Abstract
Rigorous data regarding fetal central nervous system (CNS) exposure after antidepressant exposure are sparse. The magnitude of serotonin reuptake inhibitor (SRI) CNS exposure was measured in three groups of rats using ex vivo autoradiography of the serotonin transporter (SERT): 1) in utero, 2) postnatal clearance after birth, and 3) exposure through lactation. Rats were exposed to one of five SRI-type antidepressants (escitalopram, fluoxetine, paroxetine, sertraline, and venlafaxine) administered continuously via osmotic minipumps to pregnant or nursing dams. Dam dosing was adjusted to reflect the 50th and 85th percentiles of serum concentrations observed in pregnant women. Embryonic day 21 rat pups exposed in utero exhibited >80% SERT occupancy in brain tissue, which is equivalent to that of the pregnant dam and similar to that reported for human pharmacotherapy. Venlafaxine was the exception with occupancies ranging from 61 to 92% across different litters. The magnitude of SERT occupancy is essentially equivalent between dams and fetuses. By postnatal day 4, high SERT occupancy was observed only in fluoxetine-exposed pups (41–92% occupancy). Significantly less, but measurable, exposure occurred via breast milk exposure even in the absence of detectable drug concentrations in nursing pup sera. Pups exposed to SRIs via breast milk for 3 or 7 days exhibited varying SERT occupancies (0–57% depending on the individual medication and dam dose). These data highlight the need for animal modeling of fetal and nursing infant drug exposure using clinically meaningful dosing strategies and appropriate CNS measures to develop rational treatment guidelines that systematically minimize fetal and neonatal medication exposure in humans.
Introduction
Considering the high prevalence of mental disorders among women during the childbearing years (Weissman and Olfson, 1995; Narrow et al., 2002), the high rates of unplanned conception, and the increasing number of women planning to nurse, the clinician is likely to encounter such situations. Recent data from nearly 199,000 deliveries across seven health-care databases indicate that 6.6% of women were prescribed an antidepressant during pregnancy (Andrade et al., 2008). Common clinical practice has been to discontinue antidepressant therapy during pregnancy and lactation to avert infant exposure; however, prospective data indicate that there is a significant increased risk of depression relapse during pregnancy after antidepressant discontinuation (Cohen et al., 2006). The American College of Obstetrics and Gynecology Practice Bulletins (ACOG Committee on Practice Bulletins–Obstetrics, 2008) underscore the potential adverse consequences of untreated and/or poorly treated maternal mental illness during pregnancy. Such risks include a higher incidence of preeclampsia, preterm delivery, low birth weight neonates, and neonatal intensive-care unit admissions (Steer et al., 1992; Chung et al., 2001). Likewise, maternal mental depression and anxiety during pregnancy has also been associated with childhood attention-deficit hyperactivity disorder, aggression, and anxiety (O'Connor et al., 2003). Unfortunately, clinicians and patients faced with this clinical situation make decisions in the absence of systematically derived data on fetal and neonatal central nervous system (CNS) exposure.
The last 25 years have seen serotonin reuptake inhibitors (SRIs) emerge as first-line treatments for depression and anxiety. These compounds typically offer greater tolerability and enhanced safety to nongravid patients, and although reproductive safety data have grown steadily there are discordant results. Our group and others have demonstrated that all psychotropic medications studied to date are detectable in human amniotic fluid (AF), umbilical cord serum, and breast milk (Stowe et al., 1997; Newport et al., 2009, 2011). The mechanism of placental transport and passage into breast milk is thought to be principally by passive diffusion that depends on duration of exposure and several properties of the individual medication such as molecular size, percentage of protein binding, polarity, and lipid solubility (Schröder, 1995). Despite evidence of significant differences in placental passage rates and detailed investigations of nursing infant exposure (Newport et al., 2009, 2011) the clinical relevance of these data remains obscure. In addition, there is a dearth of data regarding the neonatal clearance of antidepressants. One study reported higher nursing infant serum fluoxetine concentrations in those with exposure during pregnancy (Birnbaum et al., 1999). A natural extension of the extant clinical data, and arguably more important, is to characterize and quantify fetal CNS exposure to antidepressants during pregnancy, CNS neonatal clearance, and infant CNS exposure via breast milk.
Because of the findings showing placental passage of SRIs and the presence of these drugs in AF (Loughhead et al., 2006), it can be assumed that SRIs are present in the fetal brain as well; however, clinical studies afford no opportunity to confirm or quantify fetal CNS exposure. Serotonin plays an important role in brain morphogenesis, directing the development of several neuronal systems, including the serotonin system itself (Whitaker-Azmitia et al., 1996; Bonnin et al., 2007). Serotonin and the serotonin transporter (SERT) are critical in shaping human behavior, and the possible effects of alteration of serotonergic activity during this developmental window have not yet been adequately investigated. Human neuroimaging studies have shown that clinically efficacious doses of SRIs result in approximately 80% plus SERT occupancy (Meyer et al., 2004). This magnitude of occupancy can be assumed to result in significant changes in synaptic serotonin concentrations and serotonergic neurotransmission. Measures of receptor and transporter occupancy represent a more meaningful measure of functional exposure compared with analytic detection of drug within CNS tissue or blood concentrations alone. Using a clinically relevant, and verified, dosing strategy, we quantified SERT occupancy after in utero or lactational exposure to five different SRI medications.
To supplement the quantification of CNS exposure, we assessed the motor skills of adult rats exposed in utero to fluoxetine by using a beam traversing task and the defensive withdrawal paradigm to assess anxiety-like behavior. These tests were undertaken based on a report by Casper et al. (2003) that children prenatally exposed to SRIs showed deficits in motor skills when tested between 6 and 40 months of age and preclinical studies of postnatal SERT blockade that leads to anxiety-like behavior in adulthood (Ansorge et al., 2004). We sought to confirm these findings in our animal model.
Materials and Methods
Animals
Female Long Evans rats (Charles River Laboratories, Inc., Wilmington, MA) used for in utero antidepressant exposure studies arrived timed pregnant on embryonic days 10 and 11 (E10 and E11). Rats were housed singly and provided water and rat chow ad libitum in a temperature-controlled facility with a 12-h light/dark cycle (lights on at 7:30 AM). Rats were allowed 24 h to acclimate before osmotic minipump implantation. Timed pregnant rats used for breastfeeding exposure studies and foster dams arrived on E18. All animal protocols were approved by the Emory University Institutional Animal Care and Use Committee and carried out in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health (Institute for Laboratory Animal Resources, 1996).
Drug Treatment
Drugs were obtained as described in McConathy et al., 2007. All dams were dosed at rates that mimic the 50th (median) or 85th (high) percentile of serum drug concentrations obtained from a sampling of more than 750 pregnant and postpartum women from the Emory Women's Mental Health Program (Z.N. Stowe and D. J. Newport, internal data) (Table 1). Sertraline HCl was administered at 7 or 10 mg/kg/day dissolved in 60% polyethylene glycol (PEG) 400 diluted with isotonic saline. Paroxetine HCl hemihydrate was administered at 3 or 8 mg/kg/day dissolved in 40% PEG 400 diluted with isotonic saline. Venlafaxine HCl was administered at 50, 70, or 80 mg/kg/day dissolved in isotonic saline. Fluoxetine HCl was administered at 8, 11, or 12 mg/kg/day dissolved in a 50% PEG 400 diluted with isotonic saline. Escitalopram was administered as the free base at 4.25 or 10.20 mg/kg/day. Escitalopram was first dissolved in ethanol at a concentration of 250 mg/ml and then brought up to the required volume using PEG 400. All drug solutions were sonicated until dissolved. Pumps were loaded with 0.22-μm filtered drug solution and primed overnight in sterile saline solution at 37°C.
TABLE 1.
List of doses administered to rats and serum concentration goals
| SRI | Dose |
Desired Serum Concentration |
Measured Serum Compound | ||
|---|---|---|---|---|---|
| Median | High | 50th | 85th | ||
| mg/kg/day | ng/ml | ||||
| Sertraline | 7 | 10 | 25/50 | 100/150 | Sertraline/DMS |
| Paroxetine | 3 | 8 | 80 | 160 | Paroxetine |
| Fluoxetine | 8 | 11–12 | 300–400 | 800–1100 | Fluoxetine + norfluoxetine |
| Venlafaxine | 50–60 | 70–80 | 300–400 | 800–1100 | Venlafaxine + ODV |
| Escitalopram | 4.3–4.9 | 10.2–11.8 | 20 | 60 | Escitalopram |
DMS, desmethylsertraline; ODV, O-desmethylvenlafaxine.
Animal Protocols
Pump Implantation.
Animals were briefly anesthetized with methoxyflurane (Medical Developments Australia Pty Ltd., Springvale, Australia) and 14-day Alzet osmotic minipumps (model 2ML2; Durect Corporation, Cupertino, CA) were implanted subcutaneously slightly posterior to the scapulae. Dams used to quantify SRI exposure in utero or postnatal clearance (PNC) were implanted with pumps on E12. Dams used to measure breast milk exposure to pups were implanted with pumps on postnatal day 1 (PND1).
Cesarean Section.
C-sections were performed on E21 (the day before delivery) to assess SRI in utero exposure. In brief, dams were anesthetized with 40 mg/kg i.p. of sodium pentobarbital, the dam was placed in the supine position, and a transverse incision was made across the lower abdomen exposing the uterus. The fetal pup most distal to the pelvis in the right horn of the uterus was isolated. AF was removed by aspiration with a 25-gauge, 5/8th-inch needle using a 1-ml syringe. The fetal pup was removed and decapitated by using large sharp scissors. Pup brain and placenta were collected and fresh-frozen on flat blocks of dry ice. This procedure was repeated for each of the fetal pups. The incision was not closed, and dam blood was collected by cardiac puncture using a 16-gauge needle and 10-ml syringe. Dams were then decapitated and the brains were collected as above.
Cross-Fostering.
With the exception of sertraline-exposed pups, pups used to study postnatal drug clearance were cross-fostered to an untreated dam <24 h after birth to halt additional drug administration via breast milk. Based on pilot data determining sertraline clearance in adult male rats (t1/2 ∼ 6 h; M. J. Owens, unpublished observations), we assumed that after minipump removal the drug would clear sufficiently from the dam in less than a day and that cross-fostering was unnecessary. HPLC analyses of the dams' serum and breast milk showed this to be incorrect (see Results). Dams treated during gestation were anesthetized with 40 mg/kg i.p. of sodium pentobarbital; serum and brains were collected to verify appropriate dosing.
Sample Collections
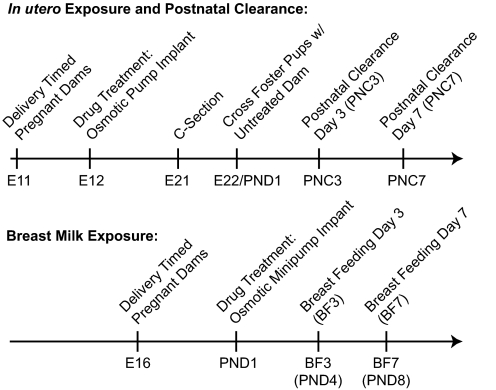
In addition to collection procedures noted above, samples were collected at E21, PNC day 3 (PNC3), PNC7, PNC14 (fluoxetine only), breastfeeding day 3 (BF3; the fourth postnatal day), or BF7 (the eighth postnatal day) (Fig. 1). Pup serum was collected by decapitation with large surgical scissors and pooled. Brains were fresh-frozen on dry ice. AF was pooled per dam and collected by using a 25-gauge, 5/8th-inch needle and 1-ml syringe. All samples were stored at −80°C.
Fig. 1.
Protocol timeline detailing dose administration and sample collection.
Serum Analysis of Drug Concentrations
Serum analysis of drug concentrations has been described previously in detail (Stowe et al., 1997; see Newport et al., 2011). In brief, after a solid-phase extraction the serum samples were analyzed via isocratic high-performance liquid chromatography separation followed by ultraviolet detection. The limit of detection for each compound was 2 ng/ml. We were unable to translate the techniques previously used to quantify the SRI presence in human breast milk (Stowe et al., 1997) to use with rodents. Breast milk samples were obtainable only by collecting pups' stomach contents and the partial digestive process rendered samples unsuitable for reliable quantification of dam breast milk concentrations.
Receptor Autoradiography
Measurement of SERT Occupancy.
Postnatal pup brains were sliced at −16°C into 20-μm sections at plate 14 containing frontal cortex and striatum by using a Paxinos and Watson, 1986 atlas and placed onto Superfrost Plus Slides (Thermo Fisher Scientific, Waltham, MA). Plate 14 exhibited both SERT and dopamine transporter (DAT) binding at separate locations ensuring a control for the radiotracer's selectivity (see below). E21 brains were sliced at + 3270-μm transverse (Foster, 1998), at a comparable location to plate 14. All slides were stored at −80°C until the assay. The cortex was targeted to measure SERT occupancy because pilot studies showed that the radioligand signal within the cortex was completely eliminated by SRI coincubation with little or no change in signal within the striatum (predominantly DAT) (Supplemental Fig. 1). The SERT autoradiography assay measuring drug transporter occupancy was based on techniques by Boja et al. (1992) with minor modifications. Most importantly, the incubation time was changed to 5 min with no preincubation. Pilot studies revealed that SERT is present at this age and easily measurable with this short incubation time for the radiotracer. This method does not achieve binding equilibrium and cannot give accurate measures of SERT Bmax but does provide an accurate assessment of SERT occupancy. Pilot studies revealed a direct relationship between unlabeled drug dissociation and preincubation time, therefore total incubation time was minimized to maintain accurate ex vivo estimates of SERT occupancy. Total binding was defined with 50 pM [125I]RTI-55 (PerkinElmer Life and Analytical Sciences (Waltham, MA). Nonspecific binding was defined using 1 μM chloroimipramine for the SERT (Sigma/RBI, Natick, MA) or 100 nM 18F-2β-carbomethoxy-3β-(4-chlorophenyl)-8-(2-fluoroethyl)nortropane for the DAT (Goodman et al., 2000). The slides were then washed twice for 10 min in 4°C assay buffer, dipped in 4°C double-distilled H2O, and dried with a stream of cold, dry air.
Measurement of SERT Protein Density.
Measurement of SERT density in cortical homogenates using Western blots (see Supplemental Data) was performed to confirm that low SERT radioligand binding measurements in the autoradiographic procedures were caused by SRI occupancy at the transporter rather than lack of the transporter protein itself as a result of drug exposure (Supplemental Fig. 2). Anti-SERT antibody was generously provided by Dr. Randy Blakely (Vanderbilt University, Nashville, TN) (Qian et al., 1995).
Image Analysis
The slides and a 125I microscale standard (GE Healthcare, Chalfont St. Giles, Buckinghamshire UK) were coexposed on Kodak Biomax MR film (Eastman Kodak, Rochester, NY) for varying times. E21 slides were exposed for 12 to 14 days; all PNC and BF slides were exposed for 7 to 10 days. SERT occupancy was assessed from cortical radioligand signal. This signal was completely eliminated by coincubation with chloroimipramine (Supplemental Fig. 1). DAT labeling was present in the caudate and signal was not altered by chloroimipramine. AIS software (version 6.0; Imaging Research, St. Catharines, ON, Canada) was used to quantify slides that were calibrated against the microscale standards. Values were expressed as a percentage of the mean of the vehicle control samples. Every brain had a single mean value that was calculated from the analysis of at least four sections.
Measurement of Transporter Density in Adulthood
Measurement of SERT and NET Bmax using single-point saturation binding was performed using methodology described previously (Owens et al., 1997). In brief, frontal cortical membranes were prepared, and a near-saturating concentration of either [3H]citalopram (6 nM final concentration) for the SERT or [3H]nisoxetine (6 nM final concentration) for the NET were performed in triplicate for both total binding and nonspecific binding (defined in Owens et al., 1997). Bmax was estimated and normalized to milligrams of membrane protein.
Behavioral Analysis
The beam traversing task and defensive withdrawal test was performed after the postnatal clearance pups had reached adulthood, PND 70 to 100. The beam traversing task and defensive withdrawal test was performed on rats exposed to fluoxetine in utero. All behavioral tapes were analyzed separately by two people blinded to the drug treatment groups and inter-rater reliability was more than 0.9.
Beam Traversing.
The beam traversing task was performed as described by Feeney et al. (1982), and the apparatus was built to the specifications listed by Goldstein and Davis (1990). In brief, the beam was 1 m long and 1.5 cm wide with four 1.5-cm pegs set up every 20 cm. It was held 1 m high with two plywood stands. The starting platform was affixed to the beam and measured approximately 10 × 15 cm. A lamp with a 75-W bulb was clipped to the starting platform, and a white noise maker was positioned approximately 500 cm from the platform. Both appliances were plugged into an extension cord so that they would turn on simultaneously when the cord was plugged in. A divider was a placed between the platform and start of the beam to prevent the rat from getting onto the beam prematurely. Rats' performances were assessed in five trials over 5 consecutive days, succeeding 3 training days. The rat was placed on the starting platform with the divider in place. Noxious stimuli were turned on as the divider was removed and turned off as the rat entered the tube at the end of the beam. Experiments were recorded on video for later analysis. Two measurements were recorded: the time the rat required traversing the beam and the number of times a rat's paw slipped off the beam. Time was started when the rat placed its third paw onto the beam and ended when the rat's nose entered the tube. Paw slips fall into two categories: A “single paw” slip was recorded when a rat gained footing on the beam and subsequently dropped its paw below the bar or when the rat reached for the beam and missed. When the rat lost balance and was anchored to the bar with only two paws, a “double paw” slip was recorded. If necessary at this point, the rat was assisted back onto the beam. A full-length mirror was positioned such that both sides of the rat's body could be seen on video. Significant differences were evaluated by one-way ANOVA followed by Student-Newman-Keuls post hoc analysis from within gender groups and by two-way ANOVA with gender and treatment assigned as the two factors. All groups consisted of at least six rats.
Defensive Withdrawal Test.
Testing took place 2 h after lights out and was conducted under dim red light. Rats were placed in a black polyvinyl chloride tube (10-cm-diameter tube × 21 cm in length, closed at one end). The tube was then placed into a white acrylic open area (1 × 1 × 0.6 m). Rats were recorded for 10 min and scored based on latency to first exit the tube and total time spent in the tube.
SRI Partition Coefficients.
Partition coefficients (logD7.4) were measured in a 10-ml volume containing 5 ml of octanol and 5 ml of phosphate-buffered saline, pH 7.4. For citalopram and paroxetine measurements, [3H]citalopram or [3H]paroxetine were used to spike the 10-ml solution. The solution was vortexed, and 10 μl of the aqueous was added to scintillation fluid, counted on a liquid scintillation counter, and compared with the total amount of radioactivity added. Other compounds were measured by adding 10 μg of compound to 10 ml of the octanol/phosphate-buffered saline solution as above. The solution was vortexed for 1 h, and a small portion of the octanol phase was diluted directly with the mobile phase for HPLC-UV quantification as described previously (Stowe et al., 1997). Citalopram and escitalopram were measured by different detection methods, but their partition coefficients were identical. This result validates the two different methods of compound detection because citalopram and escitalopram have identical partition coefficients (Supplemental Table 1). Data are the mean of three separate experiments, each prepared and extracted on different days.
Data Analysis.
In an ideal design, each litter would provide a single pup, and six to eight litters would then need to be provided for each treatment group so that no single pregnancy was over-represented. This was both impractical and not cost effective, particularly when all of the major SRI medications were to be compared and we were focusing predominantly on the pharmacokinetic properties of medication exposure rather than pharmacological actions on the offspring. For these studies, two litters of each treatment condition (e.g., vehicle, median dose drug, and high dose drug) were produced for each medication under study. Four to six pups were randomly used from each litter, and these were treated as a representative treatment group of n = four to six for analysis (i.e., each pup was treated as a single observation). The mean signal intensity (nCi/g tissue) from four to six pups in each of two vehicle litters was used to provide a control value for signal intensity and percentage of occupancy from each of the drug treatment group pups used this value for all calculations. Signal intensity between pups in different vehicle control litters varied by no more than 10 to 20%. Each different SRI was treated as a separate study, and all slides for that study were incubated in a single assay in a random fashion. The data were then analyzed by one-way ANOVA followed by Newman-Keuls post hoc analyses. All statistical tests were two-tailed, and all data are shown as either mean or mean ± S.E.M. All statistics and graphs were run using Prism software, version 3.0 (GraphPad Software Inc., San Diego, CA).
Results
The dams and pups used for study were chosen after verification of targeted serum drug concentrations. The pups were measured for SERT occupancy only if their dam's serum drug concentrations were near our established goals (Table 1), which are comparable with median and high (85th percentile) human drug concentrations (Z. N. Stowe and D. J. Newport, unpublished observations from the Emory Women's Mental Health Program; http://www.emorywomensprogram.org/). Pups were grouped as “median” and “high” at each measured time point (i.e., PNC3 high would indicate rat pups measured at PNC3 that had been exposed in utero to 85th percentile human serum drug concentrations; PNC3 median represents the 50th percentile human serum drug concentrations).
SERT occupancy and pup serum drug concentrations were measured in pups dosed with SRIs in utero. Postnatal clearance of the SRIs was measured 3 and 7 days after the cessation of drug administration, i.e., at delivery (Table 2; Fig. 2). With the exception of venlafaxine, all drug-exposed groups at E21 exhibited SERT occupancy equivalent to or in excess of that proposed to be needed for therapeutic efficacy (i.e., occupancy of ∼80%) (p < 0.001). Venlafaxine-exposed E21 pups exhibited a mean SERT occupancy range of 62 to 92% (p < 0.001). Pup AF drug concentrations (Table 2) varied from undetectable, in the case of sertraline and paroxetine, to within ranges exhibited in our human population, in the case of fluoxetine, venlafaxine, and escitalopram.
TABLE 2.
SERT occupancies and serum drug concentrations after in utero exposure and postnatal clearance
| Drug | Litter | E21 |
PNC3 |
PNC7 |
PNC14 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Occupancy Pups (mean ± S.E.M.) | Pup Drug AF Concentration | Dam Drug Serum Concentration. | Occupancy Pups (mean ± S.E.M.) | Pup Drug Serum Concentration | Dam Drug Serum Concentration | Occupancy Pups (mean ± S.E.M.) | Pup Drug Serum Concentration | Dam Drug Serum Concentration | Occupancy Pups (mean ± S.E.M.) | Pup Drug Serum Concentration | Dam Drug Serum Concentration | ||
| % | ng/ml | % | ng/ml | % | ng/ml | % | ng/ml | ||||||
| Sertraline | |||||||||||||
| Median | 1 | 93.8 ± 1.3*** | <2/<2 | 28.8/130.7 | 13.9 ± 19.0 | −21.02 ± 8.72 | |||||||
| Median | 2 | 92.8 ± 2.9*** | <2/<2 | 29.1/43.16 | −7.8 ± 4.8 | 17.68 ± 11.49 | |||||||
| High | 3 | 85.3 ± 2.2*** | <2/<2 | 143/21 | 9.1 ± 2.6 | −10.28 ± 9.5 | |||||||
| High | 4 | 82.1 ± 4.0*** | <2/<2 | 202/76 | 45.8 ± 8.6** | −21.13 ± 18.7 | |||||||
| Paroxetine | |||||||||||||
| Median | 1 | 97.9 ± 0.6*** | <2 | 63 | −12.22 ± 4.4 | <2 | 33 | −17.1 ± 7.2 | <2 | 111 | |||
| Median | 2 | 97.5 ± 0.4*** | <2 | 24 | 11.8 ± 2.1 | 30 | 66 | −12.2 ± 2.7 | <2 | 89 | |||
| High | 3 | 96.8 ± 0.8*** | <2 | 110 | 25.1 ± 3.3 | 24 | 68 | ||||||
| High | 4 | 99.2 ± 0.2*** | 3 | 132 | |||||||||
| Fluoxetine | |||||||||||||
| Median | 1 | 100.0 ± 0.0*** | <2/152 | 148/265 | 40.8 ± 1.8*** | 34/20 | 40/166 | 9.1 ± 5.1 | <2/<2 | 48/439 | 9.7 ± 7.0 | 10/50 | 84/484 |
| Median | 2 | 98.2 ± 1.0*** | <2/158 | 119/216 | 74.2 ± 0.6*** | 68/60 | 44/309 | −20.3 ± 4.2** | <2/<2 | 42/414 | 11.1 ± 5.6** | 18/32 | 18/263 |
| High | 3 | 96.2 ± 1.6*** | Lost | 224/741 | 91.5 ± 0.4*** | 58/64 | 96/480 | −33.3 ± 6.6** | <2/<2 | 70/620 | 5.2 ± 6.4 | 10/19 | 108/576 |
| High | 4 | 100.0 ± 0.0*** | 28/167 | 168/593 | 88.1 ± 0.4*** | 67/66 | 144/886 | 40.2 ± 6.8*** | <2/10 | 132/668 | 3.7 ± 3.2 | 2/12 | 280/548 |
| Venlafaxine | |||||||||||||
| Median | 1 | 64.5 ± 6.1*** | 66/201 | 49/350 | 1.5 ± 3.0 | <2/<2 | 210/51 | −14.96 ± 4.6 | <2/<2 | 65/358 | |||
| Median | 2 | 60.9 ± 6.9*** | 81/344 | 72/537 | 1.3 ± 1.2 | <2/<2 | 236/80 | 11.2 ± 18.0 | <2/<2 | 35/255 | |||
| High | 3 | 70.1 ± 2.2*** | 82/476 | 111/702 | 14.3 ± 6.6 | <2/<2 | 327/67 | 5.7 ± 6.0 | <2/<2 | 63/522 | |||
| High | 4 | 92.0 ± 2.1*** | 120/344 | 232/1002 | 5.4 ± 0.5 | <2/<2 | 348/81 | 0.3 ± 2.3 | <2/<2 | 147/941 | |||
| High | 5 | 71.6 ± 3.1*** | 164/475 | 177/692 | 9.1 ± 3.4 | <2/<2 | 408/62 | ||||||
| Escitalopram | |||||||||||||
| Median | 1 | 85.0 ± 2.1*** | 18 | 20 | 6.2 ± 6.5 | <2 | 21 | 41.1 ± 3.7*** | <2 | 39 | |||
| Median | 2 | 89.9 ± 5.0*** | 15 | 23 | −9.3 ± 7.6 | <2 | 37 | 31.0 ± 4.4*** | <2 | 21 | |||
| High | 3 | 92.0 ± 1.3*** | Lost | 54 | −11.7 ± 1.5 | <2 | 74 | 3.9 ± 3.5 | <2 | 75 | |||
| High | 4 | 96.8 ± 0.2*** | Lost | 52 | 2.3 ± 5.4 | <2 | 88 | 1.4 ± 3.7 | <2 | 43 | |||
, p < 0.01;
, p < 0.001.
Fig. 2.
SERT occupancy after in utero exposure and subsequent postnatal clearance (days last drug exposure in utero). Each bar represents the mean of two separate litters each consisting of four to six rats. *, p < 0.05; ***, p < 0.001. All comparisons are versus vehicle control (percentage occupancy). All data are mean ± S.E.M. Shaded gray area above and below 0% occupancy represents the variability (S.E.M.) in vehicle control samples.
The rate of PNC varied among the different SRIs as would be expected for drugs having different rates of metabolic clearance (Fig. 2; Table 2). At PNC3, all litters exposed to fluoxetine in utero exhibited mean SERT occupancies ranging from 40 to 91.5% (p < 0.001). Sertraline PNC3 high, venlafaxine PNC3 high, and paroxetine PNC3 high litters exhibited SERT occupancy of 27% (p < 0.001), 10% (p < 0.001), and 18% (p < 0.001), respectively. Fluoxetine-exposed litters exhibited detectable drug serum concentrations, although much less than that observed in the dam at the time of delivery suggesting that the rats' pups were actively clearing the medication. Sertraline-exposed and venlafaxine-exposed litters, despite quantifiable transporter occupancies, did not show detectable serum concentrations at PNC3. Paroxetine-exposed litters exhibited detectable drug serum concentrations, but SERT occupancies were not significantly different from controls although some occupancy was numerically apparent. With the exception of PNC7 (see below), escitalopram-exposed litters did not exhibit detectable serum drug concentrations or SERT occupancy. In summary, all SRI PNC3 litters (with the exception of those exposed to escitalopram) exhibited measurable SERT occupancies despite varying pup SRI serum concentrations.
As expected, and with few exceptions, occupancy at PNC7 was less than that observed at PNC3 (Fig. 2; Table 2). Escitalopram-exposed PNC7 high litters exhibited a significant SERT occupancy of 36% (p < 0.001). This is surprising and not readily explained in light of the escitalopram data at PNC 3. Washout studies using long preincubations revealed no evidence of SRI-induced changes in SERT protein expression to account for this observation (C. F. Capello, unpublished observations). Western blot analysis (Supplemental Fig. 2) also showed no differences in cortical SERT protein concentrations between escitalopram- or fluoxetine- and vehicle-treated animals at E21. One litter exposed to fluoxetine in utero within the PNC7 high group exhibited a mean SERT occupancy of 40% (p < 0.001) and detectable serum norfluoxetine concentrations; however, this was not observed in a separate litter identically treated and the mean occupancy for this treatment group (two separate litters) was <10%. Because of norfluoxetine's slow clearance in both rats (see below) and humans, SERT occupancy and serum drug concentrations were measured at PNC14 only in litters exposed to fluoxetine in utero. When the litters were averaged together as median and high groups there was no evidence of SERT occupancy. All litters exhibited low, but detectable, serum drug concentrations. It is noteworthy that several groups (paroxetine PNC3 median, paroxetine PNC7 median, paroxetine PNC7 high, sertraline PNC7 median, and sertraline PNC7 high) exhibited significant negative percentage occupancy of SERT. This could possibly be interpreted as assay variability or a significantly higher SERT density compared with control independent of any SRI presence (i.e., up-regulation of SERT protein). However, as noted above, Western blot analyses (Supplemental Fig. 2) and prolonged washout studies of E21 sections from drug-treated animals revealed SERT signal intensity identical to that of vehicle controls, suggesting no change in SERT protein (C. F. Capello, unpublished observations).
SERT occupancy and pooled pup serum drug concentrations were measured in pups exposed to SRIs via breast milk after 3 and 7 days of exposure (Fig. 3; Table 3). After 3 days of exposure via breast milk (BF3), sertraline BF3 high exhibited SERT occupancies, ranging from 23.6 to 34.4% (p < 0.001). Both paroxetine BF3 median and high groups showed significant SERT occupancy ranging from 48.1 to 55.9% (p < 0.001). All fluoxetine-exposed litters exhibited significant SERT occupancies, ranging from 43.2 to 57.2% (p < 0.001)). Likewise, both venlafaxine BF3 median and high groups exhibited significant SERT occupancies ranging from 5 to 46% (p < 0.01 and < 0.001, respectively). Norfluoxetine was detectable in only two of the four litters. Pup serum drug concentrations were undetectable in all other litters (Table 3).
Fig. 3.
SERT occupancy after breast milk exposure. Each bar represents the mean of two separate litters each consisting of four to six rats. *, p < 0.05; **, p < 0.01; ***, p < 0.001. All comparisons are versus vehicle control (0% occupancy). All data are mean ± S.E.M. Shaded gray area above and below 0% occupancy represents variability (S.E.M.) in vehicle control samples.
TABLE 3.
SERT occupancies and serum drug concentrations after breast milk exposure
| Drug | Litter | BF3 |
BF7 |
||||
|---|---|---|---|---|---|---|---|
| Occupancy Pups (mean ± S.E.M.) | Pup Drug Serum Concentration | Dam Drug Serum Concentration | Occupancy Pups (mean ± S.E.M.) | Pup Drug Serum Concentration | Dam Drug Serum Concentration | ||
| % | ng/ml | % | ng/ml | ||||
| Sertraline | |||||||
| Median | 1 | 8.06 ± 8.2 | 20/15 | 35.2 ± 1.5* | 19/19 | ||
| Median | 2 | 34.4 ± 2.6* | 12/6 | 18.0 ± 12.2 | 28/22 | ||
| High | 3 | 26.55 ± 6.5* | 221/11 | 23.6 ± 5.9* | 179/16 | ||
| High | 4 | 23.6 ± 5.9* | 72/6 | ||||
| Paroxetine | |||||||
| Median | 1 | 10.3 ± 3.8 | <2 | 168 | 6.5 ± 5.2 | <2 | 91 |
| Median | 2 | 53.1 ± 4.8*** | <2 | 159 | 8.1 ± 1.7 | <2 | 58 |
| High | 3 | 48.1 ± 3.7*** | <2 | 590 | −0.6 ± 2.0 | <2 | 128 |
| High | 4 | 55.9 ± 4.2*** | <2 | 372 | 28.3 ± 1.3* | <2 | 142 |
| Fluoxetine | |||||||
| Median | 1 | 45.8 ± 6.6** | <2/<2 | 18/195 | 39.0 ± 9.5*** | <2/<2 | 56/213 |
| Median | 2 | 43.2 ± 3.5* | <2/<2 | 34/155 | 34.0 ± 7.2*** | <2/<2 | 94/237 |
| High | 3 | 57.2 ± 6.2** | <2/13 | 69/228 | 47.1 ± 2.5*** | <2/<2 | 92/364 |
| High | 4 | 52.3 ± 5.8* | <2/12 | 99/258 | 40.1 ± 4.2*** | <2/<2 | 108/364 |
| Venlafaxine | |||||||
| Median | 1 | 5.9 ± 8.1 | <2/<2 | 36/266 | 20.1 ± 2.3*** | <2/<2 | 33/152 |
| Median | 2 | 34.8 ± 4.1 | <2/<2 | 56/277 | 22.0 ± 3.8*** | <2/<2 | 38/113 |
| High | 3 | 46.1 ± 17.2 | <2/<2 | 75/406 | 25.6 ± 1.2*** | <2/<2 | 52/397 |
| High | 4 | 9.8 ± 27.1 | <2/<2 | 73/364 | 27.8 ± 3.1*** | <2/<2 | 91/528 |
| High | 5 | 29.6 ± 14.6 | <2/<2 | 77/450 | |||
| Escitalopram | |||||||
| Median | 1 | −17.8 ± 8.4 | <2 | 21 | −8.11 ± 4.5 | <2 | 12 |
| Median | 2 | −16.6 ± 3.9 | <2 | 35 | 10.5 ± 6.6 | <2 | 16 |
| High | 3 | −15.9 ± 9.9 | <2 | 60 | 6.0 ± 6.1 | <2 | 39 |
| High | 4 | −10.6 ± 9.2 | <2 | 57 | 13.0 ± 3.7 | <2 | 38 |
, p < 0.05;
, p < 0.01;
, p < 0.001.
After 7 days of exposure via breast milk (BF7), SERT occupancy was observed in all sertraline groups, ranging from 18 to 35% (p < 0.001), all litters exposed to fluoxetine (range 43–57%; p < 0.001), all litters exposed to venlafaxine (range 20.1–27.8%; p < 0.001), and the escitalopram BF7 high group (range 6–13%, p < 0.05). In contrast to BF3, pups exposed to paroxetine did not exhibit significant SERT occupancy at BF7. Serum drug concentrations were below the limit of detection in all litters regardless of numerical evidence of SERT occupancy (Table 3).
Behavioral assessment in the beam traversing task of rats exposed to fluoxetine in utero are shown in Fig. 4. There were no significant differences in this measure of motor skills in any of the fluoxetine-exposed adult rats compared with vehicle controls (p > 0.05; Fig. 4, A and B). Identical results in this test were observed for each of the other four drugs (C. F. Capello, unpublished observations). Rats exposed to fluoxetine in utero did not demonstrate any anxiety-like behavior in the defensive withdrawal test (p > 0.05; Fig. 4, C and D).
Fig. 4.
Beam traversing task and defensive withdrawal test of adult rats exposed to fluoxetine in utero. Neither male nor female performance in each test was affected by in utero fluoxetine exposure in beam traversing time (A), beam traversing errors (B), latency to first exit the tube (C), or total time in the tube (D) (n = 6–12 per group).
Further assessment of adult rats exposed to fluoxetine in utero was carried out to measure transporter density in adulthood. There was no effect of sex or fluoxetine exposure in utero on SERT or NET density in frontal cortex of adult rats (p > 0.05) (Fig. 5).
Fig. 5.
Frontal cortex SERT and NET density in adult rats exposed to fluoxetine in utero. Animals were combined from multiple litters to determine mean total SERT or NET density in males (●) and females (○). There were no differences in estimates of SERT or NET Bmax in adult animals. Maternal serum concentrations of the median group were 46.5 ± 13.7 ng/ml of fluoxetine and 305.5 ± 66.6 ng/ml of norfluoxetine. Maternal serum concentrations of the high group were 138 ± 37.8 ng/ml of fluoxetine and 597.2 ± 74.2 ng/ml of norfluoxetine. Individual animal SERT or NET densities and the mean ± S.E.M. for each group are represented.
Venlafaxine and O-desmethylvenlafaxine were the least lipophilic compounds tested (Supplemental Table 1) and the only compounds measured in significant quantities in AF; however, a clear relationship between lipophilicity and occupancy or AF concentrations over the very wide range of logD7.4 values was not observed.
Discussion
Despite the importance of gathering obstetrical, neonatal, and developmental data on SRIs, this is the first study to quantify CNS exposure (i.e., SERT occupancy) in utero, during postnatal clearance, or via lactation using clinically relevant exposure. Our initiation of treatment in the pregnant dam coincides with the first appearance of SERT in the prenatal rat brain (Whitaker-Azmitia et al., 1996; Hanson et al., 1999). The period of greatest synaptogenesis and overall increase in the rate of brain weight, also known as the brain growth spurt period, occurs in different species at different times relative to birth. In rats and mice, it is primarily a postnatal event, but in human it extends from the sixth month of gestation to several years after birth (Dobbing and Sands, 1973, 1979). It has been hypothesized that this might represent the period of greatest “vulnerability;” however, this is for severe exposures such as malnutrition and whole brain irradiation, and there are many limitations including the fact that other periods of development might be just as vulnerable (Dobbing and Sands, 1979). Avishai-Eliner et al. (2002) performed a detailed review of milestones in hippocampal development in human, nonhuman primate, and rat brain. Comparisons of general development, neuronal formation, differentiation, and synaptogenesis, and afferent input between human and rat were strikingly similar as to whether these events were prenatal or postnatal. Therefore, we posit that prenatal dosing in the rat is a useful and appropriate model of prenatal medication exposure in humans, not only for the comparable development rates but also for the unique method of drug exposure (via placental passage and constant readministration via AF).
As noted previously, the SERT plays a critical role during this developmental period. Several studies have attempted to categorize possible longitudinal effects of SRI exposure during this period (Vorhees et al., 1994; Cabrera-Vera et al., 1997; Cabrera-Vera and Battaglia, 1998; Coleman et al., 1999; Christensen et al., 2000; Rayburn et al., 2000; Morrison et al., 2004; Vartazarmian et al., 2005), but these studies are of limited clinical relevance for several reasons. With the exception of a single study (Vartazarmian et al., 2005), dams were dosed only once or twice daily during gestations. Because of the SRIs' short half-lives in rodents (with the exception of norfluoxetine) parts of the 24-h cycle have little or no drug exposure. Moreover, only two of these studies confirmed that their serum drug concentrations were within appropriate human clinically observed ranges at any time during the dosing period (Morrison et al., 2004; Vartazarmian et al., 2005). For drugs with short half-lives in rodents, maintaining serum drug concentrations comparable with human treatment near the end of the dosing interval can require such a large dose that initial drug bolus serum concentrations would be much higher than that ever experienced clinically, potentially confounding results and plausibly inducing transient toxic concentrations. The dosing described in the above-mentioned studies is unlike the consequences of pharmacotherapy in humans, which results in steady-state, 24-h continuous exposure.
Ansorge et al. (2004, 2008) have investigated the use of SRIs during postnatal development and found long-term behavioral alterations in mice. Our results do not replicate their findings; however, there are several key differences that may account for this discrepancy. Perhaps most importantly, Ansorge et al. used a dosing regimen during the postnatal period (PND4–21) that has no temporal overlap with the present studies. Concise ontogenological data on human serotonergic function in the CNS is lacking, and definitive mapping of our in vivo exposure and their postnatal exposure onto human fetal/infant serotonergic development is not possible at present. Nevertheless, their dosing regimens lead to “therapeutic” levels of SERT transporter occupancy that we would argue are unlikely to be observed in human infants after parturition (i.e., exposure via lactation is relatively low and infants are unlikely to receive any type of therapeutic dose of SRIs for medical reasons for the first few years of life). In addition, we observe that peak escitalopram concentrations after subcutaneous injection are up to 12-fold higher than that observed during continuous exposure (C. H. Bourke and C. F. Capello, unpublished observations). Similar peaks, of unknown magnitude, should also be expected after subcutaneous or intraperitoneal fluoxetine administration. It is possible that these may produce transient toxic concentrations that are actually responsible for their findings but there are no data to support this possibility.
Using the osmotic minipump and dosing methodology described, SRI CNS exposure in utero can be generalized as a drug class effect despite the interdrug differences in pharmacokinetics. SERT occupancy after in utero exposure was measured at more than 60% in all pups with venlafaxine having the lowest and fluoxetine having the highest numerical occupancy (Fig. 2; Table 2). These occupancy percentages fall within or exceed established therapeutic ranges for this class of compounds (Meyer et al., 2004); therefore, it is concluded that the pups' occupancy is equal to dam's occupancy of the drug (i.e., fetal brain exposure is equivalent to the mothers'). With the exception of venlafaxine, we cannot discriminate differences in the magnitude of SERT occupancy between the remaining medications at these “therapeutic” exposures. It should be noted that the lower occupancy produced by venlafaxine is matched in both pups and dams (dam data not shown). Thus, the venlafaxine serum concentrations in these studies result in lower SERT occupancies in both dams and pups compared with the other medications under study. It is possible that the lower occupancies associated with clinically relevant venlafaxine concentrations might be related to some venlafaxine dissociation from the SERT during the 5-min radioligand incubation process because venlafaxine and its major active metabolite, O-desmethylvenlafaxine, exhibit a slightly lower affinity for the SERT than the other SRIs examined (Owens et al., 1997). These novel data are germane to the clinical decision process by informing clinicians and patients of considerable fetal CNS exposure and establishing appropriate models for investigating the long-term consequences of antidepressant use during pregnancy.
We were unable to establish a correlation between the drug/metabolite concentrations in the dam's serum and the pups' AF because 1) all AF was pooled per litter and 2) the number of animals per treatment group was not large enough. This correlation has not been established in humans either, most likely because of the complicating variables of fetal metabolism (Newport et al., 2009, 2011). From a strictly observational standpoint, it is evident that AF drug concentrations do not correlate with SERT occupancy (Table 2). All pups exhibited a very high SERT occupancy regardless of the drug administered, but AF drug concentrations ranged from undetectable to comparable with human therapeutic concentrations. Any possible relationship between SRI CNS exposure/elimination and AF concentrations warrant further investigation before any conclusions can be reached.
Pups used to study postnatal drug clearance were cross-fostered to an untreated dam less than 24 h after birth, with the exception of sertraline as explained previously. The serum and brain of the dam treated during gestation was then collected at the time of cross-fostering to confirm that in utero exposure fell within our drug exposure goals. It would have been optimal to study drug clearance within an individual litter, but we found it logistically necessary to use separate litters to measure postnatal clearance at postnatal days 3 and 7 because of the pups' small size at these ages. The HPLC drug assay is sensitive but still required pooling the blood of at least eight pups at PNC3 and six pups at PNC7 to obtain the requisite minimum amount. Based on average rat litter size, we were unable to use the same litter for both collection time points. Current studies in the laboratory using LC-MS techniques increase sensitivity by an order of magnitude (limit of detection 0.2 ng/ml). Postnatal clearance data were similar for all drugs and revealed that neonatal rats can clear drugs after parturition via normal metabolic mechanisms although the rate of clearance is medication specific.
SERT occupancy was detectable in “high” groups of venlafaxine, paroxetine, and sertraline. SERT occupancy remains substantial (within the clinically observed range) at PNC3 in all fluoxetine-treated pups. In male rats, the half-life of fluoxetine and norfluoxetine in male rats is ∼2 and ∼12 h, respectively, and ∼2 and ∼24 h in female rats (Jones et al., 2006; M. J. Owens and C. F. Capello, unpublished observations). It is assumed that it is even longer in neonatal pups because of their immature hepatic metabolism. At PNC3, paroxetine and fluoxetine are both detectable in pup serum, but SERT occupancy does not reach significance in paroxetine-treated pups (Table 2; Fig. 2). Medications were undetectable in pup serum at PNC7 except for one litter treated with fluoxetine in utero that exhibited a low concentration of norfluoxetine. By PNC14, all but one of the fluoxetine litters were identical to vehicle controls. Low concentrations of fluoxetine and norfluoxetine were detectable at PNC14.
These neonatal clearance data are of considerable interest in light of the publications on neonatal syndromes related to antidepressant exposure (see Moses-Kolko et al., 2005 for review). A myriad of symptoms (tremulousness, rigidity, rapid respirations, etc.) have been reported within the first 48 h of life. Neonatal symptoms associated with antidepressant exposure were first reported in 1973 (Webster, 1973), and subsequent reports have resulted in warnings both by the Food and Drug Administration and the Canadian Health System. Whether such purported symptoms are related to side effects, toxicity, withdrawal, or abstinence remains obscure. Extrapolation of these rat CNS neonatal clearance data, particularly fluoxetine, would suggest that side effects would be a more likely explanation rather than withdrawal secondary to a rapid rate of neonatal drug clearance.
Despite the limited presence of drug in pup serum, measurable and variable SERT occupancy was observed at BF3 and BF7 but did not reach clinically observed levels (e.g., >70–80%) at either time point (Fig. 3; Table 3). Unfortunately, because we were unable to quantify drug concentration in dam breast milk, we cannot correlate drug exposure with SERT occupancy or pup drug serum concentrations. We postulate that exposure within this drug class is variable because of each drug's distinct lipid solubility profile as already demonstrated in several human studies (Stowe et al., 1997; Newport et al., 2009). There is a general clinical consensus in the literature that undetectable nursing infant sera concentrations are preferred in selecting medications for nursing women. However, it is noteworthy that our data indicate CNS exposure in the absence of detectable pup serum concentrations. It should be noted that classic serum concentration-fractional occupancy curves predict that even low concentrations of SRIs will produce some magnitude of SERT occupancy. The physiological significance of low levels of SERT occupancy is unknown; however, therapeutic responses for the treatment of depression seem to require 70 to 80% occupancy as a threshold. Indeed, we are unaware of data revealing the magnitude of transporter blockade necessary to alter monoamine synaptic concentrations.
As stated previously, our primary goal was to quantify CNS exposure to SRIs during the late prenatal and early postnatal developmental periods in rats. We also conducted a longitudinal study assessing motor skills in adult rats that were exposed to fluoxetine, paroxetine, escitalopram, and venlafaxine in utero. Casper et al. (2003) followed children up to 40 months of age and concluded that children prenatally exposed to SRIs may show subtle motor development delays. By using the beam traversing task, we were attempting to measure the same skills in the adult rats of our study. Females were nonsignificantly quicker and had fewer errors than their male counterparts, probably the result of smaller body size. We were unable to show significant differences in treated versus untreated rats but it is possible that our behavioral assay does not qualify as a “subtle” motor skill and these deficits are present only during developmental periods and/or under laboratory testing protocols that Casper et al. used. Furthermore, no evidence of anxiety or differences in cortical NET and SERT density in adulthood were observed because of in utero exposure to fluoxetine (Figs. 4 and 5).
SRIs are valuable in the treatment of pregnant and nursing women; nevertheless, further modeling of drug exposure in infants combined with CNS measures will enhance guidelines that can be used to systematically minimize fetal and neonatal medication exposure. In addition, neurobehavioral outcomes need to be further scrutinized now that SRI CNS exposure has been quantified using appropriate and rationale dosing regimens.
Supplementary Material
This work was supported by the National Institutes of Health National Institute of Mental Health [Grant 68036] (to Z.N.S. and M.J.O.) and a Collaborative Research Grant from GlaxoSmithKline (to Z.N.S.).
Parts of the work have appeared previously as poster abstracts: Owens MJ, Capello CF, Goren D, and Stowe ZN (2004) Serotonin transporter occupancy in rats exposed to SSRIs in utero or through breast milk, at the Society for Neuroscience Annual Meeting; 2004 Oct 23–27; San Diego, CA; abstract 912.14; Society for Neuroscience, Washington, DC. Owens MJ, Capello CF, Goren D, and Stowe ZN (2004) Serotonin transporter occupancy in rats exposed to SSRIs in utero or through breast milk. Neuropsychopharmacology 29(Suppl 1):S160. Jones CF, Chura LR, Stowe ZN, Ritchie JC, Plotsky PM, and Owens MJ (2006) Escitalopram and fluoxetine kinetics in neonatal, prepubertal, and adult rats, at the Society for Neuroscience Annual Meeting; 2006 Oct 14–18; Atlanta, GA; abstract 287.15; Society for Neuroscience, Washington, DC. Capello CF, Stowe ZN, and Owens MJ (2006) Serotonin transporter occupancy in rats exposed to fluoxetine in utero or via breast milk, at the American Psychiatric Association Annual Meeting; 2006 May 20–25; Toronto, Canada; abstract NR571; American Psychiatric Association, Arlington, VA.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.111.183855.
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
- CNS
- central nervous system
- SRI
- serotonin reuptake inhibitor
- SERT
- serotonin transporter
- NET
- norepinephrine transporter
- DAT
- dopamine transporter
- AF
- amniotic fluid
- PNC
- postnatal clearance
- PNCn
- PNC day
- PND
- postnatal day
- BF
- breast feeding
- BFn
- BF day
- En
- embryonic day
- PEG
- polyethylene glycol
- ANOVA
- analysis of variance
- HPLC
- high-performance liquid chromatography.
Authorship Contributions
Participated in research design: Capello, Stowe, Newport, and Owens.
Conducted experiments: Capello, Bourke, Ritchie, Nemeroff, and Owens.
Contributed new reagents or analytic tools: Ritchie.
Performed data analysis: Capello, Bourke, Ritchie, and Owens.
Wrote or contributed to the writing of the manuscript: Capello, Bourke, Stowe, Newport, Nemeroff, and Owens.
References
- ACOG Committee on Practice Bulletins–Obstetrics (2008) ACOG Practice Bulletin: Clinical management guidelines for obstetrician-gynecologists number 92, April 2008 (replaces practice bulletin number 87, November 2007). Use of psychiatric medications during pregnancy and lactation. Obstet Gynecol 111:1001–1020 [DOI] [PubMed] [Google Scholar]
- Andrade SE, Raebel MA, Brown J, Lane K, Livingston J, Boudreau D, Rolnick SJ, Roblin D, Smith DH, Willy ME, et al. (2008) Use of antidepressant medications during pregnancy: a multisite study. Am J Obstet Gynecol 198:194.e1–5 [DOI] [PubMed] [Google Scholar]
- Ansorge MS, Morelli E, Gingrich JA. (2008) Inhibition of serotonin but not norepinephrine transport during development produces delayed, persistent perturbations of emotional behaviors in mice. J Neurosci 28:199–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansorge MS, Zhou M, Lira A, Hen R, Gingrich JA. (2004) Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science 306:879–881 [DOI] [PubMed] [Google Scholar]
- Avishai-Eliner S, Brunson KL, Sandman CA, Baram TZ. (2002) Stressed-out, or in (utero)? Trends Neurosci 25:518–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum CS, Cohen LS, Bailey JW, Grush LR, Robertson LM, Stowe ZN. (1999) Serum concentrations of antidepressants and benzodiazepines in nursing infants: A case series. Pediatrics 104:e11. [DOI] [PubMed] [Google Scholar]
- Boja JW, Mitchell WM, Patel A, Kopajtic TA, Carroll FI, Lewin AH, Abraham P, Kuhar MJ. (1992) High-affinity binding of [125I]RTI-55 to dopamine and serotonin transporters in rat brain. Synapse 12:27–36 [DOI] [PubMed] [Google Scholar]
- Bonnin A, Torii M, Wang L, Rakic P, Levitt P. (2007) Serotonin modulates the response of embryonic thalamocortical axons to netrin-1. Nat Neurosci 10:588–597 [DOI] [PubMed] [Google Scholar]
- Cabrera-Vera TM, Battaglia G. (1998) Prenatal exposure to fluoxetine (Prozac) produces site-specific and age-dependent alterations in brain serotonin transporters in rat progeny: evidence from autoradiographic studies. J Pharmacol Exp Ther 286:1474–1481 [PubMed] [Google Scholar]
- Cabrera-Vera TM, Garcia F, Pinto W, Battaglia G. (1997) Effect of prenatal fluoxetine (Prozac) exposure on brain serotonin neurons in prepubescent and adult male rat offspring. J Pharmacol Exp Ther 280:138–145 [PubMed] [Google Scholar]
- Casper RC, Fleisher BE, Lee-Ancajas JC, Gilles A, Gaylor E, DeBattista A, Hoyme HE. (2003) Follow-up of children of depressed mothers exposed or not exposed to antidepressant drugs during pregnancy. J Pediatr 142:402–408 [DOI] [PubMed] [Google Scholar]
- Christensen HD, Rayburn WF, Gonzalez CL. (2000) Chronic prenatal exposure to paroxetine (Paxil) and cognitive development of mice offspring. Neurotoxicol Teratol 22:733–739 [DOI] [PubMed] [Google Scholar]
- Chung TK, Lau TK, Yip AS, Chiu HF, Lee DT. (2001) Antepartum depressive symptomatology is associated with adverse obstetric and neonatal outcomes. Psychosom Med 63:830–834 [DOI] [PubMed] [Google Scholar]
- Cohen LS, Altshuler LL, Harlow BL, Nonacs R, Newport DJ, Viguera AC, Suri R, Burt VK, Hendrick V, Reminick AM, et al. (2006) Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA 295:499–507 [DOI] [PubMed] [Google Scholar]
- Coleman FH, Christensen HD, Gonzalez CL, Rayburn WF. (1999) Behavioral changes in developing mice after prenatal exposure to paroxetine (Paxil). Am J Obstet Gynecol 181:1166–1171 [DOI] [PubMed] [Google Scholar]
- Dobbing J, Sands J. (1973) Quantitative growth and development of human brain. Arch Dis Child 48:757–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbing J, Sands J. (1979) Comparative aspects of the brain growth spurt. Early Hum Dev 3:79–83 [DOI] [PubMed] [Google Scholar]
- Feeney DM, Gonzalez A, Law WA. (1982) Amphetamine, haloperidol, and experience interact to affect rate of recovery after motor cortex injury. Science 217:855–857 [DOI] [PubMed] [Google Scholar]
- Foster GA. (1998) Chemical Neuroanatomy of the Prenatal Rat Brain: A Developmental Atlas, Oxford University Press Inc., New York [Google Scholar]
- Goldstein LB, Davis JN. (1990) Beam walking in rats: studies towards developing an animal model of functional recovery after brain injury. J Neurosci Methods 31:101–107 [DOI] [PubMed] [Google Scholar]
- Goodman MM, Kilts CD, Keil R, Shi B, Martarello L, Xing D, Votaw J, Ely TD, Lambert P, Owens MJ, et al. (2000) 18F-labeled FECNT: a selective radioligand for PET imaging of brain dopamine transporters. Nucl Med Biol 27:1–12 [DOI] [PubMed] [Google Scholar]
- Hansson SR, Mezey E, Hoffman BJ. (1999) Serotonin transporter messenger RNA expression in neural crest-derived structures and sensory pathways of the developing rat embryo. Neuroscience 89:243–265 [DOI] [PubMed] [Google Scholar]
- Institute for Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals 7th ed Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington DC [Google Scholar]
- Jones CF, Chura LR, Stowe ZN, Ritchie JC, Plotsky PM, Owens MJ. (2006) Escitalopram and fluoxetine kinetics in neonatal, prepubertal, and adult rats, at the Society for Neuroscience Annual Meeting; 2006 Oct 14–18; Atlanta, GA; abstract 287.15 Society for Neuroscience, Washington, DC [Google Scholar]
- Loughhead AM, Fisher AD, Newport DJ, Ritchie JC, Owens MJ, DeVane CL, Stowe ZN. (2006) Antidepressants in amniotic fluid: another route of fetal exposure. Am J Psychiat 163:145–147 [DOI] [PubMed] [Google Scholar]
- McConathy J, Capello C, Jarkas N, Stowe ZN, Owens MJ. (2007) Preparation of antidepressants for use in preclinical research. Int J Neuropsychopharmacol 10:759–763 [DOI] [PubMed] [Google Scholar]
- Meyer JH, Wilson AA, Sagrati S, Hussey D, Carella A, Potter WZ, Ginovart N, Spencer EP, Cheok A, Houle S. (2004) Serotonin transporter occupancy of five selective serotonin reuptake inhibitors at different doses: an [11C]DASB positron emission tomography study. Am J Psychiatry 161:826–835 [DOI] [PubMed] [Google Scholar]
- Morrison JL, Riggs KW, Chien C, Gruber N, McMillen IC, Rurak DW. (2004) Chronic maternal fluoxetine infusion in pregnant sheep: effects on the maternal and fetal hypothalamic-pituitary-adrenal axes. Pediatr Res 56:40–46 [DOI] [PubMed] [Google Scholar]
- Moses-Kolko EL, Bogen D, Perel J, Bregar A, Uhl K, Levin B, Wisner KL. (2005) Neonatal signs after late in utero exposure to serotonin reuptake inhibitors: literature review and implications for clinical applications. JAMA 293:2372–2383 [DOI] [PubMed] [Google Scholar]
- Narrow WE, Rae DS, Robins LN, Regier DA. (2002) Revised prevalence estimates of mental disorders in the United States: using a clinical significance criterion to reconcile 2 surveys' estimates. Arch Gen Psychiatry 59:115–123 [DOI] [PubMed] [Google Scholar]
- Newport DJ, Fernandez SV, Juric S, Stowe ZN. (2009) Psychopharmacology during pregnancy and lactation, in Textbook of Psychopharmacology, 4th Ed (Schatzberg AF, Nemeroff CB. eds) pp 1373–1412, American Psychiatric Publishing, Inc., Arlington, VA [Google Scholar]
- Newport DJ, Ji S, Long Q, Knight BT, Zach EB, Smith EN, Morris NJ, Stowe ZN. (2011) Maternal depression and anxiety differentially impact fetal exposures during pregnancy. J Clin Psychiatry doi:10.4088/JCP.10m06783 [DOI] [PubMed] [Google Scholar]
- O'Connor TG, Heron J, Golding J, Glover V. (2003) Maternal antenatal anxiety and behavioral/emotional problems in children: a test of a programming hypothesis. J Child Psychol Psychiatry 44:1025–1036 [DOI] [PubMed] [Google Scholar]
- Owens MJ, Morgan WN, Plott SJ, Nemeroff CB. (1997) Neurotransmitter receptor and transporter binding profile of antidepressants and their metabolites. J Pharmacol Exp Ther 283:1305–1322 [PubMed] [Google Scholar]
- Paxinos GWC, Watson C. (1986) The Rat Brain in Stereotaxic Coordinates. Academic Press, New York [Google Scholar]
- Qian Y, Melikian HE, Rye DB, Levey AI, Blakely RD. (1995) Identification and characterization of antidepressant-sensitive serotonin transporter proteins using site-specific antibodies. J Neurosci 15:1261–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayburn WF, Gonzalez CL, Christensen HD, Kupiec TC, Jacobsen JA, Stewart JD. (2000) Effect of antenatal exposure to paroxetine (paxil) on growth and physical maturation of mice offspring. J Matern Fetal Med 9:136–141 [DOI] [PubMed] [Google Scholar]
- Schröder HJ. (1995) Comparative aspects of placental exchange functions. Eur J Obstet Gynecol Reprod Biol 63:81–90 [DOI] [PubMed] [Google Scholar]
- Steer RA, Scholl TO, Hediger ML, Fischer RL. (1992) Self-reported depression and negative pregnancy outcomes. J Clin Epidemiol 45:1093–1099 [DOI] [PubMed] [Google Scholar]
- Stowe ZN, Owens MJ, Landry JC, Kilts CD, Ely T, Llewellyn A, Nemeroff CB. (1997) Sertraline and desmethylsertraline in human breast milk and nursing infants. Am J Psychiatry 154:1255–1260 [DOI] [PubMed] [Google Scholar]
- Vartazarmian R, Malik S, Baker GB, Boksa P. (2005) Long-term effects of fluoxetine or vehicle administration during pregnancy on behavioral outcomes in guinea pig offspring. Psychopharmacology (Berl) 178:328–338 [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Acuff-Smith KD, Schilling MA, Fisher JE, Moran MS, Buelke-Sam J. (1994) A developmental neurotoxicity evaluation of the effects of prenatal exposure to fluoxetine in rats. Fundam Appl Toxicol 23:194–205 [DOI] [PubMed] [Google Scholar]
- Webster PA. (1973) Withdrawal symptoms in neonates associated with maternal antidepressant therapy. Lancet 2:318–319 [DOI] [PubMed] [Google Scholar]
- Weissman MM, Olfson M. (1995) Depression in women: implications for health care research. Science 269:799–801 [DOI] [PubMed] [Google Scholar]
- Whitaker-Azmitia PM, Druse M, Walker P, Lauder JM. (1996) Serotonin as a developmental signal. Behav Brain Res 73:19–29 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.