Abstract
Background
Anemia is an established negative prognostic factor in myelodysplastic syndromes but the relationship between its degree and clinical outcome is poorly defined. We, therefore, studied the relationship between severity of anemia and outcome in myelodysplastic syndrome patients.
Design and Methods
We studied 840 consecutive patients diagnosed with myelodysplastic syndromes at the Fondazione IRCCS Policlinico San Matteo, Pavia, Italy, and 504 patients seen at the Heinrich-Heine-University Hospital, Düsseldorf, Germany. Hemoglobin levels were monitored longitudinally and analyzed by means of time-dependent Cox’s proportional hazards regression models.
Results
Hemoglobin levels lower than 9 g/dL in males (HR 5.56, P=0.018) and 8 g/dL in females (HR=5.35, P=0.026) were independently related to reduced overall survival, higher risk of non-leukemic death and cardiac death (P<0.001). Severe anemia, defined as hemoglobin below these thresholds, was found to be as effective as transfusion-dependency in the prognostic assessment. After integrating this definition of severe anemia into the WHO classification-based Prognostic Scoring System, time-dependent regression and landmark analyses showed that the refined model was able to identify risk groups with different survivals at any time during follow up.
Conclusions
Accounting for severity of anemia through the WHO classification-based Prognostic Scoring System provides an objective criterion for prognostic assessment and implementation of risk-adapted treatment strategies in myelodysplastic syndrome patients.
Keywords: anemia, myelodysplastic syndromes, outcome, WPSS
Introduction
Myelodysplastic syndromes (MDS) are heterogeneous myeloid neoplasms ranging from indolent conditions with a near-normal life expectancy to forms approaching acute myeloid leukemia (AML).1 The World Health Organization (WHO) classification of myeloid neoplasms2 represents a very useful tool for defining the different subtypes, and also provides prognostic information.3 Additional disease-related factors of considerable prognostic relevance include cytogenetic abnormalities,4 degree of bone marrow failure, i.e. number and severity of peripheral cytopenias, and bone marrow fibrosis.5
In order to evaluate the prognosis of the individual patient with MDS, several prognostic scoring systems have been developed. They include the International Prognostic Scoring System (IPSS),6 the WHO classification-based prognostic scoring system (WPSS),7 and the MD Anderson Prognostic Scoring System (MPSS).8 These prognostic models have advantages and limitations that we have analyzed elsewhere.9,10
The WPSS is able to classify patients into five risk groups showing different survival and probability of leukemic evolution, and can be applied at any time during follow up. Although it supports the evidence-based1 and also intuitive notion that the need for red cell transfusions predicts for a worse prognosis,11 the use of transfusion dependency has been criticized as being too subjective a criterion.12 The use of hemoglobin thresholds would clearly overcome this limitation, but the relationship between degree of anemia and clinical outcome in MDS is currently poorly defined. A re-analysis of the international MDS risk analysis workshop (IMRAW) indicated that hemoglobin levels lower than 10 g/dL had additive prognostic value to IPSS in the low-risk groups.13 However, studies of the Düsseldorf MDS registry14,15 had previously shown that hemoglobin values lower than or equal to 9 g/dL represented an independent negative prognostic factor.
In this work, we studied the relationship between degree of anemia and clinical outcome in MDS patients, and integrated the definition of severe anemia into a refined WPSS.
Design and Methods
These investigations were approved by the local ethics committees and the procedures followed were in accordance with the Helsinki Declaration of 1975, as revised in 2000.
Patients
We studied 840 consecutive patients diagnosed with MDS at the Fondazione IRCCS Policlinico San Matteo, Pavia, Italy, between 1992 and 2007 (learning cohort), and 504 patients seen at the Heinrich-Heine-University Hospital, Düsseldorf, Germany, between 1982 and 2006 (validation cohort). Until 2001, diagnosis of MDS was made according to the FAB criteria;16 in 2002 all cases were reclassified according to the 2001 WHO classification criteria.17 More recently, the 2008 updated WHO classification criteria2,18 have been retrospectively applied. The main clinical features of the two patient cohorts are reported in Table 1.
Table 1.
Clinical and hematologic characteristics of the Italian and German cohorts of MDS patients classified according to the 2008 WHO criteria.
In order to analyze the relationship between degree of anemia and outcome, variation in hemoglobin was monitored during the time course until the onset of red cell transfusion requirement. Decreases in hemoglobin were grouped in categories of 1 g/dL: from values above 13 g/dL to values below 8 g/dL for males, and from values above 12 g/dL to values below 8 g/dL for females. Transfusion-dependent patients were classified in the hemoglobin category according to their pre-transfusion hemoglobin value.
Red blood cell (RBC) transfusion-dependency was defined as the absence of an interval longer than 28 days free from RBC transfusion over a surveillance period of eight weeks: this represents a need for approximately at least one unit of RBC per month. Cardiac disease was defined according to Sorror et al.19 and included coronary artery disease (one or more vessel-coronary artery stenosis requiring medical treatment, stent, or bypass graft), congestive heart failure, myocardial infarction or ejection fraction lower than or equal to 50%.
Statistical analyses
Numerical variables were summarized by median and range; categorical variables were described with count and relative frequency (%) of subjects in each category. Comparison of numerical variables between groups was carried out using a non-parametric approach (Mann-Whitney test or Kruskall Wallis ANOVA). Comparison of the distribution of categorical variables in different groups was performed with either Fisher’s exact test (2x2 tables) or the χ2 test (larger tables).
Survival analyses were made with the Kaplan-Meier method. Overall survival (OS) was defined as the time (in months) between the date of diagnosis and the date of death (for cases) or last follow up (for censored patients). Leukemia-free survival (LFS) was defined as the time (in months) between the date of diagnosis and the date of leukemic transformation (for cases) or last follow up (for censored patients). When estimating the occurrence of non-leukemic death (NLD), only deaths for all causes except leukemic evolution were considered as events. Patients who underwent allogeneic transplantation or AML-like chemotherapy were censored at the time of the therapeutic procedure. When appropriate, clinical variables were analyzed as time-dependent risk factors.
Uni- and multivariable survival analyses with both fixed and time-dependent covariates were performed by means of Cox’s proportional hazards regression models. Likelihood ratio (LR) tests were carried out to compare nested models with different covariates and parameterizations (e.g. categorical vs. continuous), and to test for interaction between covariates. Cumulative hazard was estimated with the Aalen-Nelson method. In order to compare different statistical models, we used the Akaike information criterion (AIC).20 AIC allows evaluation of a model by combining goodness of fit and complexity; a lower AIC indicates a better trade-off between fit and complexity.
In order to illustrate the effect of time-dependent prognostic factors, landmark analyses at six, 12, 24, 36 and 60 months were performed.21 A landmark analysis consists of carrying out a survival analysis in which follow up is set to start some time (namely, the landmark time) after the initial time of entry in the study. This implies that only patients who have survived up to the landmark time point are included in the analysis. The time-dependent risk factors were evaluated at the landmark time point and analyzed as fixed covariates.
Analyses were performed using Statistica 7.0 (Statsoft Inc, Tulsa, OK, USA), and Stata 9 (StataCorp LP, College Station, TX, USA) software.
More detailed information about statistical methods is available at: http://www.statsoft.com/textbook/ and http://www.stata.com/support/faqs/stat/
Results
Relationship between degree of anemia, cardiac morbidity and mortality
In order to analyze the relationship between the degree of anemia and clinical outcome, we performed a univariable Cox’s survival analysis stratified by sex, including hemoglobin as time-dependent covariate. To this purpose, we longitudinally monitored the decrease in hemoglobin in patients with MDS, and grouped hemoglobin level in categories of 1 g/dL. A decrease in hemoglobin was associated with a progressive worsening of OS, and statistical significance was obtained starting from hemoglobin lower than 11 g/dL in males (HR from 5.23 to 15.56, P values from 0.029 to <0.001) and lower than 9 g/dL in females (HR from 6.26 to 11.82, P values from 0.013 to <0.001).
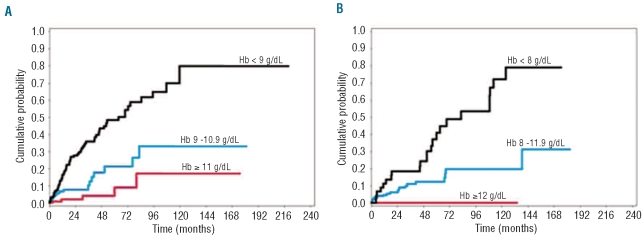
We then performed sex-specific multivariable Cox’s regression analyses with OS as outcome including age, hemoglobin categories, WHO subgroups, cytogenetic risk groups categorized according to the IPSS, number of cytopenias and cardiac disease as time-dependent covariates, and found a significantly higher risk of reduced OS for hemoglobin lower than 9 g/dL in males (HR 5.56, P=0.018) and lower than 8 g/dL in females (HR=5.35, P=0.026). These hemoglobin thresholds were also found to be associated with a significantly higher risk of NLD (HR 4.35, P<0.001) (Figure 1), and of progression to acute myeloid leukemia (AML) (HR 1.47, P=0.046).
Figure 1.
Prognostic relevance of the degree of anemia in patients with MDS. These curves were estimated from Cox’s regression analyses with time-dependent covariates in the learning cohort of MDS patients. (A) Probability of non-leukemic death according to the degree of anemia in males (hemoglobin categories that showed no significantly different probabilities of NLD were plotted together). (B) Probability of non-leukemic death according to the degree of anemia in females (hemoglobin categories that showed no significantly different probabilities of NLD were plotted together).
In order to further investigate the impact of anemia on outcome, we analyzed the relationship between hemoglobin levels and extra-hematologic comorbidity and causes of NLD. As previously reported, one or more comorbidities were present at diagnosis in 455 out of 840 (54%) patients.22 Cardiac disease was the most frequently observed comorbidity: it was observed in 211 of 840 patients (25%) at the time of diagnosis whereas it occurred during follow up in 79 patients. Cardiac disease included congestive heart failure (19%), coronary artery disease or myocardial infarction (8%), arrhythmia (7%), heart valve disease (2%). Non-leukemic death was observed in 141 patients and accounted for 54% of deaths. The main causes of NLD included cardiac failure (63%), infection (23%), hemorrhage (7%), and other causes (7%). Cardiac disease and death were significantly more frequent in patients with severe anemia, defined as a hemoglobin level lower than 9 g/dL in males or lower than 8 g/dL in females, compared to those with higher hemoglobin levels (IRR 1.70, P<0.001 and IRR 4.22, P<0.001, respectively). No significant association was found between severe anemia and death for infection and hemorrhage (P=0.27 and P=0.38, respectively).
Cox’s regression analyses were then performed including the sex-specific hemoglobin thresholds as a time-dependent covariate and death for cardiac disease, infection and hemorrhage as outcomes. We found that patients developing severe anemia at any time during their follow up had a significantly higher risk of cardiac death (HR 4.35, P<0.001). This result was confirmed in a multivariable Cox’s regression analysis adjusted for sex, and with age and cardiac comorbidity as time-dependent covariates (HR 3.62, P<0.001). No significant association was observed between severe anemia and the risk of death for infection or hemorrhage (P=0.68 and P=0.6, respectively).
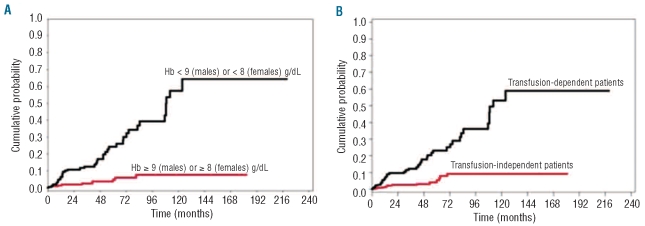
Finally, to further study the effect of severe anemia on cardiac morbidity and mortality, we focused on patients without cardiac comorbidity at diagnosis, and defined a Cox’s regression model on a combined outcome of either cardiac disease or cardiac death, with severe anemia as a time-dependent covariate. This analysis showed that patients with severe anemia had an increased risk of cardiac complications compared to those with higher hemoglobin levels (HR 3.85, P<0.001) (Figure 2A). The statistical significance of severe anemia was retained when adjusting the analysis for age and sex (HR 4.43, P<0.001).
Figure 2.
Relationship between severe anemia and cardiac disease. Data were generated in the learning cohort of MDS patients. (A) Probability of developing cardiac disease and death according to the degree of anemia. (B) Probability of developing cardiac disease and death according to the presence or absence of transfusion dependency as defined in this work (i.e. absence of a period free from transfusion longer than 28 days over a surveillance period of eight weeks).
Relationship between severe anemia, transfusion requirement, secondary iron overload, and cardiac morbidity and mortality
In order to evaluate the concordance in time between severe anemia and transfusion-dependency, we first calculated the time elapsed between the onset of severe anemia and the beginning of RBC transfusion therapy (median time 0, range 0–24 months). We then calculated the ratio between the person-time covered by transfusion therapy and the person-time of exposure to severe anemia, which was 0.92. Finally, in order to evaluate whether the time between the onset of severe anemia and the beginning of RBC transfusion therapy may affect the outcome, we performed Cox’s regression analyses on survival including the person-time as covariate. No significant effect of exposure to severe anemia without receiving transfusion was noticed (HR 1.3, P=0.15).
We then investigated the relationship between RBC transfusion-dependency and cardiac morbidity and mortality. Cardiac disease and death were significantly more frequent in transfusion-dependent patients compared to those who did not require regular RBC transfusion, with incidence relative ratios comparable to those observed in patients with and without severe anemia (IRR 1.35, P=0.018 and IRR 4.28, P<0.001, respectively). A multivariable Cox’s regression analysis on cardiac death, including transfusion-dependency, age, sex and cardiac disease as time-dependent or fixed covariates, showed that transfusion-dependent patients had a significantly higher risk of cardiac death compared to those without regular transfusion need (HR 4.12, P<0.001). In patients without cardiac disease at diagnosis, a multivariable Cox’s regression on the combined outcome of cardiac disease or death confirmed that transfusion-dependent patients had an increased risk of cardiac complications compared to those without regular transfusion need (HR 2.88, P<0.001) (Figure 2B).
Next we evaluated the impact of the intensity of transfusion requirement on cardiac disease and death in transfusion-dependent patients. The severity of transfusion requirement, calculated as the number of packed red cell (PRC) units per month, was significantly associated with cardiac complications (HR 1.82, P<0.001). When analyzed as a categorical covariate, a transfusion requirement higher than 3 PRC units per month carried a significantly higher risk of cardiac complications compared to a transfusion need lower than one PRC unit per month (P=0.023) (LR test for categorical vs. continuous covariate not significant). A relationship was also found between the total number of PRC units and the risk of cardiac disease or death (HR 1.30, P=0.007). When the number of PRC units was categorized, the risk of cardiac complications was found to be significantly higher for patients who had received 100 or more units compared to those who had received less than 20 units (P=0.009) (LR test for categorical vs. continuous covariate not significant).
Finally, we evaluated the effect of iron overload assessed by serum ferritin on survival, probability of NLD, and cardiac complications. Data on repeated measurements of serum ferritin were available in 227 transfusion-dependent patients. In a time-dependent Cox’s analysis, serum ferritin concentration significantly affected OS and risk of NLD (HR 1.34 and HR 1.51, respectively, for a 500 ng/mL increase in serum ferritin, P<0.001), and increased the risk of cardiac disease or death in patients with low and intermediate WPSS (HR 1.46, P=0.001). When analyzed as a categorical variable, serum ferritin values of more than 3,000 ng/mL involved a significantly higher risk of cardiac complications (P<0.001) (LR test for categorical vs. continuous variable not significant).
Integration of the definition of severe anemia into the WPSS
We first investigated whether the sex-specific hemoglobin thresholds identified as having a significant prognostic value in multivariable analysis, i.e. hemoglobin lower than 9 g/dL in males and lower than 8 g/dL in females, could be as effective as transfusion-dependency in the WPSS assessment of MDS patients. The unfavorable prognostic impact of the sex-specific hemoglobin thresholds was comparable to that of transfusion-dependency in both uni-variable (HR=3.96, P<0.001, and HR 4.09, P<0.001, respectively) and multivariable time-dependent Cox’s regression (HR=3.26, P<0.001, and HR=2.89, P<0.001, respectively). The substantial equivalence of these two models was confirmed by the Akaike information criterion (AIC 2967 vs. 2970, respectively). Based on these results, we recalculated the WPSS risk groups, using sex-specific hemoglobin thresholds instead of red cell transfusion requirement, and obtained highly concordant risk categories (Kendall tau coefficient 0.94). Moreover, results by both Cox’s regression and Kaplan-Meier time-dependent analyses were essentially indistinguishable.
The prognostic value of the modified WPSS was then tested in the validation cohort from the Heinrich-Heine-University Hospital, Düsseldorf, Germany. Multivariable analyses confirmed that the modified WPSS analyzed as a continuous variable retained a significant prognostic value with hazard ratios of comparable size to those obtained in the learning cohort and largely overlapping confidence intervals on both OS (validation cohort: HR 2.08, 95% CI 1.77–2.44; learning cohort: HR 2.28, 95% CI 1.99–2.63), and risk of AML (validation cohort: HR 2.76, 95% CI 1.99–3.84; learning cohort: HR 3.33, 95% CI 2.75–4.02).
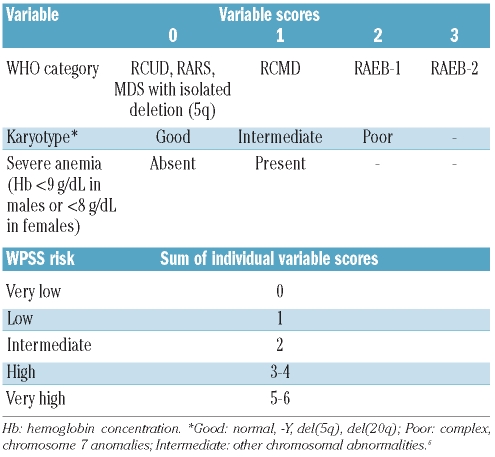
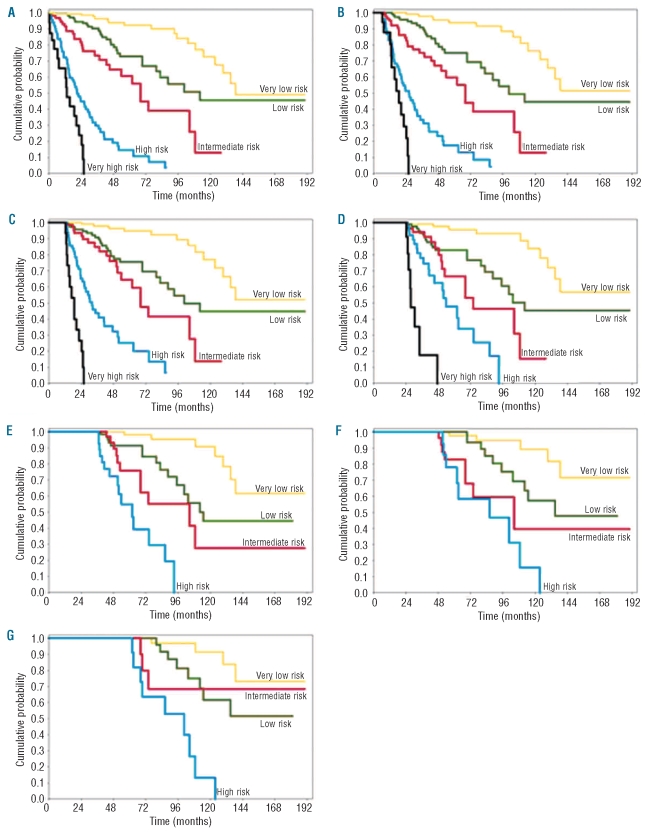
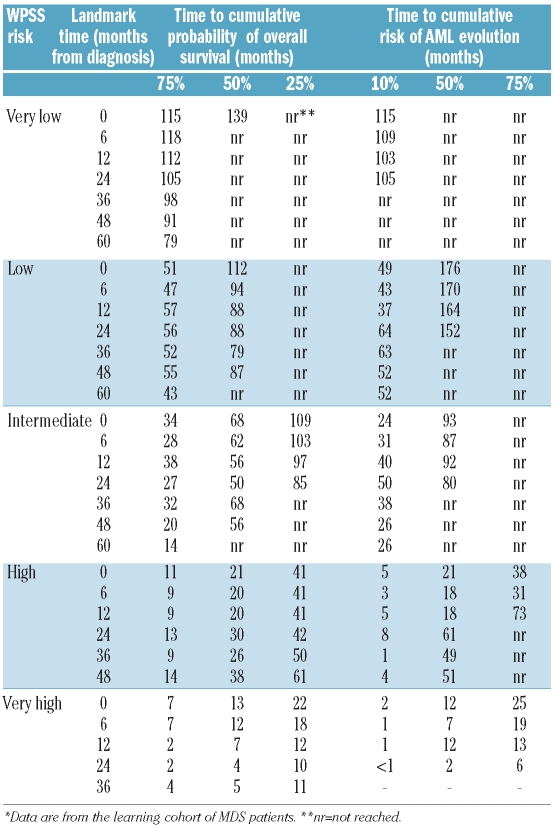
Based on the above analyses, we refined the original WPSS7 as reported in Table 2. As shown in Figure 3 and Table 3, a landmark analysis at fixed time points from the diagnosis (six, 12, 24, 36, 60 months) showed that the refined WPSS was able to identify five risk groups with clearly different survival.
Table 2.
WHO classification–based Prognostic Scoring System (WPSS)7 as refined by the findings on prognostic significance of the degree of anemia obtained in this work.
Figure 3.
Probability of overall survival according to the refined WPSS risk. Data were generated in the learning cohort of MDS patients. (A) Probability of overall survival of the refined WPSS risk groups at the time of diagnosis. (B-G) Landmark analyses at (B) six, (C) 12, (D) 24, (E) 36, (F) 48 and (G) 60 months from diagnosis.
Table 3.
Landmark analysis at fixed time points from diagnosis (six, 12, 24, 36 60 months) showing the probability of survival and of AML evolution of patients who have survived up to these time points, according to their current WPSS risk.*
Discussion
The working hypothesis that anemia, an MDS-related factor, may interact with cardiac disease, present as a comorbid condition in a significant portion of MDS patients, thus negatively influencing patient outcome, is supported by several observations. In a prospective population-based study of all 65 to 84-year old residents in a small Italian town, risks of mortality and hospitalization were significantly higher among mildly anemic elderly subjects compared with non-anemic subjects.23 Moreover, chronic heart failure is often associated with anemia of chronic disease,24 and the presence of persistent anemia confers these patients with the poorest survival.25 In a meta-analysis of more than 150,000 patients with chronic heart failure,26 anemia was present in one-third of the population studied and represented an independent risk prognosticator for mortality.
In this study we identified sex-specific hemoglobin thresholds that are independently associated with significant morbidity and mortality in MDS. In fact, male and female patients with hemoglobin lower than 9 and 8 g/dL, respectively, were found to be at higher risk of morbidity and mortality, mainly due to an increased risk of cardiac complications. Despite some discrepancy among studies in the reported prevalence, cardiac events have been identified as a major extra-hematologic comorbidity and cause of death in patients with MDS.27–32 Our finding confirms previous observations of the Düsseldorf registry14,15 and expands our previous finding that transfusion-dependency, an indicator of severe symptomatic anemia, is associated with an increased probability of cardiac death.3,7 We also found a significant interaction between severe anemia and cardiac disease on the risk of NLD, suggesting that cardiac comorbidity not only has an additive detrimental effect per se but also interacts with anemia in worsening the clinical course of the disease.22 In addition, high serum ferritin levels were associated with a significantly higher risk of NLD in transfusion-dependent patients belonging to the low and intermediate WPSS risk groups. In particular, patients with serum ferritin levels higher than 3,000 ng/mL were found to have a high risk of cardiac complications.
The WPSS accounts for WHO categories, cytogenetic abnormalities and red cell transfusion dependency. Although the prognostic value of this latter parameter was shown in both single center7 and population-based studies,30 and was also adopted by the US Food and Drug Administration for approval of drugs for treatment of low-risk MDS (i.e. lenalidomide and 5-azacytidine), the use of transfusion dependency has been considered as to be too subjective a criterion.12 The analysis performed in this study allowed us to identify hemoglobin thresholds that are independently associated with significant morbidity and mortality, and are as effective as transfusion dependency in the prognostic assessment of MDS patients. In addition, in this study we adopted a more stringent definition of transfusion-dependency with respect to our previous proposal,7 defining transfusion-dependency as no period free from transfusion longer than 28 days over a surveillance period of eight weeks. The concordance shown in this study between this definition of transfusion dependency and that of severe anemia (hemoglobin level lower than 9 g/dL in males and 8 g/dL in females) supports its adoption. More generally, the use of sex-specific hemoglobin thresholds in combination with this stringent definition of transfusion dependency in the WPSS now provides an objective criterion for accounting for severe anemia in MDS patients.
The other potential significant limitation of WPSS was considered to be multilineage dysplasia.12 We previously showed that, within MDS patients without excess of blasts, there was a significant difference in OS between those with unilineage dysplasia and those with multilineage dysplasia (median survival 108 vs. 49 months).3 The 2008 WHO classification of MDS18 has reaffirmed the prognostic relevance of distinguishing between unilineage dysplasia, mainly involving the erythroid lineage, and multilineage dysplasia. Therefore, risk assessment in MDS should consider this parameter irrespective of the fact that its definition requires considerable expertise.10
We and others have reported that treatment of anemia in MDS with erythropoietin in selected patients has a positive impact on outcome,33,34 suggesting that preventing or adequately treating symptomatic anemia in MDS may provide a survival benefit. The findings of this study suggest that once symptomatic anemia occurs in MDS patients with cardiac disease, optimal management is mandatory in order to limit the negative interaction between anemia and cardiac disease. If none of the medical treatments is effective, a transfusion regimen able to maintain pre-transfusion hemoglobin levels above 9 g/dL in males and above 8 g/dL in females should be adopted.
The negative impact of severe anemia on the outcome of patients with MDS should be carefully considered when planning delayed treatment strategies, such as allogeneic stem cell transplantation, in patients in the low WPSS risk groups.35,36 In fact, patients developing severe anemia have a significantly higher risk of also developing cardiac disease, the occurrence of which might compromise their eligibility for allogeneic transplantation.37
In conclusion, severe anemia should be considered as a major criterion for deciding type and timing of intervention in MDS patients.38 The refined WPSS illustrated in Table 2 allows clinicians to account for the severity of anemia and may, therefore, be used for implementing therapeutic strategies aimed to increase hemoglobin levels in MDS patients.
Footnotes
Funding: this work was supported by grants from the Associazione Italiana per la Ricerca sul Cancro (AIRC), the Fondazione Cariplo and the Regione Lombardia, Milan, Italy.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Cazzola M, Malcovati L. Myelodysplastic syndromes--coping with ineffective hematopoiesis. N Engl J Med. 2005;352(6):536–8. doi: 10.1056/NEJMp048266. [DOI] [PubMed] [Google Scholar]
- 2.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon: IARC; 2008. [Google Scholar]
- 3.Malcovati L, Porta MG, Pascutto C, Invernizzi R, Boni M, Travaglino E, et al. Prognostic factors and life expectancy in myelodysplastic syndromes classified according to WHO criteria: a basis for clinical decision making. J Clin Oncol. 2005;23(30):7594–603. doi: 10.1200/JCO.2005.01.7038. [DOI] [PubMed] [Google Scholar]
- 4.Haase D, Germing U, Schanz J, Pfeilstocker M, Nosslinger T, Hildebrandt B, et al. New insights into the prognostic impact of the karyotype in MDS and correlation with subtypes: evidence from a core dataset of 2124 patients. Blood. 2007;110(13):4385–95. doi: 10.1182/blood-2007-03-082404. [DOI] [PubMed] [Google Scholar]
- 5.Della Porta MG, Malcovati L, Boveri E, Travaglino E, Pietra D, Pascutto C, et al. Clinical relevance of bone marrow fibrosis and CD34-positive cell clusters in primary myelodysplastic syndromes. J Clin Oncol. 2009;27(5):754–62. doi: 10.1200/JCO.2008.18.2246. [DOI] [PubMed] [Google Scholar]
- 6.Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89(6):2079–88. [PubMed] [Google Scholar]
- 7.Malcovati L, Germing U, Kuendgen A, Della Porta MG, Pascutto C, Invernizzi R, et al. Time-dependent prognostic scoring system for predicting survival and leukemic evolution in myelodysplastic syndromes. J Clin Oncol. 2007;25(23):3503–10. doi: 10.1200/JCO.2006.08.5696. [DOI] [PubMed] [Google Scholar]
- 8.Kantarjian H, O’Brien S, Ravandi F, Cortes J, Shan J, Bennett JM, et al. Proposal for a new risk model in myelodysplastic syndrome that accounts for events not considered in the original International Prognostic Scoring System. Cancer. 2008;113(6):1351–61. doi: 10.1002/cncr.23697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cazzola M, Malcovati L. Prognostic classification and risk assessment in myelodys-plastic syndromes. Hematol Oncol Clin North Am. 2010;24(2):459–68. doi: 10.1016/j.hoc.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Cazzola M. Risk assessment in myelodys-plastic syndromes and myelodysplastic/myeloproliferative neoplasms. Haematologica. 2011;96(3):349–52. doi: 10.3324/haematol.2010.030023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stone RM. How I treat patients with myelodysplastic syndromes. Blood. 2009;113(25):6296–303. doi: 10.1182/blood-2008-09-038935. [DOI] [PubMed] [Google Scholar]
- 12.Bowen DT, Fenaux P, Hellstrom-Lindberg E, de Witte T. Time-dependent prognostic scoring system for myelodysplastic syndromes has significant limitations that may influence its reproducibility and practical application. J Clin Oncol. 2008;26(7):1180. doi: 10.1200/JCO.2007.15.2926. author reply 1–2. [DOI] [PubMed] [Google Scholar]
- 13.Kao JM, McMillan A, Greenberg PL. International MDS risk analysis workshop (IMRAW)/IPSS reanalyzed: impact of cytopenias on clinical outcomes in myelodysplastic syndromes. Am J Hematol. 2008;83(10):765–70. doi: 10.1002/ajh.21249. [DOI] [PubMed] [Google Scholar]
- 14.Aul C, Gattermann N, Heyll A, Germing U, Derigs G, Schneider W. Primary myelodys-plastic syndromes: analysis of prognostic factors in 235 patients and proposals for an improved scoring system. Leukemia. 1992;6(1):52–9. [PubMed] [Google Scholar]
- 15.Aul C, Gattermann N, Germing U, Runde V, Heyll A, Schneider W. Risk assessment in primary myelodysplastic syndromes: validation of the Dusseldorf score. Leukemia. 1994;8(11):1906–13. [PubMed] [Google Scholar]
- 16.Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, et al. Proposals for the classification of the myelodysplastic syndromes. Br J Haematol. 1982;51(2):189–99. [PubMed] [Google Scholar]
- 17.Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood. 2002;100(7):2292–302. doi: 10.1182/blood-2002-04-1199. [DOI] [PubMed] [Google Scholar]
- 18.Brunning RD, Orazi A, Germing U, Le Beau MM, Porwit A, Bauman I, et al. Myelodysplastic syndromes/Neoplasms, overview. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al., editors. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon: IARC; 2008. pp. 88–93. [Google Scholar]
- 19.Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912–9. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akaike H. A new look at the statistical model identification. IEEE Transactions on Automatic Control. 1974;19(6):716–23. [Google Scholar]
- 21.Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response and other comparisons of time-to-event by outcome variables. J Clin Oncol. 2008;26(24):3913–5. doi: 10.1200/JCO.2008.16.1000. [DOI] [PubMed] [Google Scholar]
- 22.Della Porta MG, Malcovati L, Strupp C, Ambaglio I, Kuendgen A, Zipperer E, et al. Risk stratification based on both disease status and extra-hematologic comorbidities in patients with myelodysplastic syndrome. Haematologica. 2011;96(3):441–9. doi: 10.3324/haematol.2010.033506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riva E, Tettamanti M, Mosconi P, Apolone G, Gandini F, Nobili A, et al. Association of mild anemia with hospitalization and mortality in the elderly: the Health and Anemia population-based study. Haematologica. 2009;94(1):22–8. doi: 10.3324/haematol.13449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Opasich C, Cazzola M, Scelsi L, De Feo S, Bosimini E, Lagioia R, et al. Blunted erythropoietin production and defective iron supply for erythropoiesis as major causes of anaemia in patients with chronic heart failure. Eur Heart J. 2005;26(21):2232–7. doi: 10.1093/eurheartj/ehi388. [DOI] [PubMed] [Google Scholar]
- 25.Tang WH, Tong W, Jain A, Francis GS, Harris CM, Young JB. Evaluation and long-term prognosis of new-onset, transient, and persistent anemia in ambulatory patients with chronic heart failure. J Am Coll Cardiol. 2008;51(5):569–76. doi: 10.1016/j.jacc.2007.07.094. [DOI] [PubMed] [Google Scholar]
- 26.Groenveld HF, Januzzi JL, Damman K, van Wijngaarden J, Hillege HL, van Veldhuisen DJ, et al. Anemia and mortality in heart failure patients a systematic review and meta-analysis. J Am Coll Cardiol. 2008;52(10):818–27. doi: 10.1016/j.jacc.2008.04.061. [DOI] [PubMed] [Google Scholar]
- 27.Cazzola M, Barosi G, Gobbi PG, Invernizzi R, Riccardi A, Ascari E. Natural history of idiopathic refractory sideroblastic anemia. Blood. 1988;71(2):305–12. [PubMed] [Google Scholar]
- 28.Wang R, Gross CP, Halene S, Ma X. Comorbidities and survival in a large cohort of patients with newly diagnosed myelodysplastic syndromes. Leukemia research. 2009;33(12):1594–8. doi: 10.1016/j.leukres.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dayyani F, Conley AP, Strom SS, Stevenson W, Cortes JE, Borthakur G, et al. Cause of death in patients with lower-risk myelodysplastic syndrome. Cancer. 2010;116(9):2174–9. doi: 10.1002/cncr.24984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldberg SL, Chen E, Corral M, Guo A, Mody-Patel N, Pecora AL, et al. Incidence and clinical complications of myelodysplastic syndromes among United States Medicare beneficiaries. J Clin Oncol. 2010;28(17):2847–52. doi: 10.1200/JCO.2009.25.2395. [DOI] [PubMed] [Google Scholar]
- 31.Sperr WR, Wimazal F, Kundi M, Baumgartner C, Nosslinger T, Makrai A, et al. Comorbidity as prognostic variable in MDS: comparative evaluation of the HCT-CI and CCI in a core dataset of 419 patients of the Austrian MDS Study Group. Ann Oncol. 2010;21(1):114–9. doi: 10.1093/annonc/mdp258. [DOI] [PubMed] [Google Scholar]
- 32.Breccia M, Federico V, Latagliata R, Mercanti C, D’Elia GM, Cannella L, et al. Evaluation of comorbidities at diagnosis predicts outcome in myelodysplastic syndrome patients. Leuk Res. 2011;35(2):159–62. doi: 10.1016/j.leukres.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 33.Jadersten M, Malcovati L, Dybedal I, Della Porta MG, Invernizzi R, Montgomery SM, et al. Erythropoietin and granulocyte-colony stimulating factor treatment associated with improved survival in myelodys-plastic syndrome. J Clin Oncol. 2008;26(21):3607–13. doi: 10.1200/JCO.2007.15.4906. [DOI] [PubMed] [Google Scholar]
- 34.Park S, Grabar S, Kelaidi C, Beyne-Rauzy O, Picard F, Bardet V, et al. Predictive factors of response and survival in myelodysplastic syndrome treated with erythropoietin and G-CSF: the GFM experience. Blood. 2008;111(2):574–82. doi: 10.1182/blood-2007-06-096370. [DOI] [PubMed] [Google Scholar]
- 35.Cutler CS, Lee SJ, Greenberg P, Deeg HJ, Perez WS, Anasetti C, et al. A decision analysis of allogeneic bone marrow transplantation for the myelodysplastic syndromes: delayed transplantation for low-risk myelodysplasia is associated with improved outcome. Blood. 2004;104(2):579–85. doi: 10.1182/blood-2004-01-0338. [DOI] [PubMed] [Google Scholar]
- 36.Sorror ML, Giralt S, Sandmaier BM, De Lima M, Shahjahan M, Maloney DG, et al. Hematopoietic cell transplantation specific comorbidity index as an outcome predictor for patients with acute myeloid leukemia in first remission: combined FHCRC and MDACC experiences. Blood. 2007;110(13):4606–13. doi: 10.1182/blood-2007-06-096966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alessandrino EP, Della Porta MG, Bacigalupo A, Van Lint MT, Falda M, Onida F, et al. WHO classification and WPSS predict posttransplantation outcome in patients with myelodysplastic syndrome: a study from the Gruppo Italiano Trapianto di Midollo Osseo (GITMO) Blood. 2008;112(3):895–902. doi: 10.1182/blood-2008-03-143735. [DOI] [PubMed] [Google Scholar]
- 38.Alessandrino EP, Della Porta MG, Bacigalupo A, Malcovati L, Angelucci E, Van Lint MT, et al. Prognostic impact of pre-transplantation transfusion history and secondary iron overload in patients with myelodysplastic syndrome undergoing allogeneic stem cell transplantation: a GITMO study. Haematologica. 2010;95(3):476–84. doi: 10.3324/haematol.2009.011429. [DOI] [PMC free article] [PubMed] [Google Scholar]