Chronic myeloid leukemia (CML) is a hematopoietic neoplasm characterized by uncontrolled myeloproliferation, basophilia, and the BCR/ABL oncoprotein. The natural course of CML includes a chronic phase, an accelerated phase, and a blast phase that resembles an acute leukemia. The BCR/ABL tyrosine-kinase inhibitor (TKI) imatinib is an established standard of therapy in chronic phase CML. However, despite very good long-term results concerning efficacy and safety, resistance against imatinib may occur, often in association with a BCR/ABL mutation.1,2 The treatment of patients with imatinib-resistant CML is a challenge in clinical hematology.3,4 For these patients, two effective second-generation TKI are available: dasatinib and nilotinib.3–6
Recent data suggest that these two TKI, when compared to imatinib, also exert superior anti-leukemic effects in newly diagnosed patients, with higher rates of complete cytogenetic remission and major molecular remission at 12 and 18 months.5,6 Moreover, nilotinib and dasatinib counteract early transformation to blast phase more effectively.5,6 The superior effects of these TKI may result from their strong effects on BCR/ABL (including BCR/ABL-mutants) and their effects on additional drug targets.7 However, such additional targets are also expressed in non-hematopoietic cells and may thus be responsible for non-hematologic adverse events. Whereas side effects are, in many cases, mild and manageable without organ damage, some patients have major problems and develop overt intolerance or even life-threatening adverse events.
The unique adverse event profile of nilotinib and dasatinib
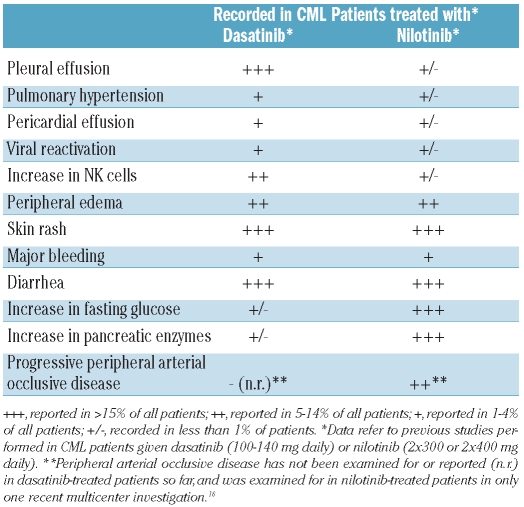
A unique spectrum of adverse events has been reported for both TKI (Table 1). For dasatinib-treated patients, the occurrence of pleural effusion is a clinical challenge,8–12 especially when pleural effusion is recurrent or accompanied by a perciardial effusion, pulmonary hypertension, or an infection. Several other non-hematologic adverse events have also been described for dasatinib, including diarrhea, skin rash, bleeding, viral re-activation, and sometimes also opportunistic infections which have been reported in patients receiving dasatinib at a dose of 2×70 mg daily (Table 1).11 The frequency of most adverse events appears to be lower when the dose of dasatinib is 100 mg once daily.9 However, even in patients receiving 100 mg once daily, pleural effusions develop and may accumulate over time.12,13
Table 1.
Non-hematologic adverse events and related laboratory abnormalities reported in CML patients treated with dasatinib or nilotinib.
Nilotinib-treated patients may develop increases in pancreatic enzymes, bilirubin, and fasting glucose level (Table 1).5,14,15 Other non-hematologic adverse events include diarrhea, a folliculitis-like skin rash, and bleeding. There are a few reports of severe peripheral arterial occlusive disease (PAOD) and other vascular occlusive events (infarction) in patients receiving nilotinib (Table 1).15,16 Several of these patients developed a rapidly progressive and highly resistant form of PAOD after switching from imatinib to nilotinib.15,16
It is remarkable that pleural effusions rarely occur in nilotinib-treated patients, that severe PAOD has not been reported in patients treated with dasatinib, and that both types of adverse events are rare in imatinib-treated patients.5,6,9,15 Thus, although not confirmed for all types of adverse events in prospective studies, these particular events are considered to be associated with drug-intake, and to occur in a TKI-specific manner. So far, little is known about factors predisposing to the development of such adverse events. From a clinical point of view, this is an essential issue as many patients are candidates for long-term treatment with TKI, or may be transplantable patients in whom comorbidities should be kept to a minimum and should be recognized as early as possible.
Preferential occurrence of severe tyrosine kinase inhibitor-related adverse events in patients who suffer from comorbidities or have other pre-existing risk factors
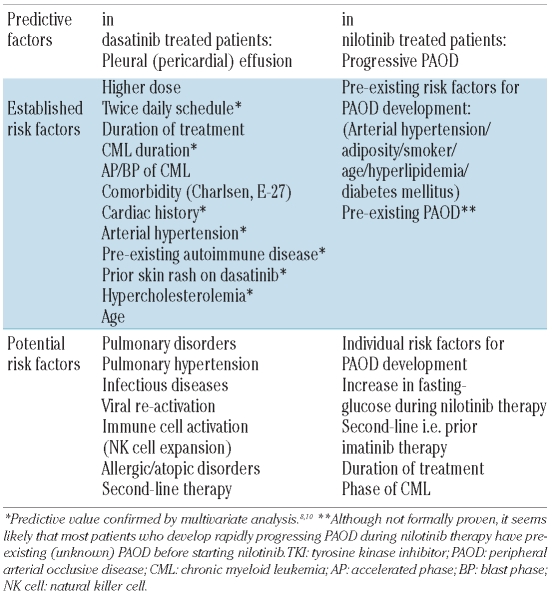
Recent data suggest that severe TKI-related adverse events (TKI-syndromes) preferentially occur in patients who have pre-existing risk factors or are already suffering from certain comorbidities. For dasatinib-induced pleural effusions, several risk factors such as a pre-existing cardiac disease, arterial hypertension, or auto-immune disorders have been described (Table 2).8–10 Other comorbidities have also been discussed as potential risk factors, but their exact predictive value remains uncertain (Table 2). The impact of comorbidities on effusion-formation in dasatinib-treated patients is confirmed by Breccia et al. in a study published in this issue of the journal.17 They applied two multi-parameter comorbidity scoring systems, the Charlsen comorbidity score and the adult comorbidity evaluation-27 score; according to both scores, comorbidities were found to predict effusion-formation.17 Apart from comorbidities, other risk factors, such as age and the phase of CML, should also be considered in dasatinib-treated patients.8 Finally, the risk of formation of an effusion is clearly higher when the drug is administered at a dose of 2×70 mg per day than at the once-daily 100 mg-dose (Table 2).9,18
Table 2.
Risk factors and comorbidities predicting specific adverse events in patients treated with second line TKI.
Comorbidities that may predispose to the development of PAOD and other vascular occlusive events in nilotinib-treated patients may be the same as those predisposing to PAOD in untreated non-leukemic patients, namely smoking, arterial hypertension, diabetes mellitus, age, and obesity (Table 2).15,16 One or more of these risk factors were identified in almost all CML patients who developed a severe form of PAOD during nilotinib therapy.15,16 It is currently unknown why some of these patients develop a rapidly progressive, severe, treatment-resistant form of PAOD, requiring repeated surgical interventions or even amputation.15,16
An important aspect is that the risk factors predisposing to the development of severe adverse events during treatment with the two TKI may overlap. Thus, age, arterial hypertension, and hypercholesterolemia are risk factors for both pleural effusion-formation during dasatinib treatment and PAOD development in patients taking nilotinib.
Potential mechanisms and treatment options
The exact mechanisms underlying TKI-induced severe adverse events remain unknown. In the case of dasatinib-induced pleural effusions, a widely discussed hypothesis is that systemic activation of the immune system (by viral reactivation, infections, auto-immune or allergic reactions) is a critical factor.10 In line with this hypothesis, an increase in natural killer cells is often seen in patients with such effusions. Many of these patients also have a skin rash, an allergic episode or a respiratory infection (shortly) before the effusion is diagnosed. In this regard, it is noteworthy that IgE-mediated histamine release from basophils is augmented by low concentrations of dasatinib.19 Moreover, therapy with glucocorticosteroids, known to suppress lymphocyte- and basophil function, exerts beneficial effects in these patients.8,18 From these observations, infections (especially bronchopulmonary infections), auto-immune disorders, and allergic reactions must be taken into account as potentially relevant for the development of effusion-formation in dasatinib-treated patients, and early recognition and management of such comorbidities may be an effective way to prevent effusion-formation. For patients with an overt pleural or pericardial effusion, drug-interruption and dose-reduction, thoracentesis (if needed), short-term glucocorticosteroids, and diuretics are recommended approaches.8,18 In those with repeated severe effusion-formation, it is advisable to discontinue dasatinib and to switch to an alternative drug.
With regard to vascular events, the exact relationship to nilotinib-exposure and the underlying mechanisms remain unclear. Potential explanations include the metabolic effect of nilotinib, direct effects of nilotinib on vascular cells, and drug effects on the coagulation and/or fibrinolytic system. There are several targets of nilotinib that may be involved in these drug-specific effects, including the discoidin domain receptor-1 (DDR1).20 Although imatinib also interacts with DDR1, the suppressive effect of nilotinib on DDR1-activity is much stronger. Whether DDR1 is indeed a critical vascular and pancreatic target of nilotinib is currently under investigation.
With regard to therapy and prevention of PAOD, it may be important to treat metabolic and vascular comorbidities and to introduce prophylactic therapy early. An unresolved question is whether a slight increase in the fasting glucose level, which is quite frequently seen in nilotinib-treated patients, should immediately lead to early intervention with anti-diabetic management/therapy. At least repeated testing of glucose levels seems justified. In addition, patients with raised fasting glucose levels should be thoroughly examined for the presence and for the development of PAOD.
Implications for first-line and second-line therapy
The observations that CML patients who suffer from metabolic, immunological, or vascular comorbidities (often elderly patients) are more likely to develop major non-hematologic adverse events during dasatinib or nilotinib treatment have clinical implications and should assist in patient-selection. In particular, certain comorbidities as well as related risk factors should be taken into account when selecting a second-line TKI. Patients should be examined for the presence and development of risk factors and comorbidities before starting TKI treatment as well as during the therapy. In the case of front-line therapy, it should always be considered that many patients may take TKI for many years or even decades, and that no long-term safety data for dasatinib or nilotinib are available, in contrast to the well-established and superior long-term safety profile of imatinib. It should also be mentioned that predisposing risk factors and comorbidities may develop in most patients with age, even when the factors were not present at the start of therapy. Finally, the safety issues concerning dasatinib and nilotinib are not trivial and the underlying mechanisms are not understood. Many experts do, therefore, consider that, for the time being, imatinib should remain standard front-line therapy in chronic phase CML, at least for patients with Sokal low-risk CML and for older patients suffering from certain comorbidities. In the future this view may change, especially when the mechanisms underlying adverse events are better understood, when studies have shown that the risk can be reduced by appropriate selection of patients and/or comedication, or when CML eradication can be achieved within a relatively short period. A related reasonable concept might be to eradicate most of the more malignant subclones with second- or third-line TKI first, and then switch back to imatinib maintenance therapy at the time of complete molecular remission.
Summary and future perspectives
Based on the notion that certain comorbidities predispose to the development of severe adverse events in patients receiving dasatinib or nilotinib, front-line and second-line treatment of CML must be adapted to the individual situation in each patient. In many instances the use of such TKI and their superior activity as front-line therapy must be balanced against their potential risk. Even in patients without comorbidities, the risk must be calculated as patients may be treated for many years if not decades and may acquire risk factors over time. Therefore, parameters exploring the risk and detecting TKI-associated adverse events early should be included in the follow up.
Footnotes
Financial and other disclosures provided by the author using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are available with the full text of this paper at www.haematologica.org.
References
- 1.O'Hare T, Eide CA, Deininger MW. Bcr-Abl kinase domain mutations, drug resistance, and the road to a cure for chronic myeloid leukemia. Blood. 2007;110(7):2242–9. doi: 10.1182/blood-2007-03-066936. [DOI] [PubMed] [Google Scholar]
- 2.Quintás-Cardama A, Kantarjian HM, Cortes JE. Mechanisms of primary and secondary resistance to imatinib in chronic myeloid leukemia. Cancer Control. 2009;16(2):122–31. doi: 10.1177/107327480901600204. [DOI] [PubMed] [Google Scholar]
- 3.Martinelli G, Soverini S, Rosti G, Cilloni D, Baccarani M. New tyrosine kinase inhibitors in chronic myeloid leukemia. Haematologica. 2005;90(4):534–41. [PubMed] [Google Scholar]
- 4.Cervantes F, Mauro M. Practical management of patients with chronic myeloid leukemia. Cancer. 2011 Mar 16; doi: 10.1002/cncr.26062. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 5.Saglio G, Kim DW, Issaragrisil S, le Coutre P, Etienne G, Lobo C, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 2010;362(24):2251–9. doi: 10.1056/NEJMoa0912614. [DOI] [PubMed] [Google Scholar]
- 6.Kantarjian H, Shah NP, Hochhaus A, Cortes J, Shah S, Ayala M, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2010;362(24):2260–70. doi: 10.1056/NEJMoa1002315. [DOI] [PubMed] [Google Scholar]
- 7.Rix U, Hantschel O, Dürnberger G, Remsing Rix LL, Planyavsky M, et al. Chemical proteomic profiles of the BCR-ABL inhibitors imatinib, nilotinib, and dasatinib reveal novel kinase and nonkinase targets. Blood. 2007;110(12):4055–63. doi: 10.1182/blood-2007-07-102061. [DOI] [PubMed] [Google Scholar]
- 8.Quintás-Cardama A, Kantarjian H, O'brien S, Borthakur G, Bruzzi J, Munden R, et al. Pleural effusion in patients with chronic myelogenous leukemia treated with dasatinib after imatinib failure. J Clin Oncol. 2007;25(25):3908–14. doi: 10.1200/JCO.2007.12.0329. [DOI] [PubMed] [Google Scholar]
- 9.Shah NP, Kantarjian HM, Kim DW, Réa D, Dorlhiac-Llacer PE, Milone JH, et al. Intermittent target inhibition with dasatinib 100 mg once daily preserves efficacy and improves tolerability in imatinib-resistant and –intolerant chronic-phase chronic myeloid leukemia. J Clin Oncol. 2008;26(19):3204–12. doi: 10.1200/JCO.2007.14.9260. [DOI] [PubMed] [Google Scholar]
- 10.de Lavallade H, Punnialingam S, Milojkovic D, Bua M, Khorashad JS, Gabriel IH, et al. Pleural effusions in patients with chronic myeloid leukaemia treated with dasatinib may have an immune-mediated pathogenesis. Br J Haematol. 2008;141(5):745–7. doi: 10.1111/j.1365-2141.2008.07108.x. [DOI] [PubMed] [Google Scholar]
- 11.Sillaber C, Herrmann H, Bennett K, Rix U, Baumgartner C, Böhm A, et al. Immunosuppression and atypical infections in CML patients treated with dasatinib at 140 mg daily. Eur J Clin Invest. 2009;39(12):1098–109. doi: 10.1111/j.1365-2362.2009.02206.x. [DOI] [PubMed] [Google Scholar]
- 12.Krauth MT, Herndlhofer S, Schmook MT, Mitterbauer-Hohendanner G, Schlögl E, Valent P. Extensive pleural and pericardial effusion in chronic myeloid leukemia during treatment with dasatinib at 100 mg or 50 mg daily. Haematologica. 2011;96(1):163–6. doi: 10.3324/haematol.2010.030494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eskazan AE, Soysal T, Ongoren S, Gulturk E, Ferhanoglu B, Aydin Y. Pleural and pericardial effusions in chronic myeloid leukemia patients receiving low-dose dasatinib therapy. Haematologica. 2011;96(3):e15. doi: 10.3324/haematol.2011.040048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Breccia M, Muscaritoli M, Gentilini F, Latagliata R, Carmosino I, Rossi Fanelli F, et al. Impaired fasting glucose level as metabolic side effect of nilotinib in non-diabetic chronic myeloid leukemia patients resistant to imatinib. Leuk Res. 2007;31(12):1770–2. doi: 10.1016/j.leukres.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 15.Aichberger KJ, Herndlhofer S, Schernthaner GH, Schillinger M, Mitterbauer-Hohendanner G, Sillaber C, et al. Progressive peripheral arterial occlusive disease and other vascular events during nilotinib therapy in CML. Am J Hematol. 2011;86(7):533–9. doi: 10.1002/ajh.22037. [DOI] [PubMed] [Google Scholar]
- 16.Le Coutre P, Rea D, Abruzzese E, Dombret H, Trawinska MM, Herndlhofer S, et al. Severe peripheral arterial disease during nilotinib therapy. J Natl Cancer Inst. 2011;103(17):1347–8. doi: 10.1093/jnci/djr292. [DOI] [PubMed] [Google Scholar]
- 17.Breccia M, Latagliata R, Stagno F, Luciano L, Gozzini A, Castagnetti F, et al. Charlson comorbidity index and adult comorbidity evaluation-27 scores might predict treatment compliance and development of pleural effusions in elderly patients with chronic myeloid leukemia treated with second-line dasatinib. Haematologica. 2011;96(10):1457–61. doi: 10.3324/haematol.2011.041251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Porkka K, Khoury HJ, Paquette RL, Matloub Y, Sinha R, Cortes JE. Dasatinib 100 mg once daily minimizes the occurrence of pleural effusion in patients with chronic myeloid leukemia in chronic phase and efficacy is unaffected in patients who develop pleural effusion. Cancer. 2010;116(2):377–86. doi: 10.1002/cncr.24734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kneidinger M, Schmidt U, Rix U, Gleixner KV, Vales A, Baumgartner C, et al. The effects of dasatinib on IgE receptor-dependent activation and histamine release in human basophils. Blood. 2008;111(6):3097–107. doi: 10.1182/blood-2007-08-104372. [DOI] [PubMed] [Google Scholar]
- 20.Franco C, Ahmad PJ, Hou G, Wong E, Bendeck MP. Increased cell and matrix accumulation during atherogenesis in mice with vessel wall-specific deletion of discoidin domain receptor 1. Circ Res. 2010;106(11):1775–83. doi: 10.1161/CIRCRESAHA.109.213637. [DOI] [PubMed] [Google Scholar]