Abstract
Evaluation of the discriminative stimulus effects of drugs is a useful procedure for identification of receptor mediation of in vivo drug effects. This assay can be enhanced when the stimulus effects of different doses of agonist are evaluated. In the present study, rats were trained to discriminate small or large doses of nicotine from saline, and interactions of these effects with nicotinic receptor antagonists and partial agonists were determined. The insurmountable nicotine antagonist mecamylamine blocked both the discriminative stimulus and response rate-reducing effects of nicotine but was less effective against the large dose of nicotine. The α4β2*-selective, competitive antagonist dihydro-β-erythrodine (DHβE) antagonized the discriminative stimulus effects of both doses but was less effective against the larger training dose of nicotine. Schild analyses of DHβE suggested that different nicotinic receptor populations may be mediating the stimulus effects of large and small doses of nicotine. This suggestion was supported by observations that the discriminative stimulus effects of the partial agonist cytisine were more like those of the large dose than of the small dose of nicotine and that cytisine antagonized the effects of only the small nicotine dose. Varenicline produced nicotine-like effects in both training dose groups but reduced the discriminative stimulus effects of intermediate doses of nicotine in the group trained to the small dose of nicotine. Overall, these results suggest that small doses of nicotine produce their stimulus effects via α4β2* nicotine receptors, whereas larger doses of nicotine recruit additional nicotine receptor subtypes, as revealed by drug discrimination assays in rats.
Introduction
Drug discrimination assays are useful for identifying compounds that have interoceptive stimulus effects in common. In these assays, animals (typically rats, pigeons, or nonhuman primates) are trained over several sessions to make one response to receive food if they have been given a particular centrally active drug and to make another response if they have been given saline. Once a particular drug has been established as a discriminative stimulus, animals make the drug-appropriate response when they have been given a test compound that has interoceptive stimulus properties like those of the training drug; otherwise, they make the saline-appropriate response (Colpaert and Rosecrans, 1978).
Not only are drug discrimination assays useful for categorizing drugs with common stimulus effects, but they are also among the best systems for obtaining quantitative in vivo information for pharmacological analysis. Selection of the drug-appropriate response is dependent on the dose of the training drug delivered, and monotonic dose-response curves can be described. Drugs with stimulus properties in common with a specific test drug but with lesser efficacy produce an amount of drug-appropriate responding that reflects their relative efficacies. Dose-response curves established with the training drug or with a drug with similar discriminative stimulus effects are shifted appropriately to the right after administration of surmountable antagonists (e.g., Colpaert, 1978; Holtzman, 1985).
This last generalized finding has allowed several investigators to use drug discrimination procedures to determine apparent pA2 values in vivo (Rowlett et al., 1999; McMahon, 2006). The pA2 is a descriptive statistic that, when certain assumptions are met, reflects the affinity of an antagonist for its receptor (for review, see Kenakin, 1982). The pA2 also permits a determination of whether two or more agonists are acting at the same receptor: if a single antagonist yields the same pA2 value when interacting with two agonists, the agonists are probably producing the measured effects through actions on the same receptor (Walker et al., 1994), whereas different pA2 values indicate action through different receptors (Comer et al., 1993; Picker et al., 1993).
It has been found that establishment of a discriminative stimulus effect of two doses of a single agonist in separate groups of animals can also provide information about the receptors through which the drug acts. In the opioid system, for example, receptor-selective partial agonists are more likely to produce drug-appropriate responding to a small dose of a full agonist than to a large dose of a full agonist (Shannon and Holtzman, 1979; Koek and Woods, 1989; Picker et al., 1992, 1994; Grabus et al., 1999).
Nicotinic acetylcholine receptors (nAChRs) are ionotropic channels comprising five subunits. As such, they exist in several subtypes that are somewhat distinct with respect to their location and function. Although considerable effort remains to establish a clear relation between the various receptor subtypes and their functions, there is evidence that the reinforcing effects of nicotine, which are thought to be important in abuse of nicotine-containing products (Ator and Griffiths, 2003), are mediated through the α4β2* subtype of the nAChR (Corrigall et al., 1994; Watkins et al., 1999; Mansbach et al., 2000; Liu et al., 2007; Le Foll et al., 2009). Drug discrimination assays have been used in attempts to isolate the receptor that is most relevant to nicotine's interoceptive effects, and several lines of evidence indicate that these are also mediated through the α4β2 subtype of the nAChR (for review, see Smith and Stolerman, 2009). Nevertheless, genetic studies in particular have implicated other nicotinic receptor subtypes, such as the α3, α5, α6, and β4 subunits, in the abuse liability of nicotine (Improgo et al., 2010; Sherva et al., 2010; Russo et al., 2011).
The current study had three aims: 1) to evaluate nicotinic partial agonist effects under different nicotine training conditions, 2) to characterize antagonism of small and large nicotine dose discriminative stimulus effects, and 3) to perform, for the first time, a quantitative evaluation of the discriminative stimulus effects of nicotine and the receptor subtypes that mediate these effects. The antagonists used were mecamylamine (Stone et al., 1956; Martin et al., 1989), a nicotine receptor ion channel blocker that is uncompetitive in its actions, and dihydro-β-erythroidine (DHβE) (Williams and Robinson, 1984; Sabey et al., 1999; Shoaib et al., 2000), a competitive, α4β2-selective nicotine antagonist. The partial agonists were varenicline (Coe et al., 2005) and cytisine (Barlow and McLeod, 1969; Romano et al., 1981; Sloan et al., 1988). Interaction studies were performed in rats trained to discriminate between saline and a large or a small dose of nicotine. The goal was to obtain a more thorough understanding of the receptor basis for nicotine's interoceptive stimulus effects and to provide a procedural framework for the development of nicotine antagonists that are selective at this receptor and might be suitable for treatment of tobacco abuse.
Materials and Methods
Subjects.
Male Sprague-Dawley rats were obtained from Harlan (Indianapolis, IN) and singly housed in polycarbonate cages with water continuously available. All rats weighed approximately 280 to 290 g at the start of the experiment. A food-restricted diet of Purina rodent food was used to maintain body weight at approximately 350 to 375 g. Housing and experimental rooms were maintained on a 12-h light/dark cycle with lights on at 7:00 AM with an average temperature of 21°C. A group of six rats was used in each of the two nicotine-training dose groups. After extensive training and testing, the group of rats trained to discriminate the small dose of nicotine was replaced with a second group of six rats.
Apparatus.
Drug discrimination procedures were performed in six standard operant conditioning chambers with an area of 30.5 × 24.1 × 21.0 cm and stainless steel grid floors (ENV-008; Med Associates, St. Albans, VT) contained within ventilated, sound-attenuating boxes. Each chamber was equipped with two nose-poke devices with apertures containing yellow LED lights on either side of a dipper capable of delivering 50 μl of fluid into a third opening (H14–05R; Coulbourn Instruments, Whitehall, PA).
Discrimination Training.
Before discrimination training was started, all subjects were trained to respond on a fixed-ratio (FR) 10 schedule of reinforcement with responding in either aperture resulting in 10-s access to 50 μl of vanilla-flavored Ensure (Abbott Laboratories, Abbott Park IL). After evaluation of side or aperture biases, the saline- or nicotine-associated nose-poke apertures were assigned to each rat in the following format to minimize biases: 1) approximately 50% of rats had the drug-associated aperture assigned on the left and 50% of the rats had the drug-associated aperture assigned on the right and 2) the drug-associated aperture was assigned to the nonpreferred side in 50% of subjects.
For discrimination training, rats were injected with saline or nicotine and immediately placed in the operant chamber for a 5-min blackout followed by a 20-min period during which response contingencies were in effect (SD period). Rats were trained to discriminate either 0.32 mg/kg nicotine (small nicotine dose) from saline or 1.78 mg/kg nicotine (large nicotine dose) from saline. Discrimination training continued until the following criteria were met: 1) responding on first FR of the session was completed on the injection-appropriate aperture, and 2) >85% of total session responses were made on the injection-appropriate aperture. Nicotine discrimination was obtained in 20 to 50 sessions, depending on the rat.
Discrimination Testing and Maintenance.
After the drug discrimination was established, the duration of the SD period was decreased to 5 min over a period of approximately 5 days. At this point, daily sessions comprised one to four 10-min components, each consisting of a 5-min blackout period and a 5-min SD period. Rats were injected with either saline or the appropriate dose of nicotine and immediately placed into the chamber with the blackout in effect. At the end of the blackout, both nose-poke aperture lights were illuminated and responding (FR10) on the injection-appropriate aperture was reinforced with 10-s access to 50 μl of Ensure. Each reinforcer delivery was followed by a 10-s timeout. FR completion in the incorrect aperture led directly to the 10-s timeout without reinforcer presentation. At the end of the 5-min SD period, the rat was removed from the operant chamber, given another injection, and returned to the chamber for the next 5-min blackout and the following 5-min response period. Multiple-component training sessions consisted of two to four consecutive administrations of saline (saline-saline, saline-saline-saline, or saline-saline-saline-saline) or zero to three components of consecutive administrations of saline followed by nicotine administration (nicotine only, saline-nicotine, saline-saline-nicotine, or saline-saline-saline-nicotine).
Test sessions were conducted only after 3 training days during which all criteria listed above were met. Test sessions consisted of four components, and completed FRs on either nose-poke aperture were reinforced with 10-s access to Ensure. Drug was administered before the start of each component as described previously, with doses increasing across injections in a cumulative fashion. For evaluation of antagonist effects, a dose of the selected antagonist was administered in the home cage 15 min before the start of the session. Increasing doses of nicotine were then given in the four components of the test session. Rats received no more than two test sessions per week; at least once per month, cumulative dose-effect curves with nicotine were determined to monitor for changes in drug sensitivity. Full or complete generalization to a discriminative cue was defined as >85% of responding on the drug-associated aperture and completing at least one FR.
Data Analysis.
All dose-effect curves were calculated from an average of five to six rats. Cytisine substitution curves and nicotine dose-effect curves after cytisine and varenicline pretreatments were calculated from a combination of two groups of six rats trained to discriminate the small dose of nicotine (10–12 rats total). Discrimination data are expressed as a percentage of responses occurring on the nicotine-associated aperture out of the total number of responses on both the drug- and saline-associated apertures. Rates of responding were calculated by dividing the total number of responses by the total duration of SD presentations within each component. Data were averaged across five to six rats in every dose condition unless otherwise described under Results. Response rates were included in group averages only if at least one FR was completed.
To determine changes in agonist potency in the presence of the antagonist DHβE (percentage drug responding only), individual dose-response curves and ED50 values were analyzed using GraphPad Prism 4.03 (GraphPad Software Inc., San Diego, CA). To calculate ED50 values for each rat, the 50% level of maximum effect was determined from the straight line analysis of percentage drug responding, including only one dose that produced <10% and one dose that produced >90% drug-appropriate responding. For the small-dose preparation, pA2 values were calculated for DHβE. Schild plots were determined by expressing the average logarithm of the dose ratio − 1 as a function of the negative logarithm of the molar dose (moles per kilogram) of DHβE (Arunlakshana and Schild, 1959). Dose ratios [ratio of the ED50 dose of an agonist in the presence of antagonist (A′) to the ED50 dose in the absence of the antagonist (A)] were calculated for individual rats. Using GraphPad Prism 4.03, a straight line model of nonlinear regression was fit to the equation: log(dose ratio − 1) = −log(DHβE) × slope + x-intercept at y = 0. Apparent pA2 values were calculated with unconstrained slopes as well as slopes constrained to −1. To determine whether the slope of the regression line differed significantly from a straight line, a run test after linear regression was performed (GraphPad Prism). For the large nicotine dose condition, only two doses of DHβE were evaluated. This permitted measurement of the pKB value, which is an apparent affinity estimate for a single dose of antagonist, thus yielding one pKB value per dose of DHβE, which was obtained for each of the two doses of DHβE using the equation: pKB = −log[(DHβE molar dose)/dose ratio − 1].
Drugs.
(−)-Nicotine hydrogen tartrate salt, (±)-epibatidine dihydrochloride hydrate, DHβE, and mecamylamine were obtained from Sigma-Aldrich (St. Louis, MO). Cytisine hydrobromide was obtained from Xingcheng Chempharm Co., Ltd. (Taizhou, Zhejiang, China) and then purified. Varenicline tartrate was obtained from the National Institute on Drug Abuse Drug Supply Program. All drug doses were calculated on the basis of the salt form of the drugs.
Results
Agonist Effects.
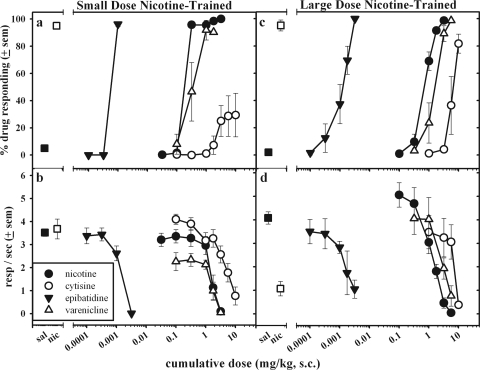
Figure 1 displays the effects of a range of doses of nicotine, epibatidine, and the partial agonists, cytisine and varenicline, in rats trained to discriminate between saline and 0.32 mg/kg nicotine (Fig. 1, a and b) or 1.78 mg/kg nicotine (Fig. 1, c and d). Saline injections during training sessions administered in any component generated very little or no responding in the drug-associated aperture in any of the groups, and rats made on average 3.5 to 4 responses/s in the presence of the SD after saline administration. In the rats trained to discriminate a small dose of nicotine from saline (Fig. 1, a and b), doses of nicotine of 0.32 mg/kg and greater occasioned selection of the nicotine-appropriate aperture; response rates decreased to less than 0.2 response/s on average after administration of a cumulative dose of 3.2 mg/kg nicotine. Epibatidine occasioned nicotine-appropriate responding at a dose of 0.001 mg/kg in this group of rats; a larger dose decreased response rates. In addition, in the small-dose nicotine-trained group, varenicline at a dose of 1 mg/kg produced nicotine-appropriate responding. There was a 50% decrease in response rate after administration of 1.78 mg/kg varenicline, and responding was nearly eliminated after administration of 3.2 mg/kg varenicline. Cytisine produced approximately 30% generalization to the small-dose nicotine discriminative cue, but the cytisine effects were variable across subjects trained to discriminate the small dose of nicotine. Across the 10 rats in the two different small-dose nicotine-trained groups, four rats did not show nicotine-appropriate responding at any cytisine dose tested, even at rate-suppressing doses, whereas two rats showed complete nicotine-appropriate responding after cytisine administration and four rats showed partial nicotine-appropriate responding (from 20 to 73%). Doses of cytisine that produced a nicotine-like discriminative cue did not consistently decrease responding, but the largest dose of cytisine (10 mg/kg) decreased rates by 78% (Fig. 1b).
Fig. 1.
The effects of cumulative doses (subcutaneous) of nicotine (●), epibatidine (▾), cytisine (○), and varenicline (▵) substitutions in rats trained to discriminate either a small dose of nicotine (nic) (0.32 mg/kg) or a large dose of nicotine (1.78 mg/kg) from saline (sal) on percentage drug-associated responding (a and c) or response rate (b and d). The percentage drug responding and response rate on training days are demonstrated by ■ (saline) or □ (training dose). Symbols and error bars show the group average ± S.E.M. All substitution dose-effect curves were calculated from the average of five to six rats except the cytisine dose-effect curve in the small-dose nicotine-trained rats, which was calculated from 10 to 12 rats. resp, responses.
Figure 1, c and d, shows nicotine-appropriate responding in the rats trained to discriminate the large dose (1.78 mg/kg) of nicotine from saline. Nicotine produced full nicotine-appropriate responding at a cumulative dose of 1.78 mg/kg. Nicotine decreased rates of responding by 80 to 90% after administration of 3.2 mg/kg, and a cumulative dose of 5.6 mg/kg nearly eliminated responding in these rats. Epibatidine at a dose of 0.003 mg/kg engendered complete nicotine-appropriate responding in the large-dose nicotine-trained group, and this dose decreased response rates by approximately 70% compared with control. One rat in this group was particularly sensitive to the rate-decreasing effects of epibatidine, producing only 60% nicotine-appropriate responding at a dose of 0.001 mg/kg and showing complete suppression of responding at a dose of 0.0018 mg/kg. Varenicline produced complete large-dose nicotine-appropriate responding after administration of 3.2 mg/kg and higher, and 5.6 mg/kg varenicline decreased response rates by 80% on average. In this group of rats, cytisine produced an average of 82% selection of the nicotine-appropriate manipulandum. Three rats selected the nicotine-appropriate response after administration of 10 mg/kg cytisine, two rats showing partial selection of this option (64 and 79%) at this dose, and one rat did not select the nicotine-appropriate response at rate-suppressing doses of cytisine. The largest dose of 10 mg/kg cytisine decreased response rates to approximately 10% of control rates.
Antagonist Effects.
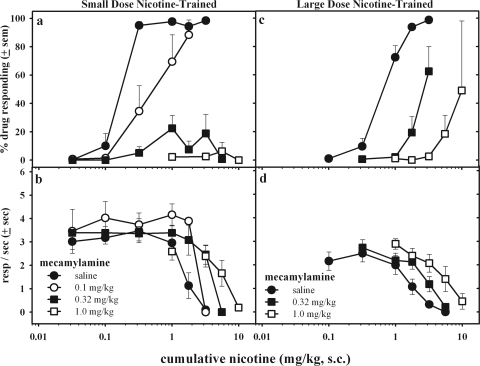
Figure 2 shows the effects of mecamylamine given prior to obtaining nicotine cumulative dose-effect curves in the two nicotine dose training conditions. Mecamylamine produced rightward and downward shifts in discrimination of nicotine in both nicotine-trained groups, and it also attenuated nicotine's rate suppressant effects. In the group trained with a small dose of nicotine, a dose of 0.1 mg/kg mecamylamine produced an approximate 4-fold rightward shift in nicotine's discriminative potency. In this group, nicotine doses as large as 10 mg/kg occasioned selection of the saline-appropriate aperture after administration of 0.32 and 1 mg/kg mecamylamine, demonstrating the insurmountable nature of mecamylamine's action. In large-dose nicotine-trained rats, mecamylamine also produced rightward and downward shifts in the nicotine dose-effect curves; however, mecamylamine's antagonist properties were partially surmountable in some large-dose nicotine-trained rats when response rates were not suppressed completely. For example, after administration of 0.32 mg/kg mecamylamine, all six rats responded on the nicotine-appropriate aperture to some extent (ranging between 20 and 97%). However, after administration of 1 mg/kg mecamylamine, 10 mg/kg nicotine did not decrease responding significantly in two of the six rats, and, of these two rats, one rat fully generalized to the large-dose discriminative stimulus and one rat responded in the saline-appropriate aperture. The large dose of mecamylamine produced an approximate 3-fold shift in the rate-decreasing effects of nicotine in small- and large-dose nicotine-trained rats.
Fig. 2.
The effects of mecamylamine pretreatment (15 min, subcutaneously) on the discriminative stimulus (a and c) and rate-decreasing effects (b and d) of cumulative doses of nicotine in small-dose (left) or large-dose (right) nicotine-trained rats. All dose-effect curves were established from averaged data across five to six rats. resp, responses.
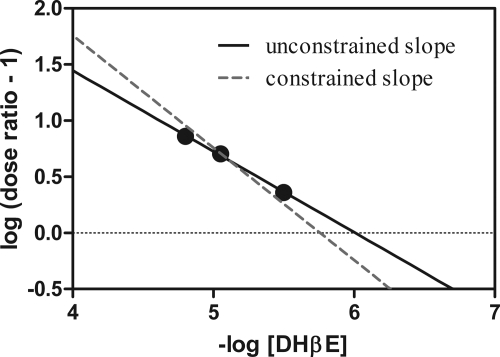
The α4β2*-selective, competitive nicotine antagonist, DHβE, produced surmountable, rightward shifts in the percent drug generalization curves in both nicotine groups (Fig. 3, a and c). Unlike mecamylamine, DHβE did not produce rightward shifts in the rate-decreasing effects of nicotine; however, DHβE produced some minimal antagonism of the initial nicotine doses that decreased response rates (Fig. 3, b and d). Figure 4 presents the Schild plot for DHβE antagonism of the discriminative effect of the small dose of nicotine. The goodness of fit for linear regression (r2) was 0.99 for these data. The unconstrained slope (95% confidence intervals) was −0.72 (−1.18 to −0.26) and was not significantly different from a slope of −1. On the basis of this slope, the pA2 value of DHβE was 6.01 (5.63–7.62) for the small-dose nicotine-trained group. If the slope was constrained to −1, the pA2 value for DHβE (95% confidence interval) was 5.76 (5.7–5.83). In the large-dose nicotine-trained group, single-dose apparent affinity estimates [pKB values (95% confidence intervals)] for DHβE were lower: 5.41 (4.91–5.91) for 3.2 mg/kg DHβE and 4.79 (4.0–5.6) for 5.6 mg/kg DHβE.
Fig. 3.
The effects of DHβE pretreatment (15 min, subcutaneously) on the discriminative stimulus (a and c) and rate-decreasing effects (b and d) of cumulative doses of nicotine in small-dose (left) or large-dose (right) nicotine-trained rats. All dose-effect curves were established from averaged data across five to six rats. resp, responses.
Fig. 4.
Schild plot for DHβE antagonism in small-dose nicotine-trained rats by expressing the average logarithm of the dose ratio − 1 as a function of the negative logarithm of the molar dose (moles per kilogram) of DHβE for the group of rats (Arunlakshana and Schild, 1959). The solid black line indicates the unconstrained slope for each plot and the dotted gray line indicates the slope constrained to −1 (unity).
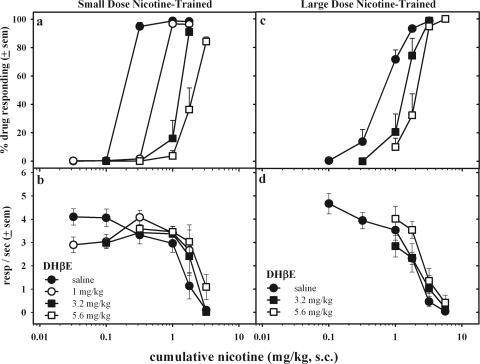
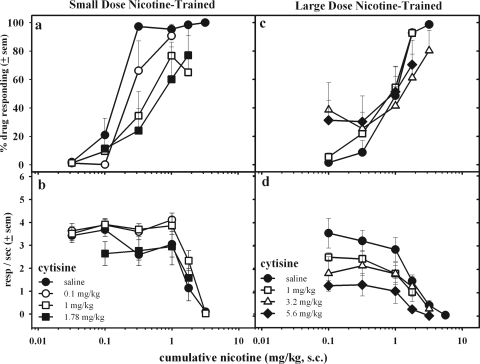
Figure 5 shows the effect of cytisine pretreatment on the discriminative and rate-suppressing effects of nicotine. In the small-dose nicotine-trained rats (Fig. 5, a and b), cytisine pretreatment produced small, rightward shifts in nicotine's discriminative potency. Cytisine pretreatment did not significantly alter rates of responding. In the large-dose nicotine-trained rats, small doses of cytisine (1 and 3.2 mg/kg) did not greatly alter the nicotine dose-effect curve. The two largest cytisine pretreatment doses increased responding in the nicotine-associated aperture after administration of initial nicotine doses, without significantly altering response patterns after later administration of large doses of nicotine.
Fig. 5.
The effects of cytisine pretreatment (15 min, subcutaneously) on the discriminative stimulus (a and c) and rate-decreasing effects (b and d) of cumulative doses of nicotine in small-dose (left) or large-dose (right) nicotine-trained rats. Dose-effect curves after cytisine pretreatments were determined from data averaged across 10 to 12 rats. resp, responses.
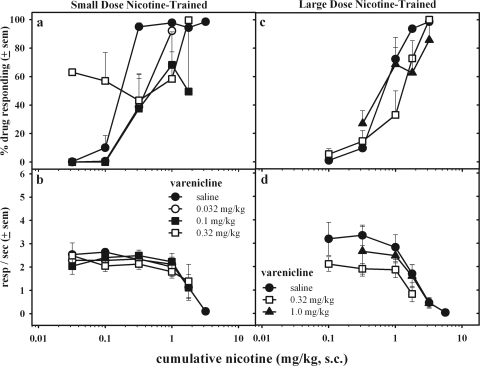
Figure 6 shows the effects of varenicline pretreatment on the discriminative and response rate-suppressing effects of nicotine. In small-dose nicotine-trained rats (Fig. 6, a and b), small doses of varenicline (0.032 and 0.1 mg/kg) produced rightward shifts in the nicotine dose-effect curve; however, a larger dose of varenicline (0.32 mg/kg) increased responding on the drug-associated aperture after administration of the initial doses of nicotine and decreased responding on the drug-associated aperture after subsequent administration of larger doses of nicotine. Varenicline pretreatment did not significantly alter response rates. In large-dose nicotine-trained rats (Fig. 6, c and d), varenicline produced a modest reduction in potency of nicotine without robustly altering rates of responding. Larger doses of varenicline pretreatment were not given because these doses produced full generalization to the nicotine discriminative cue in the large-dose nicotine-trained rats (Fig. 1c).
Fig. 6.
The effects of varenicline pretreatment (15 min, subcutaneously) on the discriminative stimulus (a and c) and rate-decreasing effects (b and d) of cumulative doses of nicotine in small-dose (left) or large-dose (right) nicotine-trained rats. Dose-effect curves after varenicline pretreatments were averaged across 10 to 12 rats. resp, responses.
Discussion
The data described in this report establish that nicotine acts on multiple sites to produce qualitatively different behavioral outcomes within drug discrimination procedures. Action at one specific site is apparent in the discriminative stimulus effects produced by a small dose (0.32 mg/kg) of nicotine, whereas action at multiple sites was observed in the stimulus effects produced by a larger dose (1.78 mg/kg) of nicotine. The small-dose action is probably via the α4β2* nicotine receptor; the large-dose action is probably through multiple nicotinic receptors, including the α4β2* receptor and a site/sites that have not yet been identified.
Several lines of evidence, shown in this report and in the work of other investigators, draw us to the above generalization. The nicotinic basis for the discriminative stimulus and rate-suppressing effects of both doses of nicotine is demonstrated by the ability of the nicotinic antagonist, mecamylamine, to attenuate these effects (Chandler and Stolerman, 1997; Young and Glennon, 2002; Zakharova et al., 2005; Zaniewska et al., 2006; Gatch et al., 2008). To some extent, mecamylamine differentially altered the discriminative stimulus effects of small and large nicotine doses. These data may suggest that combinations of small or large nicotine doses with mecamylamine generate different interoceptive effects. However, these data more likely indicate that somewhat distinct populations of nicotinic receptors mediate the discriminative stimulus effects of nicotine in small and large dose-trained rats. This interpretation is supported by our finding that a dose of 0.32 mg/kg mecamylamine produced an insurmountable antagonism in the small-dose group, yet produced surmountable antagonism in the large-dose group.
Evidence that the small-dose nicotine discriminative stimulus effect is mediated through the α4β2* receptor lies in the ability of the selective, competitive antagonist DHβE to produce surmountable shifts in the discriminative stimulus effects produced by the small dose, as shown here and by others (Stolerman et al., 1997; Gommans et al., 2000). Evidence that the general rate-suppressing effects of nicotine are not mediated to a large extent through this receptor is present in the limited ability of DHβE to modify these effects in either group of rats (Stolerman et al., 1997; Gommans et al., 2000). That the large dose discriminative stimulus effect is mediated through sites in addition to the α4β2* receptor is found in the even more modest ability of this antagonist to modify the discriminative stimulus effects of the large dose.
The difference between the ability of DHβE to antagonize the discriminative stimulus effects of the two doses of nicotine was quantified with a pA2 and a pKB analysis. These are quantitative methods of determining whether two effects that are reduced to some extent by the same antagonist are mediated through the same receptor. If these discriminative stimulus effects of small and large doses of nicotine were mediated through the same receptor, then it would be expected that the pA2 and pKB values would be the same. The negative unity slope of the Schild plot for the interaction of DHβE with discrimination produced by the small dose of nicotine is consistent with a competitive, reversible interaction (Arunlakshana and Schild, 1959; Tallarida, 1995). The pA2 value of this interaction (5.76) was different from the single-dose apparent affinity estimates (pKB) of the potency of DHβE to reduce the discriminative stimulus effects of the large dose of nicotine (5.41 and 4.79); however, it is important to note that these shifts are measured over small dose ranges, unlike that found with in vivo pA2 analyses in the opioid literature. This observation highlights the difficulty of identifying selective nicotinic subtype-mediated behaviors and the narrow dose ranges that may be considered subtype-selective. Nevertheless, these observations provide pharmacological evidence that these two effects may be mediated through distinct, albeit overlapping, receptor populations. Additional competitive antagonists, selective for different receptor populations, are needed in this area of pharmacology to confirm these findings.
The effects of the partial agonists cytisine and varenicline in the two groups of rats also support the notion of different populations of receptor-mediated effects. Cytisine was a full agonist in the rats trained with the large dose of nicotine, in which it was unable to produce any antagonism, but it had small and variable effects as an agonist in the rats trained with the small dose of nicotine, in which it was an effective antagonist. LeSage et al. (2009) evaluated cytisine in rats trained to a relatively large dose of nicotine (0.4 mg/kg base) and also found little agonist effect of this partial agonist up to 3 mg/kg; however, they found that cytisine had little nicotine antagonist effect, which is surprising, given its lack of agonist action. The pattern of partial agonist effects that we observed is quite different from what would be expected if the effects of the two doses were mediated through a single receptor. Partial agonists in single-receptor systems are more effective in blocking large-dose effects and have more effects in common with small-dose effects (Schaefer and Holtzman, 1981; Holtzman, 1982; Colpaert and Janssen, 1986; Colpaert, 1988; Picker et al., 1996; Dykstra et al., 1997; Holtzman, 1997; Grabus et al., 1999). Cytisine appears to be a full agonist at the receptors that mediate the discriminative stimulus effects of the large dose of nicotine and a low-efficacy partial agonist at the α4β2* receptor that mediates the discriminative stimulus effects of the small dose of nicotine.
Varenicline had a profile of activity that was somewhat different from that of cytisine. It was a full agonist in both the small- and large-dose nicotine-trained rats, indicating a greater efficacy than cytisine (LeSage et al., 2009). Similar to cytisine, varenicline also showed no antagonist actions when given as a pretreatment to the rats trained to the large dose of nicotine. However, in the small-dose nicotine-trained rats, varenicline had a variety of interesting effects. It produced modest rightward shifts in the discriminative stimulus potency of nicotine at doses below those that had no nicotine-like stimulus effects on their own. LeSage et al. (2009) found a similar effect of varenicline in rats trained to discriminate a dose of 0.4 mg/kg nicotine (base). In addition, a larger dose of varenicline that produced some nicotine-appropriate responding in these animals enhanced the effects of small doses of nicotine, antagonized the effects of intermediate doses of nicotine, and did not modify the effects of large doses of nicotine. If, as we suggest, these small-dose nicotine-trained animals are responsive to the actions of nicotine at the α4β2* receptor, varenicline appears to be a relatively high efficacy partial agonist at this receptor. In vitro data support this suggestion (e.g., Rollema et al., 2007). Varenicline's ability to produce large-dose nicotine effects and no large dose antagonism indicates that it may be a full agonist at the nicotinic receptors that apparently mediate the large-dose nicotine effects.
It is of particular interest and importance that the effects being evaluated in this report are the interoceptive effects of nicotine. The interoceptive effects of nicotine are also probably closely related to the reinforcing effects of tobacco. Nicotine and nicotine-containing products are unusual drugs of abuse in that they appear to be strongly addictive insofar as many people find it very difficult to quit using tobacco- or nicotine-containing products. However, the reinforcing effects of nicotine in most animal species are often difficult to detect, suggesting that they may be weak and that conditioned stimuli or other components of tobacco-containing products may enhance the reinforcing properties of nicotine (Le Foll and Goldberg, 2009). Nicotine is also unusual among drugs of abuse in that users report that intake of large doses may be aversive (Le Foll and Goldberg, 2009). These findings combine to suggest that small doses of nicotine, those antagonized by DHβE, may be responsible for the positive reinforcing effects of nicotine, whereas the larger doses, those acting on other nicotine receptors, may be involved in the other effects of nicotine or at least contribute less to the abuse-related aspects of this drug. If true, this hypothesis indicates that DHβE, by effectively blocking these small-dose effects, may be an effective antagonist of nicotine's reinforcing effects (Watkins et al., 1999) and might, in a long-acting form of a pharmacological equivalent, be able to be developed as a treatment for tobacco abuse. Currently, it is not known whether nicotinic antagonists, especially subtype-selective antagonists, would be useful smoking cessation treatments in humans, but future studies should investigate this possibility, considering that antagonists are useful pharmacotherapies for opioid addiction (Comer et al., 2002, 2006; Sullivan et al., 2006; for review, see Lobmaier et al., 2008).
In conclusion, the present findings demonstrate that nicotine drug discrimination assays, particularly those that evaluate the discriminative stimulus effects of two doses of nicotine, can provide helpful insights into receptor subtypes mediating the behavioral effects of nicotine. The interaction of the antagonists and partial agonists with the small nicotine training dose indicated that 1) small doses of nicotine are acting at a single receptor site, one that both mecamylamine and DHβE act functionally upon, 2) this single receptor site alone does not mediate the rate-decreasing effect of nicotine, and 3) cytisine appears to be a lower efficacy agonist at this receptor than varenicline.
The interactions with the larger dose of nicotine were more complex. These doses were more able to override the antagonist effects of the uncompetitive antagonist, were shifted less by the selective, competitive antagonist, and were not affected to any significant extent by the antagonist actions of the partial agonists. The large nicotine dose appears to incorporate other nicotine receptors that include the α4β2* subtype and others, and cytisine and varenicline also act as agonists at these unidentified receptors.
This additional support for the evidence that the α4β2* subtype of nAChR selectively mediates the discriminative stimulus effects of small training doses of nicotine emphasizes the need for development of antagonists that are selective at this site, because such an antagonist might provide clinical blockade of nicotine's reinforcing effect with minimal disruption of the effects of acetylcholine on other nicotinic receptor subtypes.
Acknowledgments
We thank Dr. Gail Winger for helpful suggestions and editing of this manuscript.
This work was supported in part by the Intramural Research Programs of the National Institutes of Health National Institute on Drug Abuse and the National Institute on Alcohol Abuse and Alcoholism; the National Institutes of Health National Institute on Drug Abuse [Grant T32-DA007268]; and the University of Michigan Tobacco Research Network.
The contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute on Drug Abuse.
Parts of this work were previously presented at the following conference: Jutkiewicz EM and Woods JH (2008) The nicotinic receptor partial agonist cytisine attenuated some, but not all, behavioral effects of nicotine in rodents. 70th Annual Scientific Meeting of the College on Problems of Drug Dependence; 2008 June 14–19; San Juan, Puerto Rico. Poster 74. College on Problems of Drug Dependence, Philadelphia, PA.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.111.182170.
- nAChR
- nicotinic acetylcholine receptor
- DHβE
- dihydro-β-erythroidine
- FR
- fixed-ratio.
Authorship Contributions
Participated in research design: Jutkiewicz and Woods.
Conducted experiments: Jutkiewicz, Brooks, and Kynaston.
Contributed new reagents or analytic tools: Rice.
Performed data analysis: Jutkiewicz.
Wrote or contributed to the writing of the manuscript: Jutkiewicz and Woods.
References
- Arunlakshana O, Schild HO. (1959) Some quantitative uses of drug antagonists. Br J Pharmacol 14:48–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ator NA, Griffiths RR. (2003) Principles of drug abuse liability assessment in laboratory animals. Drug Alcohol Depend 70 (Suppl 1):S55–S72 [DOI] [PubMed] [Google Scholar]
- Barlow RB, McLeod LJ. (1969) Some studies on cytisine and its methylated derivatives. Br J Pharmacol 35:161–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler CJ, Stolerman IP. (1997) Discriminative stimulus properties of the nicotinic agonist cytisine. Psychopharmacology 129:257–264 [DOI] [PubMed] [Google Scholar]
- Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, Sands SB, Davis TI, Lebel LA, Fox CB, et al. (2005) Varenicline: an α4β2 nicotinic receptor partial agonist for smoking cessation. J Med Chem 48:3474–3477 [DOI] [PubMed] [Google Scholar]
- Colpaert FC. (1978) Discriminative stimulus properties of narcotic analgesic drugs. Pharmacol Biochem Behav 9:863–887 [DOI] [PubMed] [Google Scholar]
- Colpaert FC. (1988) Intrinsic activity and discriminative effects of drugs. Psychopharmacol Ser 4:154–160 [DOI] [PubMed] [Google Scholar]
- Colpaert FC, Janssen PA. (1986) Agonist and antagonist effects of prototype opiate drugs in fentanyl dose-dose discrimination. Psychopharmacology 90:222–228 [DOI] [PubMed] [Google Scholar]
- Colpaert FC, Rosecrans JA. editors (1978) Stimulus Properties of Drugs: Ten Years of Progress: International Symposium on Drugs as Discriminative Stimuli Beerse, Belgium Elsevier/North-Holland Biomedical Press, Amsterdam [Google Scholar]
- Comer SD, Collins ED, Kleber HD, Nuwayser ES, Kerrigan JH, Fischman MW. (2002) Depot naltrexone: long-lasting antagonism of the effects of heroin in humans. Psychopharmacology 159:351–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, McNutt RW, Chang KJ, De Costa BR, Mosberg HI, Woods JH. (1993) Discriminative stimulus effects of BW373U86: a nonpeptide ligand with selectivity for delta opioid receptors. J Pharmacol Exp Ther 267:866–874 [PubMed] [Google Scholar]
- Comer SD, Sullivan MA, Yu E, Rothenberg JL, Kleber HD, Kampman K, Dackis C, O'Brien CP. (2006) Injectable, sustained-release naltrexone for the treatment of opioid dependence: a randomized, placebo-controlled trial. Arch Gen Psychiatry 63:210–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, Adamson KL. (1994) Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res 653:278–284 [DOI] [PubMed] [Google Scholar]
- Dykstra LA, Preston KL, Bigelow GE. (1997) Discriminative stimulus and subjective effects of opioids with mu and kappa activity: data from laboratory animals and human subjects. Psychopharmacology 130:14–27 [DOI] [PubMed] [Google Scholar]
- Gatch MB, Flors E, Forster MJ. (2008). Nicotine and methamphetamine share discriminative stimulus effects. Drug Alcohol Depend 93:63–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gommans J, Stolerman IP, Shoaib M. (2000) Antagonism of the discriminative and aversive stimulus properties of nicotine in C57BL/6J mice. Neuropharmacology 39:2840–2847 [DOI] [PubMed] [Google Scholar]
- Grabus SD, Smurthwaite ST, Riley AL. (1999) Nalorphine's ability to substitute for morphine in a drug discrimination procedure is a function of training dose. Pharmacol Biochem Behav 63:481–488 [DOI] [PubMed] [Google Scholar]
- Holtzman SG. (1982) Phencyclidine-like discriminative stimulus properties of opioids in the squirrel monkey. Psychopharmacology 77:295–300 [DOI] [PubMed] [Google Scholar]
- Holtzman SG. (1985) Drug discrimination studies. Drug Alcohol Depend 14:263–282 [DOI] [PubMed] [Google Scholar]
- Holtzman SG. (1997) Discriminative stimulus effects of buprenorphine in the rat. Psychopharmacology 130:292–299 [DOI] [PubMed] [Google Scholar]
- Improgo MR, Scofield MD, Tapper AR, Gardner PD. (2010) The nicotinic acetylcholine receptor CHRNA5/A3/B4 gene cluster: dual role in nicotine addiction and lung cancer. Prog Neurobiol 92:212–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenakin TP. (1982) The Schild regression in the process of receptor classification. Can J Physiol Pharmacol 60:249–265 [DOI] [PubMed] [Google Scholar]
- Koek W, Woods JH. (1989) Partial generalization in pigeons trained to discriminate morphine from saline: application of receptor theory. Drug Dev Res 15:169–181 [Google Scholar]
- Le Foll B, Chefer SI, Kimes AS, Shumway D, Stein EA, Mukhin AG, Goldberg SR. (2009) Baseline expression of α4β2* nicotinic acetylcholine receptors predicts motivation to self-administer nicotine. Biol Psychiatry 65:714–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR. (2009) Effects of nicotine in experimental animals and humans: an update on addictive properties. Handb Exp Pharmacol 192:335–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeSage MG, Shelley D, Ross JT, Carroll FI, Corrigall WA. (2009) Effects of the nicotinic receptor partial agonists varenicline and cytisine on the discriminative stimulus effects of nicotine in rats. Pharmacol Biochem Behav 91:461–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Palmatier MI, Caggiula AR, Donny EC, Sved AF. (2007) Reinforcement enhancing effect of nicotine and its attenuation by nicotinic antagonists in rats. Psychopharmacology 194:463–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobmaier P, Kornør H, Kunøe N, Bjørndal A. (2008) Sustained-release naltrexone for opioid dependence. Cochrane Database Syst Rev 16:CD006140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansbach RS, Chambers LK, Rovetti CC. (2000) Effects of the competitive nicotinic antagonist erysodine on behavior occasioned or maintained by nicotine: comparison with mecamylamine. Psychopharmacology 148:234–242 [DOI] [PubMed] [Google Scholar]
- Martin BR, Onivi ES, Martin TJ. (1989) What is the nature of mecamylamine's antagonism of the central effects of nicotine? Biochemical Pharmacol 38:3391–3397 [DOI] [PubMed] [Google Scholar]
- McMahon LR. (2006) Characterization of cannabinoid agonists and apparent pA2 analysis of cannabinoid antagonists in rhesus monkeys discriminating Δ9-tetrahydrocannabinol. J Pharmacol Exp Ther 319:1211–1218 [DOI] [PubMed] [Google Scholar]
- Picker MJ, Benyas S, Horwitz JA, Thompson K, Mathewson C, Smith MA. (1996) Discriminative stimulus effects of butorphanol: influence of training dose on the substitution patterns produced by mu, kappa and delta opioid agonists. J Pharmacol Exp Ther 279:1130–1141 [PubMed] [Google Scholar]
- Picker MJ, Craft RM, Negus SS, Powell KR, Mattox SR, Jones SR, Hargrove BK, Dykstra LA. (1992) Intermediate efficacy mu opioids: examination of their morphine-like stimulus effects and response rate-decreasing effects in morphine-tolerant rats. J Pharmacol Exp Ther 263:668–681 [PubMed] [Google Scholar]
- Picker MJ, Smith MA, Morgan D. (1994) Assessment of the relative intrinsic efficacy of profadol and meperidine in a pigeon drug discrimination procedure: relevance to partial substitution patterns. Behav Pharmacol 5:61–70 [DOI] [PubMed] [Google Scholar]
- Picker MJ, Yarbrough J, Hughes CE, Smith MA, Morgan D, Dykstra LA. (1993) Agonist and antagonist effects of mixed action opioids in the pigeon drug discrimination procedure: influence of training dose, intrinsic efficacy and interanimal differences. J Pharmacol Exp Ther 266:756–767 [PubMed] [Google Scholar]
- Rollema H, Chambers LK, Coe JW, Glowa J, Hurst RS, Lebel LA, Lu Y, Mansbach RS, Mather RJ, Rovetti CC, et al. (2007) Pharmacological profile of the α4β2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology 52:985–994 [DOI] [PubMed] [Google Scholar]
- Romano C, Goldstein A, Jewell NP. (1981) Characterization of the receptor mediating the nicotine discriminative stimulus. Psychopharmacology 74:310–315 [DOI] [PubMed] [Google Scholar]
- Rowlett JK, Wilcox KM, Woolverton WL. (1999) Discriminative stimulus effects of ethyl-β-carboline-3-carboxylate at two training doses in rats. Psychopharmacology 145:324–332 [DOI] [PubMed] [Google Scholar]
- Russo P, Cesario A, Rutella S, Veronesi G, Spaggiari L, Galetta D, Margaritora S, Granone P, Greenberg DS. (2011) Impact of genetic variability in nicotinic acetylcholine receptors on nicotine addiction and smoking cessation treatment. Curr Med Chem 18:91–112 [DOI] [PubMed] [Google Scholar]
- Sabey K, Paradiso K, Zhang J, Steinbach JH. (1999) Ligand binding and activation of rat nicotinic α4β2 receptors stably expressed in HEK293 cells. Mol Pharmacol 55:58–66 [PubMed] [Google Scholar]
- Schaefer GJ, Holtzman SG. (1981) Morphine-like stimulus effects in the monkey: opioids with antagonist properties. Pharmacol Biochem Behav 14:241–245 [DOI] [PubMed] [Google Scholar]
- Shannon HE, Holtzman SG. (1979) Morphine training dose: a determinant of stimulus generalization to narcotic antagonists in the rat. Psychopharmacology 61:239–244 [DOI] [PubMed] [Google Scholar]
- Sherva R, Kranzler HR, Yu Y, Logue MW, Poling J, Arias AJ, Anton RF, Oslin D, Farrer LA, Gelernter J. (2010) Variation in nicotinic acetylcholine receptor genes is associated with multiple substance dependence phenotypes. Neuropsychopharmacology 35:1921–1931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoaib M, Zubaran C, Stolerman IP. (2000) Antagonism of stimulus properties of nicotine by dihydro-β-erythroidine (DHβE) in rats. Psychopharmacology 149:140–146 [DOI] [PubMed] [Google Scholar]
- Sloan JW, Martin WR, Bostwick M, Hook R, Wala E. (1988) The comparative binding characteristics of nicotinic ligands and their pharmacology. Pharmacol Biochem Behav 30:255–267 [DOI] [PubMed] [Google Scholar]
- Smith JW, Stolerman IP. (2009) Recognising nicotine: the neurobiological basis of nicotine discrimination. Handb Exp Pharmacol 192:295–333 [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Chandler CJ, Garcha HS, Newton JM. (1997) Selective antagonism of behavioural effects of nicotine by dihydro-β-erythroidine in rats. Psychopharmacology 129:390–397 [DOI] [PubMed] [Google Scholar]
- Stone CA, Torchiana ML, Navarro A, Beyer KH. (1956) Ganglionic blocking properties of 3-methylaminoisocamphane hydrochloride (mecamylamine): a secondary amine. J Pharmacol Exp Ther 117:169–183 [PubMed] [Google Scholar]
- Sullivan MA, Vosburg SK, Comer SD. (2006) Depot naltrexone: antagonism of the reinforcing, subjective, and physiological effects of heroin. Psychopharmacology 189:37–46 [DOI] [PubMed] [Google Scholar]
- Tallarida RJ. (1995) Receptor discrimination and control of agonist-antagonist binding. Am J Physiol 269:E379–E391 [DOI] [PubMed] [Google Scholar]
- Walker EA, Makhay MM, House JD, Young AM. (1994) In vivo apparent pA2 analysis for naltrexone antagonism of discriminative stimulus and analgesic effects of opiate agonists in rats. J Pharmacol Exp Ther 271:959–968 [PubMed] [Google Scholar]
- Watkins SS, Epping-Jordan MP, Koob GF, Markou A. (1999) Blockade of nicotine self-administration with nicotinic antagonists in rats. Pharmacol Biochem Behav 62:743–751 [DOI] [PubMed] [Google Scholar]
- Williams M, Robinson JL. (1984) Binding of the nicotinic cholinergic antagonist, dihydro-β-erythroidine, to rat brain tissue. J Neurosci 4:2906–2911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R, Glennon RA. (2002) Nicotine and bupropion share a similar discriminative stimulus effect. Eur J Pharmacol 443:113–118 [DOI] [PubMed] [Google Scholar]
- Zakharova ES, Danysz W, Bespalov AY. (2005) Drug discrimination analysis of NMDA receptor channel blockers as nicotinic receptor antagonists in rats. Psychopharmacology 179:128–135 [DOI] [PubMed] [Google Scholar]
- Zaniewska M, McCreary AC, Przegaliński E, Filip M. (2006) Evaluation of the role of nicotine acetylcholine receptor subtypes and cannabinoid system in the discriminative stimulus effects of nicotine in rats. Eur J Pharmacol 540:96–106 [DOI] [PubMed] [Google Scholar]