-RVLM hemin; indeed, NPLA eliminated the interstrain differences in the hemin-evoked neurochemical (RVLM NOx and cGMP) and hypotensive responses (Figs. 4, 6, and 7). Finally, the similarity of the HO-independent hypotensive responses elicited by intra-RVLM CORM-3 in both strains of rats seems to highlight the importance of the HO products, biliverdin/bilirubin, in the exaggerated neurochemical and hypotensive responses elicited by intra-RVLM hemin in the SHR. Nonetheless, more neurochemical studies are needed to more definitively delineate the relative role of CO versus biliverdin/bilirubin in the observed responses. This is because the release of equal CO amounts in the RVLM, by intra-RVLM CORM-3, elicited similar short-lived hypotensive response in the two strains of rats (Fig. 8). On the other hand, the exaggerated HO activation by hemin produced larger amounts of bilirubin (used as index of HO activity in the present study) and CO in the RVLM of the SHR. Collectively, these findings infer that the HO products biliverdin/bilirubin contribute to the larger neurochemical and cardiovascular responses elicited by HO, at least in part via activation of nNOS, in the RVLM of the SHR.
In summary, we report the first evidence of higher basal HO catalytic activity and HO-1 expression in the RVLM of SHRs. These findings are functionally relevant because intra-RVLM activation (hemin) or inhibition (ZnPPIX) of HO caused substantially more neurochemical and blood pressure responses in SHRs. The present findings support the hypothesis that in the RVLM of the SHR, which exhibits oxidative stress, the increase in HO-1 expression/catalytic activity guards against exacerbation of hypertension. Moreover, the study implicates an important role for nNOS activation in mediating the exaggerated hemin-evoked hypotensive response in the SHR. Therefore, the present study yields insights into the important role of RVLM HO-1 in blood pressure control in genetic hypertension. More studies are warranted to investigate the role of HO-nNOS cross-talk in the RVLM of hypertensive animals to elucidate the underlying mechanisms that modulate the antioxidant activity of RVLM HO-1 so that novel therapeutics can be developed for the treatment of hypertension.
Acknowledgments
We thank Dr. Shouquan Huo (Department of Chemistry, East Carolina University) for synthesis of CORM-3 and Kui Sun and Jeannie Register for technical assistance.
This work was supported by the National Institutes of Health National Institute on Alcohol Abuse and Alcoholism [Grant 2R01 AA07839-18].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.111.183368.
- CO
- carbon monoxide
- CORM-3
- CO-releasing molecule 3
- HO
- hemeoxygenase
- HO-1
- HO isoform 1
- HO-2
- HO isoform 2
- nNOS
- neuronal nitric-oxide synthase
- p-nNOS
- phosphorylated nNOS
- NOx
- nitrite/nitrate concentration
- NPLA
- N-propyl-l-arginine
- NTS
- nucleus tractus solitarius
- RVLM
- rostral ventrolateral medulla
- GC
- guanylyl cyclase
- sGC
- soluble GC
- ZnPPIX
- zinc protoporphyrin IX
- SHR
- spontaneously hypertensive rat
- WKY
- Wistar Kyoto
- aCSF
- artificial cerebrospinal fluid
- ANOVA
- analysis of variance
- MAP
- mean arterial pressure
- NE
- norepinephrine.
Authorship Contributions
Participated in research design: Nassar, Li, and Abdel-Rahman.
Conducted experiments: Nassar, Li, and Strat.
Performed data analysis: Nassar and Li.
Wrote or contributed to the writing of the manuscript: Nassar and Abdel-Rahman.
References
- Bardgett ME, McCarthy JJ, Stocker SD. (2010) Glutamatergic receptor activation in the rostral ventrolateral medulla mediates the sympathoexcitatory response to hyperinsulinemia. Hypertension 55:284–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke PG, Li Q, Costin ML, McMullan S, Pilowsky PM, Goodchild AK. (2008) Somatostatin 2A receptor-expressing presympathetic neurons in the rostral ventrolateral medulla maintain blood pressure. Hypertension 52:1127–1133 [DOI] [PubMed] [Google Scholar]
- Carraway MS, Ghio AJ, Taylor JL, Piantadosi CA. (1998) Induction of ferritin and heme oxygenase-1 by endotoxin in the lung. Am J Physiol Lung Cell Mol Physiol 275:L583–L592 [DOI] [PubMed] [Google Scholar]
- Chatterjee PK. (2004) Water-soluble carbon monoxide-releasing molecules: helping to elucidate the vascular activity of the ‘silent killer’. Br J Pharmacol 142:391–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai KY, Chan SH, Chang AY. (2010) Heme oxygenase-1 plays a pro-life role in experimental brain stem death via nitric oxide synthase I/protein kinase G signaling at rostral ventrolateral medulla. J Biomed Sci 17:72–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dampney RA, Horiuchi J, Tagawa T, Fontes MA, Potts PD, Polson JW. (2003) Medullary and supramedullary mechanisms regulating sympathetic vasomotor tone. Acta Physiol Scand 177:209–218 [DOI] [PubMed] [Google Scholar]
- De Backer O, Lefebvre RA. (2008) Investigation of a possible interaction between the heme oxygenase/biliverdin reductase and nitric oxide synthase pathway in murine gastric fundus and jejunum. Eur J Pharmacol 590:369–376 [DOI] [PubMed] [Google Scholar]
- El-Mas MM, Fan M, Abdel-Rahman AA. (2009) Facilitation of myocardial PI3K/Akt/nNOS signaling contributes to ethanol-evoked hypotension in female rats. Alcohol Clin Exp Res 33:1158–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foresti R, Hammad J, Clark JE, Johnson TR, Mann BE, Friebe A, Green CJ, Motterlini R. (2004) Vasoactive properties of CORM-3, a novel water-soluble carbon monoxide-releasing molecule. Br J Pharmacol 142:453–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L, Wang W, Zucker IH. (2008) Simvastatin inhibits central sympathetic outflow in heart failure by a nitric-oxide synthase mechanism. J Pharmacol Exp Ther 326:278–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Stein AB, Wu WJ, Tan W, Zhu X, Li QH, Dawn B, Motterlini R, Bolli R. (2004) Administration of a CO-releasing molecule at the time of reperfusion reduces infarct size in vivo. Am J Physiol Heart Circ Physiol 286:H1649–H1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Darnall RA, Riley TA. (1990) Rostral ventrolateral medulla and sympathorespiratory integration in rats. Am J Physiol Regul Integr Comp Physiol 259:R1063–R1074 [DOI] [PubMed] [Google Scholar]
- Hirakawa H, Hayashida Y. (2006) Autonomic cardiovascular responses to heme oxygenase inhibition in conscious rats. Hypertension 48:1124–1129 [DOI] [PubMed] [Google Scholar]
- Huang Y, Wu L, Xu C, Yang B, Wang R. (2006) Increased HO-1 expression and decreased iNOS expression in the hippocampus from adult spontaneously hypertensive rats. Cell Biochem Biophys 46:35–42 [DOI] [PubMed] [Google Scholar]
- Johnson FK, Teran FJ, Prieto-Carrasquero M, Johnson RA. (2002) Vascular effects of a heme oxygenase inhibitor are enhanced in the absence of nitric oxide. Am J Hypertens 15:1074–1080 [DOI] [PubMed] [Google Scholar]
- Johnson RA, Colombari E, Colombari DS, Lavesa M, Talman WT, Nasjletti A. (1997) Role of endogenous carbon monoxide in central regulation of arterial pressure. Hypertension 30:962–967 [DOI] [PubMed] [Google Scholar]
- Johnson RA, Lavesa M, Askari B, Abraham NG, Nasjletti A. (1995) A heme oxygenase product, presumably carbon monoxide, mediates a vasodepressor function in rats. Hypertension 25:166–169 [DOI] [PubMed] [Google Scholar]
- Johnson RA, Lavesa M, DeSeyn K, Scholer MJ, Nasjletti A. (1996) Heme oxygenase substrates acutely lower blood pressure in hypertensive rats. Am J Physiol Heart Circ Physiol 271:H1132–H1138 [DOI] [PubMed] [Google Scholar]
- Kim JH, Yang JI, Jung MH, Hwa JS, Kang KR, Kang KR, Park DJ, Roh GS, Cho GJ, Choi WS, et al. (2006) Heme oxygenase-1 protects rat kidney from ureteral obstruction via an antiapoptotic pathway. J Am Soc Nephrol 17:1373–1381 [DOI] [PubMed] [Google Scholar]
- Lamon BD, Zhang FF, Puri N, Brodsky SV, Goligorsky MS, Nasjletti A. (2009) Dual pathways of carbon monoxide-mediated vasoregulation: modulation by redox mechanisms. Circ Res 105:775–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CY, Yen MH. (2009) Nitric oxide and carbon monoxide, collaborative and competitive regulators of hypertension. Chang Gung Med J 32:12–21 [PubMed] [Google Scholar]
- Li G, Abdel-Rahman AA. (2009) Estrogen-dependent enhancement of NO production in the nucleus tractus solitarius contributes to ethanol-induced hypotension in conscious female rats. Alcohol Clin Exp Res 33:366–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Wang X, Abdel-Rahman AA. (2005) Brainstem norepinephrine neurons mediate ethanol-evoked pressor response but not baroreflex dysfunction. Alcohol Clin Exp Res 29:639–647 [DOI] [PubMed] [Google Scholar]
- Lipski J, Kanjhan R, Kruszewska B, Rong WF. (1995) Criteria for intracellular identification of pre-sympathetic neurons in the rostral ventrolateral medulla in the rat. Clin Exp Hypertens 17:51–65 [DOI] [PubMed] [Google Scholar]
- Lo WC, Hsiao M, Tung CS, Tseng CJ. (2004) The cardiovascular effects of nitric oxide and carbon monoxide in the nucleus tractus solitarii of rats. J Hypertens 22:1182–1190 [DOI] [PubMed] [Google Scholar]
- Lo WC, Jan CR, Chiang HT, Tseng CJ. (2000) Modulatory effects of carbon monoxide on baroreflex activation in nucleus tractus solitarii of rats. Hypertension 35:1253–1257 [DOI] [PubMed] [Google Scholar]
- Lo WC, Lu PJ, Ho WY, Hsiao M, Tseng CJ. (2006) Induction of heme oxygenase-1 is involved in carbon monoxide-mediated central cardiovascular regulation. J Pharmacol Exp Ther 318:8–16 [DOI] [PubMed] [Google Scholar]
- Mao L, Li G, Abdel-Rahman AA. (2003) Effect of ethanol on reductions in norepinephrine electrochemical signal in the rostral ventrolateral medulla and hypotension elicited by I1-receptor activation in spontaneously hypertensive rats. Alcohol Clin Exp Res 27:1471–1480 [DOI] [PubMed] [Google Scholar]
- Mazza E, Thakkar-Varia S, Tozzi CA, Neubauer JA. (2001) Expression of heme oxygenase in the oxygen-sensing regions of the rostral ventrolateral medulla. J Appl Physiol 91:379–385 [DOI] [PubMed] [Google Scholar]
- Nassar N, Abdel-Rahman AA. (2009) Brainstem adenosine A1 receptor signaling masks phosphorylated extracellular signal-regulated kinase 1/2-dependent hypotensive action of clonidine in conscious normotensive rats. J Pharmacol Exp Ther 328:83–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndisang JF, Lane N, Syed N, Jadhav A. (2010) Up-regulating the heme oxygenase system with hemin improves insulin sensitivity and glucose metabolism in adult spontaneously hypertensive rats. Endocrinology 151:549–560 [DOI] [PubMed] [Google Scholar]
- Ndisang JF, Wang R. (2003) Age-related alterations in soluble guanylyl cyclase and cGMP pathway in spontaneously hypertensive rats. J Hypertens 21:1117–1124 [DOI] [PubMed] [Google Scholar]
- Peterson SJ, Frishman WH, Abraham NG. (2009) Targeting heme oxygenase: therapeutic implications for diseases of the cardiovascular system. Cardiol Rev 17:99–111 [DOI] [PubMed] [Google Scholar]
- Pflueger A, Croatt AJ, Peterson TE, Smith LA, d'Uscio LV, Katusic ZS, Nath KA. (2005) The hyperbilirubinemic Gunn rat is resistant to the pressor effects of angiotensin II. Am J Physiol Renal Physiol 288:F552–F558 [DOI] [PubMed] [Google Scholar]
- Powers-Martin K, Barron AM, Auckland CH, McCooke JK, McKitrick DJ, Arnolda LF, Phillips JK. (2008) Immunohistochemical assessment of cyclic guanosine monophosphate (cGMP) and soluble guanylate cyclase (sGC) within the rostral ventrolateral medulla. J Biomed Sci 15:801–812 [DOI] [PubMed] [Google Scholar]
- Ruetten H, Zabel U, Linz W, Schmidt HH. (1999) Downregulation of soluble guanylyl cyclase in young and aging spontaneously hypertensive rats. Circ Res 85:534–541 [DOI] [PubMed] [Google Scholar]
- Siu PM, Donley DA, Bryner RW, Alway SE. (2004) Myogenin and oxidative enzyme gene expression levels are elevated in rat soleus muscles after endurance training. J Appl Physiol 97:277–285 [DOI] [PubMed] [Google Scholar]
- Tsukamoto K, Ito S, Katsunuma N, Hiratsuka M, Masubuchi Y, Kanai T, Kawabe T, Yajima Y, Kanmatsuse K. (2002) Effect of phenylephrine injected into the nucleus tractus solitarius of Sprague-Dawley rats and spontaneously hypertensive rats. Brain Res Bull 58:351–356 [DOI] [PubMed] [Google Scholar]
- Xia CM, Chen J, Wang J, Fan MX, Xiao F, Cao YX, Li L, Shen LL, Zhu DN. (2008) Differential expressions of nNOS and iNOS in the rostral ventrolateral medulla induced by electroacupuncture in acute myocardial ischemia rats. Acta Physiol Sinica 60:453–461 [PubMed] [Google Scholar]