Abstract
Background
KANK1-PDGFRB is a fusion gene generated by the t(5;9) translocation between KANK1 and the platelet-derived growth factor receptor beta gene PDGFRB. This hybrid was identified in a myeloproliferative neoplasm featuring severe thrombocythemia, in the absence of the JAK2 V617F mutation.
Design and Methods
KANK1-PDGFRB was transduced into Ba/F3 cells and CD34+ human progenitor cells to gain insights into the mechanisms whereby this fusion gene transforms cells.
Results
Although platelet-derived growth factor receptors are capable of activating JAK2, KANK1-PDGFRβ did not induce JAK2 phosphorylation in hematopoietic cells and a JAK inhibitor did not affect KANK1-PDGFRβ-induced cell growth. Like JAK2 V617F, KANK1-PDGFRβ constitutively activated STAT5 transcription factors, but this did not require JAK kinases. In addition KANK1-PDGFRβ induced the phosphorylation of phospholipase C-γ, ERK1 and ERK2, like wild-type PDGFRβ and TEL-PDGFRβ, another hybrid protein found in myeloid malignancies. We next tested various mutant forms of KANK1-PDGFRβ in Ba/F3 cells and human CD34+ hematopoietic progenitors. The three coiled-coil domains located in the N-terminus of KANK1 were required for KANK1-PDGFRβ-induced cell growth and signaling via STAT5 and ERK. However, the coiled-coils were not essential for KANK1-PDGFRβ oligomerization, which could be mediated by another new oligomerization domain. KANK1-PDGFRβ formed homotrimeric complexes and heavier oligomers.
Conclusions
KANK1-PDGFRB is a unique example of a thrombocythemia-associated oncogene that does not signal via JAK2. The fusion protein is activated by multiple oligomerization domains, which are required for signaling and cell growth stimulation.
Keywords: receptor tyrosine kinase, PDGF receptor, myeloproliferative disease, KANK, ANKRD15, thrombocytosis
Introduction
Alterations in two tyrosine kinase genes, ABL1 and JAK2, account for the majority of myeloproliferative neoplasms. The BCR-ABL1 fusion is the hallmark of chronic myeloid leukemia while JAK2 point mutations are found in most cases of polycythemia vera and in about 50% of patients with essential thrombocythemia or primary myelofibrosis.1–3 Essential thrombocythemia and primary myelofibrosis can also be caused by mutations in the thrombopoietin receptor, which activates JAK2.4 In rare cases of myeloproliferative neoplasms, mutations are found in other tyrosine kinases, such as platelet-derived growth factor receptor (PDGFR) α or β.5 Chromosomal rearrangements of the PDGFR genes produce constitutively activated fusion receptors that are responsible for myeloid neoplasms associated with eosinophilia.5 Like chronic myeloid leukemia, these diseases are efficiently treated with tyrosine kinase inhibitors such as imatinib.6 Whether myeloproliferative neoplasms associated with JAK2 mutations can also benefit from a treatment based on specific tyrosine kinase inhibitors is currently under investigation.7
The best characterized PDGFR fusion product arises from the translocation between the genes TEL (also known as ETV6) and PDGFRB, which encodes PDGFRβ. The fusion protein retains the N-terminal pointed (PNT) domain of TEL and the PDGFRβ tyrosine kinase domain. The PNT domain promotes the oligomerization of the hybrid protein, mimicking ligand-induced dimerization and thereby activating the PDGFRβ kinase domain by trans-phosphorylation.5 In addition, we showed that this fusion protein escapes ubiquitination and degradation, which PDGF receptors normally undergo upon activation.8 TEL-PDGFRβ and most other PDGFRβ hybrid proteins retain the PDGFRβ transmembrane domain, which does not affect the cytosolic localization of these oncoproteins but contributes to their acquiring the optimal active conformation.9 TEL-PDGFRβ was shown to activate various signal transduction mediators, among which the transcription factor STAT5 plays a prominent role.10 Whether the same mechanisms apply to other PDGFRB translocation products is not clear, as none of the alternative fusion partners includes a PNT domain. Various types of dimerization domains, such as coiled coils, were suggested to substitute for the PNT in these proteins, but this has not been established experimentally.5 In HIP1-PDGFRβ, the coiled-coil/leucine zipper domain is dispensable for oligomerization and cell transformation.11 In another hybrid, H4-PDGFRβ, a similar domain was shown to be required to sustain Ba/F3 cell proliferation but its function was not further studied.12 In BCR-ABL1, the coiled-coil domain of BCR promotes multimerization and activation of the tyrosine kinase required for the BCR-ABL-induced cell transformation. A mutant lacking this domain fails to induce myeloproliferative neoplasms in mice.13 Smith et al. showed that the sole function of the BCR-ABL coiled-coil domain is to disrupt the autoinhibited conformation through oligomerization and intermolecular autophosphorylation.14
We recently identified a new chromosomal translocation between the potential tumor suppressor gene KANK1 and PDGFRB in a case of thrombocythemia.15 KANK1 (also known as KANK or ANKRD15) is part of a family of proteins that regulates actin polymerization and cell motility.16 These proteins feature multiple N-terminal coiled-coil domains and C-terminal ankyrin domains. Loss of KANK1 expression has been associated with renal cell carcinoma and cerebral palsy.17,18 We have shown that the KANK1-PDGFRβ fusion protein (KPβ) stimulates Ba/F3 cell growth and the activation of the STAT5 transcription factor.15
In the present study, we further analyzed the mechanisms of hematopoietic cell transformation by KPβ. Since JAK2 is a key mediator of essential thrombocythemia and was shown to be activated by wild-type PDGF receptors in different cell types,19–21 we first tested whether JAK2 activates STAT downstream of KPβ. Next, we identified the domains responsible for signaling and activation of KPβ in hematopoietic cells.
Design and Methods
Antibodies, inhibitors and constructs
Anti-PDGFRβ (958), anti-phosphotyrosine (PY99) and anti-STAT5 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-phospho STAT5 (tyr694), anti-phospho JAK2 (tyr1007-1008), anti-phospho PLCγ1 (tyr783) and anti-phospho ERK1/2 (thr202 and tyr204) antibodies were purchased from Cell Signaling. Mouse monoclonal antibodies against FLAG (M5) and β-actin (clone AC-15) were purchased from Sigma and the anti-JAK2 antibody from Millipore (#06-1310). The anti-PDGFR (CED), anti-PLCγ1 and anti-ERK1 (EET) rabbit polyclonal antisera have been described elsewhere.22 JAK inhibitor I, UO126, PD98059, and SU6656 were obtained from Calbiochem and imatinib from Novartis. All cytokines were purchased from Peprotech.
The KPβ sequence was cloned in the lentiviral vector pTM898-neo as described elsewhere.15 All KANK1 constructs correspond to KANK1-S, which is the predominant isoform in hematopoietic cells.23 (Medves et al., unpublished data) The following mutants carrying deletions of the KANK1 part of KPβ were generated: m1, residues 159 to 739; m2, residues 238 to 739; m3, residues 343 to 739; m4, residues 002 to 287; m5, residues 2 to 202 and m6, residues 159 to 287; m8, residues 100 to 739; m11, residues 343 to 641; m12, residues 642 to 739; m14, residues 002 to 451 (residue numbering according to SWISS-PROT accession number #Q6PIB3). KANK1 fragments were generated by PCR amplification and introduced by restriction (AgeI, NheI) into the pTM898-neo-KPβ vector. The KANK1 residues present in KPβ (residues 1 to 739, named K1-739) were also cloned separately in pTM898-neo. All constructs were FLAG-tagged in the N-terminus region and checked by sequencing. TEL-PDGFRβ and JAK2-V617F constructs were described previously.8,24
Transfection, infection, luciferase and thymidine incorporation assay
Lentiviral particles were produced by HEK-293T cells, which were transfected by the calcium phosphate method, as described previously.8,15 The interleukin (IL)3-dependent Ba/F3 cells were infected twice15 and selected in the presence of G418. Transduced cells were cultured in the presence of fetal calf serum and IL3, except for the experiments whose results are presented in Figures 1 and 2, in which cells growing autonomously without IL3 were used.
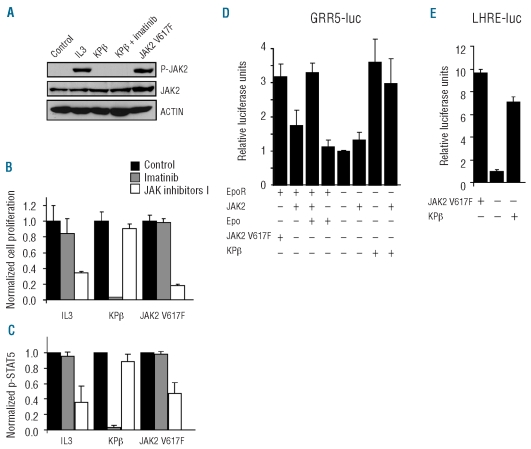
Figure 1.
JAK2 is not required for KPβ-induced cell proliferation or STAT activation. (A) Lysates of Ba/F3-KPβ or Ba/F3-JAK2-V617F cells that were cultured in the absence of IL3 were analyzed by western blot with anti-phospho-JAK2, anti-JAK2 and anti-β-actin antibodies. As a control, Ba/F3 cells transduced with the empty vector were stimulated with IL3 or left untreated (left lane). (B) The same cells were cultured in the presence of 25 nM imatinib or 0.5 μM Jak inhibitor I for 24 h. Cell proliferation was analyzed by measuring [3H]thymidine incorporation. Untreated cells were used as a reference. Total radioactivity incorporation in the DNA of Ba/F3 cells treated with IL3, Ba/F3-KPβ and Ba/F3-JAK2-V617F was 85543 ±2026 cpm, 59098 ±1577 cpm and 44899 ±4846 cpm, respectively. (C) STAT5 phosphorylation was monitored by flow cytometry using cells treated with 0.5 μM imatinib or 2 μM JAK inhibitor I for 4 h. (D, E) JAK2-deficient γ-2A cells were co-transfected with the erythropoietin receptor (EpoR), wild-type JAK2, JAK2-V617F, KPβ or the empty vector, as indicated. Cells were co-transfected with STAT5 and the luciferase reporter pGRR5-luc (D) or pLHRE-luc (E). Cells transfected with EpoR were stimulated by erythropoietin as indicated. STAT-dependent transcriptional activities were measured and normalized using the empty vector control as the reference. One representative experiment is shown with the standard deviation calculated from triplicate measurements.
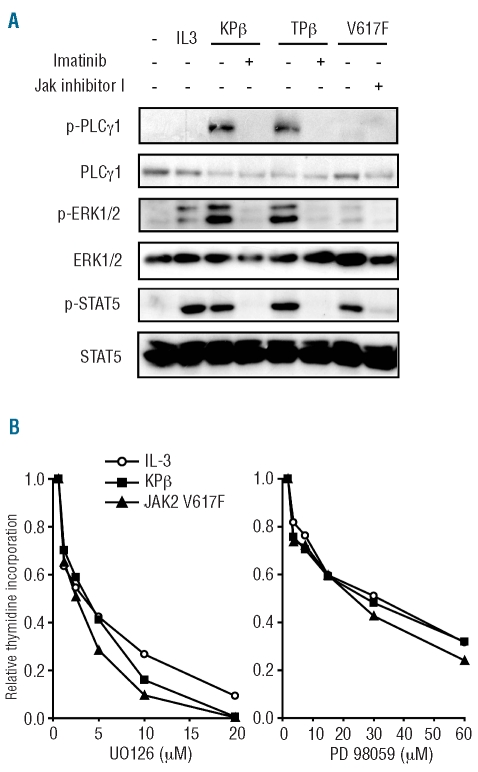
Figure 2.
Signal transduction by KPβ, TEL-PDGFRβ and activated JAK2. Ba/F3 cells were transduced with KPβ, TEL-PDGFRβ (TPβ) or JAK2-V617F and cultured in the absence of IL3. (A) Cells were treated with 0.1 μM imatinib or 2 μM JAK inhibitor I for 4 h as indicated. As a control, Ba/F3 cells expressing the empty vector were cultured with or without IL3 for 4 h (left lanes). Cell lysates were immunoblotted with antibodies against phosphorylated or total PLC-γ, ERK1/2 or STAT5. (B) Cells were washed and seeded in microtiter plates in the presence of the indicated inhibitor for 24 h. As a control, Ba/F3 cells were cultured with IL3. Tritiated thymidine was added 4 h before harvest and radioactivity incorporated into DNA was quantified. The effect of both inhibitors was statistically significant in all cell lines (P<0.05).
γ-2A JAK2-deficient human fibrosarcoma cells were transfected using lipofectamine with pGRR5-luc or pLHRE-luc reporters and the pEF-β-galactosidase vector as an internal control, as described previously.25 pGRR5-luc contains five copies of the STAT-binding site of the FcγRI gene inserted upstream of the minimal TK promoter and a luciferase gene.26,27 pLHRE-luc harbors tandem copies of the STAT5-inducible lactogenic hormone response element (LHRE) of the rat β-casein gene promoter.25 Twenty-four hours after transfection, cells were lysed and luciferase and β-galactosidase activities were recorded as described elsewhere.28–30 Cell proliferation was measured by counting cells in the presence of trypan blue as a function of time or by [3H]thymidine incorporation assays.31
Flow cytometry
Intracellular staining was performed as described previously and analyzed by flow cytometry.9 Briefly, about 106 cells were washed to remove IL3 and starved for 4 h in the presence of imatinib or JAK inhibitor I. As a positive control, some cells were restimulated with IL3 after starvation (data not shown). Cells were fixed with 2% formaldehyde in phosphate-buffered saline for 10 min at 37°C and then permeabilized with methanol on ice for 30 min. After having been washed twice with HAFA buffer (Hanks’ buffer complemented with 3% fetal bovine serum and 1% NaN3), the cells were incubated with Alexa-Fluor 647-conjugated antibodies specific for phospho-STAT5 (tyrosine 694) or phospho-ERK1/2 (threonine 202 and tyrosine 204) (BD Transduction Laboratories) or phycoerythrin-conjugated anti-PDGFRβ (#958, Santa Cruz Biotechnology) for 1 h at room temperature. Cells were washed and analyzed by flow cytometry. Results are expressed as a percentage of positive cells and normalized using KPβ as a reference.
Immunoprecipitation and western blot
HEK 293T cells were transiently transfected with KPβ or mutants and/or with K1-739 and lysed 24 h after transfection. Cell lysates were immunoprecipitated for 4 h with 1 μg of anti-PDGFRβ antibody at 4°C to capture KPβ or mutants proteins. Antibody complexes were collected by adding protein-A/G Ultralink (Pierce) for 2 h at 4°C or protein-A/G magnetic beads (New England Biolabs) for 1 h at 4°C, washed extensively and analyzed by western blot with the anti-FLAG or the anti-PDGFR antibody as described.9,22 To assess KPβ phosphorylation, Ba/F3 cells stably expressing the constructs were lyzed and KPβ was immunoprecipitated overnight and analyzed by immunoblotting using anti-phosphotyrosine antibodies as described elsewhere.9,22
CD34+ cell isolation, infection and culture
Cord blood units unsuitable for preservation were used following a procedure approved by the ethics committee of the medical faculty (reference B40320108411) within 24 h of collection. Leukocytes were isolated from fresh cord blood by centrifugation over a Ficoll-Paque density-gradient (GE Healthcare) and CD34+ cells were purified using the EasySep kit (StemCell Technologies, Vancouver, Canada). Cell purity was routinely checked by flow cytometry with an anti-CD34 antibody (Becton Dickinson) and was above 80%. Cells were cultured in Iscove’s modified Dulbecco’s medium (IMDM), supplemented with 20% fetal bovine serum, 10 U/mL penicillin, 1.0 μg/mL streptomycin, and 0.24 mM L-asparagine, 0.55 mM L-arginine, 1.5 mM L-glutamine in the presence of recombinant human stem cell factor and FLT3 ligand (SCF and FLT3L, respectively, both at 25 ng/mL). Lentiviral particles were produced with the VSV-G envelope protein and concentrated using Centricon Plus-70 (Millipore).15 One day after isolation, 5×105 CD34+ cells were re-suspended in 0.5 mL of growth medium and incubated for 24 h with lentiviral particles (0.3 mL) and polybrene (8 μg/mL). The cells were then washed and re-suspended in fresh growth medium (0.5 mL) with new viral supernatant (0.3 mL) and polybrene. Cells were centrifuged at 400 x g for 2 h, washed and cultured as described above. After 24 h, transduced cells were seeded at 30000 cells/mL in two different conditions: in the absence of cytokines or in the presence of SCF (20 ng/mL) and FLT3L (20 ng/mL), thrombopoietin (20 ng/mL) and IL6 (1 ng/mL).
Statistics
Experiments were repeated at least three times with identical results. In most figures, the average of multiple replicate experiments is shown with the standard error of the mean (SEM), unless otherwise stated. Statistical analysis was performed using the Student’s t-test (*P<0.05; **P<0.01; ***P<0.001).
Results
Role of JAK2 in KANK1-PDGFRβ-induced STAT5 activation and cell proliferation
Wild-type PDGF receptors are capable of activating JAK2 in several cell types,19–21 raising the possibility that JAK2 plays a role in hematopoietic cell transformation by KPβ. We first analyzed the phosphorylation of JAK2 by western blot in Ba/F3 cells expressing KPβ, which proliferate in the absence of IL3.15 As a positive control, we used cells treated with IL3 or expressing JAK2-V617F. Figure 1A clearly shows that JAK2 phosphorylation was not detectable in cells expressing KPβ. We next measured the proliferation rate of these cells in the presence of imatinib or JAK inhibitor I, which selectively blocks JAK kinases (Figure 1B). Ba/F3-KPβ proliferation was blunted by imatinib, as previously shown,15 but was not affected by JAK inhibitor I. By contrast, this inhibitor blocked proliferation driven by JAK2-V617F or IL3, which was not affected by imatinib.
We have shown that KPβ induces the activation of STAT5, which plays an important role in Ba/F3 proliferation.15,27 KPβ-induced STAT5 phosphorylation was measured by flow cytometry and was not affected by JAK inhibitor I, in line with the cell proliferation results (Figure 1C). To confirm these observations, we analyzed the activation of STAT by KPβ using luciferase reporter constructs in γ2A cells, which do not express JAK2.25 KPβ was capable of stimulating the activity of two promoters containing STAT responsive elements, namely GRR5 and LHRE (Figure 1D and 1E, respectively). The GRR5 promoter can bind multiple STAT while LHRE is more specific for STAT5.25,27 Wild-type JAK2 transfection did not modify the response to KPβ. By contrast, erythropoietin stimulated STAT activity only if JAK2 was reintroduced by co-transfection, in agreement with previously published results.25 Altogether these observations show that JAK2 is not involved in STAT activation and cell transformation by KPβ. Since JAK inhibitor I also targets other JAK kinases, these are unlikely to play a role in the autonomous signaling by KPβ in Ba/F3-KPβ. SRC is another tyrosine kinase that is activated by PDGF receptors and is capable of activating STAT proteins. However, a specific inhibitor of SRC family kinases had no impact on STAT5 phosphorylation (Online Supplementary Figure S1). The PDGFR kinase domain of KPβ may directly phosphorylate STAT5, as described for the wild-type receptor in other cell types.32
Signaling pathways activated by KPβ
We next compared the signaling pathways activated by KPβ, TEL-PDGFRβ and JAK2-V617F by western blot in Ba/F3 cells stably expressing these oncogenes. As expected, the three oncogenes induced the phosphorylation of STAT5 and ERK1/2 (Figure 2A). KPβ and TEL-PDGFRβ, unlike JAK2-V617F, also induced strong phosphorylation of phospholipase Cγ (Figure 2A). Specific kinase inhibitors were used to demonstrate the specificity of the signal. In conclusion, KPβ was more comparable to TEL-PDGFRβ than to JAK2-V617F in terms of signaling. Activation of ERK1/2 and phospholipase Cγ by KPβ was consistent with signal transduction by wild-type PDGF receptors.33 To test the importance of ERK signaling in cell proliferation, we used two different MEK inhibitors, namely U0126 and PD98059, which block ERK1 and ERK2 activation.28,34 Both inhibitors abolished proliferation of the Ba/F3 cell lines (Figure 2B). Using a similar approach, we did not find any convincing evidence for a role of the phospholipase C pathway in Ba/F3-KPβ cell proliferation (data not shown). Further studies are needed to determine whether PLCγ contributes to KPβ–induced signaling.
Identification of KANK1 domains required for Ba/F3-KPβ cell proliferation
To further characterize the mechanism of activation of KPβ, we constructed mutants with progressive deletions of the N-terminal KANK1 part, which includes multiple coiled-coil (CC) domains (Figure 3A). Mutants were introduced in Ba/F3 cells using lentiviral particles and their expression was tested by western blot (Figure 3C). Deletion of the first CC domain (m1 mutant) increased the protein expression significantly. However, at the mRNA level measured by quantitative PCR, m1 expression was not increased compared to KPβ (data not shown). The difference in protein expression between KPβ and m1 was partially compensated for by treating KPβ cells with the proteasome inhibitor MG132, pointing to an increased protein stability of the mutants compared to KPβ (data not shown).
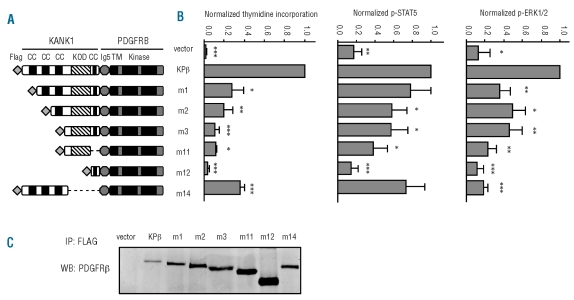
Figure 3.
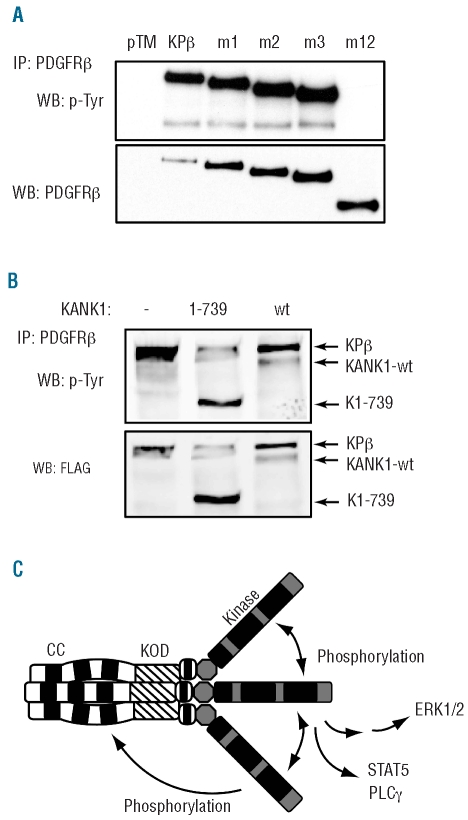
KPβ coiled coils play an important role in Ba/F3 proliferation and signaling. (A) A schematic representation of KPβ and mutants is shown. Ba/F3 cells were transduced with KPβ, one of the mutants, or the empty vector as control. CC: coiled-coil domain; KOD: KANK1 oligomerization domain; Ig5: Ig-like domain 5 of PDGFRβ; TM: transmembrane domain; Kinase: split kinase domain. (B) Cells were grown for 72 h in the absence of IL3 and proliferation was measured by [3H]thymidine incorporation. Ba/F3-KPβ cells were used as a reference. All cell lines proliferated to a similar extent in the presence of IL3 (data not shown). To quantify STAT5 and ERK1/2 phosphorylation, transduced cells were washed and cultured for 4 h without IL3. Cells were permeabilized, stained with antibodies directed against phospho-STAT5 or phospho–ERK1/2 and analyzed by flow cytometry. (C) Cell lysates were immunoprecipitated overnight with 3.3 μg of FLAG antibody at 4°C to capture KPβ or mutant proteins. Antibody complexes were collected by adding protein-A/G magnetic beads for 1 h at 4°C, washed extensively and analyzed by western blot with anti-PDGFR antibodies.
When long-term proliferation in the absence of IL3 was assessed, only m1 and, to a lesser extent, m2, but not m3 or m12, were able to sustain Ba/F3 cell growth and produce an autonomous cell line in a reproducible manner, indicating that the presence of CC is mandatory for cell transformation. To further compare the activity of all the mutants, we measured thymidine incorporation a few hours after removing IL3. Deletion of one, two or three CC domains progressively decreased Ba/F3 proliferation in the absence of IL3, even though these mutants were expressed at a higher level. A weak but significant proliferation was still detectable with cells expressing the m3 mutant, which lacks the 342 amino acids encoding the CC domains. Deletion of 641 amino acids (m12 mutant) abolished Ba/F3 cell growth (Figure 3B). This inactive mutant retained the fourth CC domain, which is thus unable to activate KPβ. Finally, deletion of the first 99 amino acids located before the CC (m8 mutant) or PDGFRβ Ig5 domain (m13 mutant) did not affect the growth of Ba/F3-KPβ (Online Supplementary Figure S2 and15). Altogether these results pointed to two important KANK1 regions in KPβ: the three first CC and the domain located after (amino acids 343-641). We constructed two additional mutants, m11 and m14, in which only one of these two regions of KANK1 was present. Both mutations decreased proliferation significantly (Figure 3B).
The phosphorylation of STAT5 and ERK1/2, measured by flow cytometry, was significantly decreased when CC were progressively deleted (m2 and m3 mutants), and reached the background level in cells expressing the m12 mutant (Figure 3B). ERK phosphorylation was more affected than STAT5 phosphorylation. Collectively these results show that multiple domains are required for cell transformation and signaling induced by KPβ.
Coiled coils are not essential for KPβ oligomerization and autophosphorylation
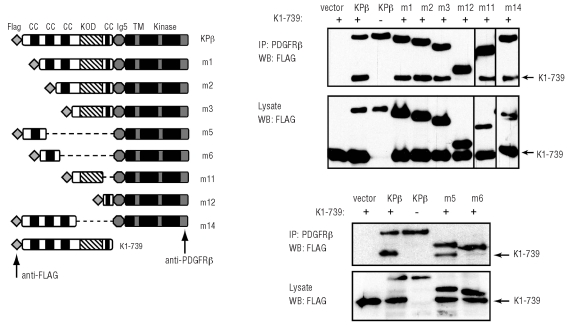
Based on the study of TEL-PDGFRβ, it is usually assumed that oligomerization domains, such as CC, found in the partner fused to PDGFR are implicated in the self-assembly of hybrid molecules. In addition, the CC domains of wild-type KANK1 were suggested to promote KANK1 dimerization (unpublished data from the study by Kakinuma et al.16). This prompted us to investigate whether KPβ forms oligomers though its CC domains. We tested the ability of KPβ and mutants to bind the first 739 residues of KANK1 (K1-739) by co-immunoprecipitation after transfection in 293T cells (Figure 4). As expected if the KANK1 part of the hybrid mediates oligomerization, KPβ was able to bind to K1-739. Surprisingly, the deletion of the three N-terminal CC domains of KPβ did not impair the association with K1-739. Thus the decreased transformation activity of the mutants lacking these CC domains cannot be explained by a defect in oligomerization. Further deletion (m12 mutant) abolished the interaction of mutant KPβ with the K1-739 fragment. Analysis of additional mutants suggested that the first CC alone (m5), but not the second one (m6), was able to induce KPβ oligomerization (Figure 4). These two mutants did not support IL3-independent Ba/F3 proliferation (Online Supplementary Figure S2) or STAT5 phosphorylation (data not shown). Collectively these observations suggested that several domains promote the oligomerization of KPβ, including the first CC domain and another sequence located between residues 343 to 641 of KANK1. This sequence is partially conserved in Danio rerio KANK1, but does not share homology with any other protein or with the other ankyrin repeat family members KANK2, KANK3 or KANK4. We will refer to this conserved region as KANK1 oligomerization domain (KOD, see hatched regions in Figures 3 and 4). By contrast to CC, most of the KOD sequence is predicted to adopt a β-sheet structure.
Figure 4.
Coiled coils are not required for KPβ oligomerization. Regions of KANK1 implicated in KPβ multimerization were identified by their ability to bind to K1-739, after transfection in HEK-293T cells. KPβ or mutants were immunoprecipitated with an anti-PDGFRβ antibody, which recognizes an epitope indicated by the arrow. Co-immunoprecipitated KPβ and K1-739 were visualized by western blot with anti-FLAG antibodies. Only m6 and m12 did not co-precipitate with K1-739. Non-relevant lanes were removed for clarity.
To confirm these results, we performed protein cross-linking experiments with lysates of Ba/F3 cells expressing KPβ.9 Addition of the BS3 cross-linker induced high molecular weight oligomeric KPβ complexes, while the monomeric form of KPβ disappeared (Online Supplementary Figure S3B). The size of the bands compared to standards was consistent with homotrimeric KPβ complexes and heavier polymers, but no dimer. The K1-739 KANK1 fragment alone also formed trimers and oligomers after cross-linking, confirming that oligomerization is driven by the KANK1 part of the fusion. Similar results were obtained with mutants lacking N-terminal CC domains. Mutant m11, which lacks all four CC domains and retains only the KOD domain fused to PDGFRβ was still efficiently cross-linked. By contrast, the combined deletions of the first CC domain and the KOD domain (m12) prevented efficient cross-linking of monomers, the amount of which was unchanged even at high BS3 concentrations (Online Supplementary Figure S3C). Nevertheless, a small proportion of KPβ m12 was still in the form of high molecular weight complexes. This may represent residual oligomerization, background protein cross-linking and/or interaction with other proteins.
Ligand-induced dimerization of wild-type PDGF receptors activates the kinase domain and induces the receptor autophosphorylation on tyrosines. We have shown that KPβ is constitutively phosphorylated on tyrosines in a process that is highly sensitive to imatinib.15 To determine which KANK1 sequences are required for autophosphorylation, we performed immunoprecipitation followed by immunoblotting with anti-phosphotyrosine antibodies. Figure 5A shows that KPβ and mutants m1, m2 and m3 were strongly phosphorylated, in contrast to mutant m12, in agreement with the oligomerization data. However, when the total amount of each mutant KPβ protein was taken into account, the normalized tyrosine phosphorylation was decreased by about 60% in m1, m2 and m3 (see figure legend for details), suggesting that the kinase activity of these mutants was lowered, which was compensated by their higher expression level compared to KPβ.
Figure 5.
The KANK1 part of KPβ is required for autophosphorylation and provides additional phosphorylated tyrosine residues. (A) Lysates of Ba/F3 cells expressing the indicated mutant were immunoprecipitated with anti-PDGFRβ antibodies and analyzed by western blot with anti-phosphotyrosine or anti-PDGFRβ antibodies. Alternatively, blots were analyzed using quantitative Odyssey technology, which indicated that normalized PDGFRβ phosphorylation (calculated as the phosphotyrosine to PDGFRβ signal ratio) was decreased by 64% in m1, 65% in m2 and 58% in m3 compared to KPβ. (B) HEK-293T cells were transfected with KPβ together with KANK1, K1-739 or an empty vector. Cells were treated for 4 h with the proteasome inhibitor MG132 (20 μM) to increase KANK1 expression level. KPβ was immunoprecipitated with anti-PDGFRβ antibodies and blotted sequentially with anti-phosphotyrosine and anti-FLAG antibodies. (C) Model for KPβ oligomerization, phosphorylation and signaling. See Figure 3 for abbreviations.
Interestingly, when KPβ was co-expressed with KANK1 wild-type or the K1-739 fragment, both proteins were also found phosphorylated on tyrosine residues (Figure 5B). This indicates that the PDGFR kinase domain is able to phosphorylate sites in the KANK1 part of KPβ, creating new docking sites for signaling proteins, in addition to the 11 characterized phosphorylated sites of PDGFRβ.
KPβ induces the proliferation of human hematopoietic progenitor cells
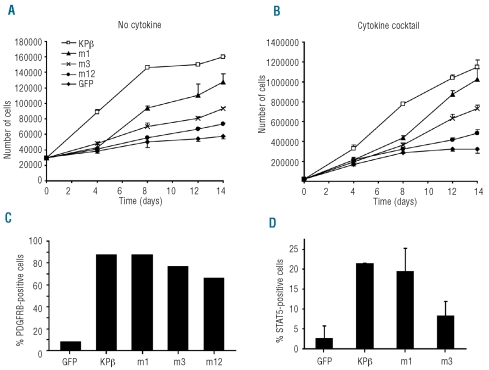
To confirm our results in a more physiological model, we introduced KPβ into human CD34+ cells isolated from cord blood. This population of cells is highly enriched in hematopoietic progenitors and stem cells, which are the origin of myeloproliferative disorders. We observed that cells transduced with KPβ proliferated in the absence of cytokines, while cells infected with control lentivirus did not (Figure 6A). Cell proliferation reached a plateau and stopped after about 2 weeks, indicating that cells were not immortalized. In the presence of an optimal growth factor cocktail (i.e. thrombopoietin, SCF, FLT3L and IL6), KPβ expression further enhanced cell growth (Figure 6B). We introduced the KPβ mutants into CD34+ cells and controlled their expression by flow cytometry on permeabilized cells with an anti-PDGFRβ antibody (Figure 6C). In line with the results obtained in Ba/F3 cells, the deletion of CC domains decreased cell proliferation significantly. KPβ also activated STAT5 in these cells (Figure 6D), which was much reduced by deletion of the CC domains. These results confirmed the importance of the CC domains for KPβ activity in hematopoietic cells. A summary of our results is presented in Online Supplementary Figure S4.
Figure 6.
KPβ stimulates the proliferation of CD34+ hematopoietic progenitor cells. CD34+ human hematopoietic cells were isolated from cord blood and transduced with lentiviral particles encoding the indicated KPβ construct or GFP. Cells were grown for 14 days in the absence of cytokines (A) or in the presence of a cytokine cocktail containing thrombopoietin, stem cell factor, FLT3-ligand and interleukin-6 (B). Viable cells were counted in the presence of trypan blue. One representative experiment is shown. At the end of the experiment, cells that had been cultured without cytokines were permeabilized and stained with anti-PDGFRβ (C) or anti-phospho-STAT5 (D) antibodies and analyzed by flow cytometry.
Discussion
The unique association of KANK1-PDGFRβ with thrombocythemia prompted us to test whether this hybrid oncoprotein could share signal transduction pathways with JAK2-V617F. We showed that KPβ does not signal via JAK2, unlike wild-type PDGFR, which can activate JAK2 in various types of cells.19–21 Similar results were published regarding cells expressing TEL-PDGFRβ.35 Like JAK2-V617F and TEL-PDGFRβ, KPβ activated ERK1/2 and STAT5. The role of STAT5 in myeloproliferative neoplasms is well established. This factor is activated downstream of all mutated kinases found in myeloproliferative neoplasms so far. The ERK pathway is an important mediator of cell transformation by BCR-ABL and was recently suggested to play a role in myeloproliferative neoplasm downstream mutant thrombopoietin receptors.36 Even though TEL-PDGFRβ and KPβ have been associated with different myeloproliferative neoplasms, we did not identify any difference in signaling by these two oncogenes.
Our data demonstrate that KPβ forms oligomers through interactions between various domains of the KANK1 part of the fusion protein, like most oncogenic fusion kinases. The cross-linking analysis suggested the existence of KPβ homotrimers and polymers (Figure 5C). This is also supported by the observation that, although K1-739 binds to KPβ, its over-expression did not reduce KPβ phosphorylation on tyrosines, as would be expected if KPβ were a dimer (data not shown). Wild-type KANK1 is likely to form trimers as well, like K1-739. This may have implications for the physiological function of KANK1 in actin regulation.
CC domains are present in most hybrid PDGFR and are generally thought to induce oligomerization. Although the first CC domain could mediate KPβ oligomerization, these domains were not absolutely essential because the KOD domain, located after the CC domains, could also do it. Thus the coiled coils and the KOD appeared to be redundant for oligomerization. All mutants that were able to form oligomers were phosphorylated on tyrosines. However, the normalized tyrosine phosphorylation indicated that the mutant polypeptides were less phosphorylated. Although this is likely to reflect decreased kinase activity of the mutants, it is also possible that the phosphorylation of specific PDGFRβ sites is affected by the mutations. Based on this potentially reduced kinase activity and on the reduced signaling efficiency of the mutants, we hypothesize that multiple oligomerization domains (CC and KOD) are required to generate the optimal active conformation of KPβ. This is reminiscent of the role of the transmembrane domain of TEL-PDGFRβ, which is required for signaling and cell transformation but not for oligomerization and autophosphorylation.9 In that case, we suggest that the transmembrane domain is required to adopt a conformation that is competent for signaling. In line with this hypothesis, wild-type PDGF receptor dimerization is not sufficient to switch on the kinase domains, which must be properly oriented to activate each other.37
In addition, it is also possible that the KANK1 part of KPβ plays a direct role in signaling. KANK1 CC domains were shown to bind to IRSp53,38 and could recruit other signaling proteins that help STAT5 or ERK activation. Phosphorylated tyrosine residues of the KANK1 part of KPβ may also act as docking sites for signaling mediators containing SH2 domains, since we showed that the PDGFR kinase domain can phosphorylate wild-type KANK1 and the K1-739 fragment on tyrosine residues. Analysis of the KANK1 sequence revealed one potential binding site for STAT5 (DNY612LV), compared to other known STAT5 recruitment sites.26,39 However, the KPβ-Y612A mutation did not affect STAT5 phosphorylation or growth stimulation (Online Supplementary Data, unpublished data). Ten other tyrosines are located in the KANK1 part of KPβ.
The subcellular localization of mutated tyrosine kinases is an important determinant of their oncogenicity.5 We confirmed that KPβ is located in the cytosol of transfected cells (data not shown), like wild-type KANK1 and TEL-PDGFRβ.9,16 However, we did not rule out the possibility that KPβ associates with a particular cytosolic structure, which could regulate signaling.
In conclusion, our results show that multiple oligomerization domains of KPβ are required for signaling and hematopoietic cell proliferation. Deciphering how these domains are organized in KPβ will require further structural studies. These data also imply that complex structural constraints dictate whether a given chromosomal breakpoint will produce an active hybrid kinase.
Acknowledgements
The authors would like to thank André Tonon for expertise in flow cytometry, Alain Buisseret and the members of the MEXP unit for their constant support, and the cord blood bank team of UZ Leuven.
Footnotes
Funding: this work was supported by grants from the Salus Sanguinis Foundation, from “Action de Recherches Concertées” (Communauté Française de Belgique, Belgium), from the Interuniversity Attraction Pole Program BCHM61B5, from Atlantic Philanthropies, New York and from the FRS-FNRS, Belgium. SM was the recipient of a fellowship from the Maurange Foundation (managed by the Roi Baudouin Foundation) and LAN is supported by a scholarship from Opération Télévie. RIA was supported by the Marie Curie ReceptEUR Network PhD fellowship.
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.James C, Ugo V, Le Couedic JP, Staerk J, Delhommeau F, Lacout C, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434(7037):1144–8. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- 2.Staerk J, Kallin A, Royer Y, Diaconu CC, Dusa A, Demoulin JB, et al. JAK2, the JAK2 V617F mutant and cytokine receptors. Pathol Biol (Paris) 2007;55(2):88–91. doi: 10.1016/j.patbio.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352(17):1779–90. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- 4.Pikman Y, Lee BH, Mercher T, McDowell E, Ebert BL, Gozo M, et al. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med. 2006;3(7):e270. doi: 10.1371/journal.pmed.0030270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toffalini F, Demoulin JB. New insights into the mechanisms of hematopoietic cell transformation by activated receptor tyrosine kinases. Blood. 2010;116(14):2429–37. doi: 10.1182/blood-2010-04-279752. [DOI] [PubMed] [Google Scholar]
- 6.David M, Cross NC, Burgstaller S, Chase A, Curtis C, Dang R, et al. Durable responses to imatinib in patients with PDGFRB fusion gene-positive and BCR-ABL-negative chronic myeloproliferative disorders. Blood. 2007;109(1):61–4. doi: 10.1182/blood-2006-05-024828. [DOI] [PubMed] [Google Scholar]
- 7.Hitoshi Y, Lin N, Payan DG, Markovtsov V. The current status and the future of JAK2 inhibitors for the treatment of myeloproliferative diseases. Int J Hematol. 2010;91(2):189–200. doi: 10.1007/s12185-010-0531-y. [DOI] [PubMed] [Google Scholar]
- 8.Toffalini F, Kallin A, Vandenberghe P, Pierre P, Michaux L, Cools J, et al. The fusion proteins TEL-PDGFRbeta and FIP1L1-PDGFRalpha escape ubiquitination and degradation. Haematologica. 2009;94(8):1085–93. doi: 10.3324/haematol.2008.001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toffalini F, Hellberg C, Demoulin JB. Critical role of the platelet-derived growth factor receptor (PDGFR)-beta transmembrane domain in the TEL-PDGFRbeta cytosolic oncoprotein. J Biol Chem. 2010;285(16):12268–78. doi: 10.1074/jbc.M109.076638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cain JA, Xiang Z, O’Neal J, Kreisel F, Colson A, Luo H, et al. Myeloproliferative disease induced by TEL-PDGFRB displays dynamic range sensitivity to Stat5 gene dosage. Blood. 2007;109(9):3906–14. doi: 10.1182/blood-2006-07-036335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ross TS, Gilliland DG. Transforming properties of the Huntingtin interacting protein 1/platelet-derived growth factor beta receptor fusion protein. J Biol Chem. 1999;274(32):22328–36. doi: 10.1074/jbc.274.32.22328. [DOI] [PubMed] [Google Scholar]
- 12.Schwaller J, Anastasiadou E, Cain D, Kutok J, Wojiski S, Williams IR, et al. H4(D10S170), a gene frequently rearranged in papillary thyroid carcinoma, is fused to the platelet-derived growth factor receptor beta gene in atypical chronic myeloid leukemia with t(5;10)(q33;q22) Blood. 2001;97(12):3910–8. doi: 10.1182/blood.v97.12.3910. [DOI] [PubMed] [Google Scholar]
- 13.Ren R. Mechanisms of BCR-ABL in the pathogenesis of chronic myelogenous leukaemia. Nat Rev Cancer. 2005;5(3):172–83. doi: 10.1038/nrc1567. [DOI] [PubMed] [Google Scholar]
- 14.Smith KM, Yacobi R, Van Etten RA. Autoinhibition of Bcr-Abl through its SH3 domain. Mol Cell. 2003;12(1):27–37. doi: 10.1016/S1097-2765(03)00274-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Medves S, Duhoux FP, Ferrant A, Toffalini F, Ameye G, Libouton JM, et al. KANK1, a candidate tumor suppressor gene, is fused to PDGFRB in an imatinib-responsive myeloid neoplasm with severe thrombocythemia. Leukemia. 2010;24(5):1052–5. doi: 10.1038/leu.2010.13. [DOI] [PubMed] [Google Scholar]
- 16.Kakinuma N, Zhu Y, Wang Y, Roy BC, Kiyama R. Kank proteins: structure, functions and diseases. Cell Mol Life Sci. 2009;66(16):2651–9. doi: 10.1007/s00018-009-0038-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roy BC, Aoyagi T, Sarkar S, Nomura K, Kanda H, Iwaya K, et al. Pathological characterization of Kank in renal cell carcinoma. Exp Mol Pathol. 2005;78(1):41–8. doi: 10.1016/j.yexmp.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 18.Lerer I, Sagi M, Meiner V, Cohen T, Zlotogora J, Abeliovich D. Deletion of the ANKRD15 gene at 9p24.3 causes parent-of-origin-dependent inheritance of familial cerebral palsy. Hum Mol Genet. 2005;14(24):3911–20. doi: 10.1093/hmg/ddi415. [DOI] [PubMed] [Google Scholar]
- 19.Vignais ML, Sadowski HB, Watling D, Rogers NC, Gilman M. Platelet-derived growth factor induces phosphorylation of multiple JAK family kinases and STAT proteins. Mol Cell Biol. 1996;16(4):1759–69. doi: 10.1128/mcb.16.4.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neeli I, Liu Z, Dronadula N, Ma ZA, Rao GN. An essential role of the Jak-2/STAT-3/cytosolic phospholipase A(2) axis in platelet-derived growth factor BB-induced vascular smooth muscle cell motility. J Biol Chem. 2004;279(44):46122–8. doi: 10.1074/jbc.M406922200. [DOI] [PubMed] [Google Scholar]
- 21.Masamune A, Satoh M, Kikuta K, Suzuki N, Shimosegawa T. Activation of JAK-STAT pathway is required for platelet-derived growth factor-induced proliferation of pancreatic stellate cells. World J Gastroenterol. 2005;11(22):3385–91. doi: 10.3748/wjg.v11.i22.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Demoulin JB, Seo JK, Ekman S, Grapengiesser E, Hellman U, Ronnstrand L, et al. Ligand-induced recruitment of Na+/H+-exchanger regulatory factor to the PDGF (platelet-derived growth factor) receptor regulates actin cytoskeleton reorganization by PDGF. Biochem J. 2003;376(Pt 2):505–10. doi: 10.1042/BJ20030385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Onishi Y, Kakinuma N, Roy BC, Aoyagi T, Kiyama R. Alternative splicing of the human Kank gene produces two types of Kank protein. Biochem Biophys Res Commun. 2005;330(4):1247–53. doi: 10.1016/j.bbrc.2005.03.106. [DOI] [PubMed] [Google Scholar]
- 24.Staerk J, Kallin A, Demoulin JB, Vainchenker W, Constantinescu SN. JAK1 and Tyk2 activation by the homologous polycythemia vera JAK2 V617F mutation: cross-talk with IGF1 receptor. J Biol Chem. 2005;280(51):41893–9. doi: 10.1074/jbc.C500358200. [DOI] [PubMed] [Google Scholar]
- 25.Dusa A, Mouton C, Pecquet C, Herman M, Constantinescu SN. JAK2 V617F constitutive activation requires JH2 residue F595: a pseudokinase domain target for specific inhibitors. PLoS One. 2010;5(6):e11157. doi: 10.1371/journal.pone.0011157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Demoulin JB, Van Roost E, Stevens M, Groner B, Renauld JC. Distinct roles for STAT1, STAT3, and STAT5 in differentiation gene induction and apoptosis inhibition by interleukin-9. J Biol Chem. 1999;274(36):25855–61. doi: 10.1074/jbc.274.36.25855. [DOI] [PubMed] [Google Scholar]
- 27.Demoulin JB, Uyttenhove C, Lejeune D, Mui A, Groner B, Renauld JC. STAT5 activation is required for interleukin-9-dependent growth and transformation of lymphoid cells. Cancer Res. 2000;60(14):3971–7. [PubMed] [Google Scholar]
- 28.Demoulin JB, Ericsson J, Kallin A, Rorsman C, Ronnstrand L, Heldin CH. Platelet-derived growth factor stimulates membrane lipid synthesis through activation of phosphatidylinositol 3-kinase and sterol regulatory element-binding proteins. J Biol Chem. 2004;279(34):35392–402. doi: 10.1074/jbc.M405924200. [DOI] [PubMed] [Google Scholar]
- 29.Essaghir A, Dif N, Marbehant CY, Coffer PJ, Demoulin JB. The transcription of FOXO genes is stimulated by FOXO3 and repressed by growth factors. J Biol Chem. 2009;284(16):10334–42. doi: 10.1074/jbc.M808848200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kallin A, Johannessen LE, Cani PD, Marbehant CY, Essaghir A, Foufelle F, et al. SREBP-1 regulates the expression of heme oxygenase 1 and the phosphatidylinositol-3 kinase regulatory subunit p55 gamma. J Lipid Res. 2007;48(7):1628–36. doi: 10.1194/jlr.M700136-JLR200. [DOI] [PubMed] [Google Scholar]
- 31.Classen JF, Henrohn D, Rorsman F, Lennartsson J, Lauwerys BR, Wikstrom G, et al. Lack of evidence of stimulatory autoantibodies to platelet-derived growth factor receptor in patients with systemic sclerosis. Arthritis Rheum. 2009;60(4):1137–44. doi: 10.1002/art.24381. [DOI] [PubMed] [Google Scholar]
- 32.Paukku K, Valgeirsdottir S, Saharinen P, Bergman M, Heldin CH, Silvennoinen O. Platelet-derived growth factor (PDGF)-induced activation of signal transducer and activator of transcription (Stat) 5 is mediated by PDGF beta-receptor and is not dependent on c-src, fyn, jak1 or jak2 kinases. Biochem J. 2000;345(Pt 3):759–66. [PMC free article] [PubMed] [Google Scholar]
- 33.Heldin CH, Östman A, Rönnstrand L. Signal transduction via platelet-derived growth factor receptors. Biochim Biophys Acta. 1998;1378(1):F79–113. doi: 10.1016/s0304-419x(98)00015-8. [DOI] [PubMed] [Google Scholar]
- 34.Demoulin JB, Louahed J, Dumoutier L, Stevens M, Renauld JC. MAP kinase activation by interleukin-9 in lymphoid and mast cell lines. Oncogene. 2003;22(12):1763–70. doi: 10.1038/sj.onc.1206253. [DOI] [PubMed] [Google Scholar]
- 35.Wilbanks AM, Mahajan S, Frank DA, Druker BJ, Gilliland DG, Carroll M. TEL/PDGFbetaR fusion protein activates STAT1 and STAT5: a common mechanism for transformation by tyrosine kinase fusion proteins. Exp Hematol. 2000;28(5):584–93. doi: 10.1016/s0301-472x(00)00138-7. [DOI] [PubMed] [Google Scholar]
- 36.Pecquet C, Staerk J, Chaligne R, Goss V, Lee KA, Zhang X, et al. Induction of myeloproliferative disorder and myelofibrosis by thrombopoietin receptor W515 mutants is mediated by cytosolic tyrosine 112 of the receptor. Blood. 2010;115(5):1037–48. doi: 10.1182/blood-2008-10-183558. [DOI] [PubMed] [Google Scholar]
- 37.Yang Y, Yuzawa S, Schlessinger J. Contacts between membrane proximal regions of the PDGF receptor ectodomain are required for receptor activation but not for receptor dimerization. Proc Natl Acad Sci USA. 2008;105(22):7681–6. doi: 10.1073/pnas.0802896105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roy BC, Kakinuma N, Kiyama R. Kank attenuates actin remodeling by preventing interaction between IRSp53 and Rac1. J Cell Biol. 2009;184(2):253–67. doi: 10.1083/jcb.200805147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Demoulin JB, Uyttenhove C, Van Roost E, de Lestre B, Donckers D, Van Snick J, et al. A single tyrosine of the interleukin-9 (IL-9) receptor is required for STAT activation, antiapoptotic activity, and growth regulation by IL-9. Mol Cell Biol. 1996;16(9):4710–6. doi: 10.1128/mcb.16.9.4710. [DOI] [PMC free article] [PubMed] [Google Scholar]