Abstract
Background
The EVI1 gene (3q26) codes for a zinc finger transcription factor with important roles in both mammalian development and leukemogenesis. Over-expression of EVI1 through either 3q26 rearrangements, MLL fusions, or other unknown mechanisms confers a poor prognosis in acute myeloid leukemia.
Design and Methods
We analyzed the prevalence and prognostic impact of EVI1 over-expression in a series of 476 patients with acute myeloid leukemia, and investigated the epigenetic modifications of the EVI1 locus which could be involved in the transcriptional regulation of this gene.
Results
Our data provide further evidence that EVI1 over-expression is a poor prognostic marker in acute myeloid leukemia patients less than 65 years old. Moreover, we found that patients with no basal expression of EVI1 had a better prognosis than patients with expression/over-expression (P=0.036). We also showed that cell lines with over-expression of EVI1 had no DNA methylation in the promoter region of the EVI1 locus, and had marks of active histone modifications: H3 and H4 acetylation, and trimethylation of histone H3 lysine 4. Conversely, cell lines with no expression of EVI1 have DNA hypermethylation and are marked by repressive trimethylation of histone H3 lysine 27 at the EVI1 promoter.
Conclusions
Our results identify EVI1 over-expression as a poor prognostic marker in a large, independent cohort of acute myeloid leukemia patients less than 65 years old, and show that the total absence of EVI1 expression has a prognostic impact on the outcome of such patients. Furthermore, we demonstrated for the first time that an aberrant epigenetic pattern involving DNA methylation, H3 and H4 acetylation, and trimethylation of histone H3 lysine 4 and histone H3 lysine 27 might play a role in the transcriptional regulation of EVI1 in acute myeloid leukemia. This study opens new avenues for a better understanding of the regulation of EVI1 expression at a transcriptional level.
Keywords: AML, EVI1, overexpression, 3q, epigenetics
Introduction
The EVI1 gene (3q26) codes for a zinc finger transcription factor with important roles in both mammalian development and leukemogenesis. Since the identification of EVI1 as a common murine locus of retroviral integration in myeloid tumors1 this evolutionarily conserved gene has been implicated in human myeloid disorders, and in the development and progression of high-risk acute myeloid leukemia (AML).2,3 Recurrent 3q26 rearrangements are the only known mechanisms that lead to EVI1 over-expression;4–7 however, over-expression of this gene has been reported in 9–20% AML with no 3q aberrations, where it is also associated with an unfavorable outcome.8–13 Moreover, a recent study showed that MLL-ENL activates the transcription of Evi1.7 Transcriptional activation of EVI1 through chromosome rearrangements or other yet to be identified mechanisms, therefore, leads to particularly aggressive forms of human myeloid leukemia.2,3 The EVI1 locus gives rise to several alternatively spliced variants,2,3,14,15 including the intergenic splicing MDS1EVI1 which codes for a larger protein with a PR domain.3,16 Besides, EVI1 is transcribed into several 5′-end mRNA transcripts that have the same translation start site (Online Supplementary Figure S1).
To date, only three studies in large series of AML patients have analyzed the prevalence and prognostic value of EVI1 over-expression, discriminating EVI1 from MDS1EVI1 (Online Supplementary Table S1).8–10,17 The first study found that EVI1-1D was over-expressed in 13.7% cases, and was significantly associated with shorter overall and event-free survival.8 Two recent studies, one by the same group, included the analyses of other EVI1 5′-end transcripts and confirmed the prevalence and the poor impact that EVI1 over-expression has in AML.9,10 Lately, this group has proposed a diagnostic assay that quantifies all EVI1 5′-end transcripts, including MDS1EVI1. In this study, high expression of EVI1/MDS1EVI1 was found in 10.7% cases, and predicted adverse disease-free and event-free survival.17
Our aim was to study the prevalence of EVI1 over-expression and its impact on survival in a large series of AML patients, and to investigate the mechanisms of regulation of EVI1. We performed extensive analyses in both cell lines and patients’ samples to investigate the genetic and epigenetic mechanisms that could control the expression of EVI1 in AML. Our results open new avenues to a better understanding of the prognostic impact of EVI1 in AML, and the regulation of its expression at a transcriptional level.
Design and Methods
Material
Samples were obtained at diagnosis from 476 patients with AML, other than acute promyelocytic leukemia: the details are given in the Online Supplementary Design and Methods. Survival analysis was performed in the 213 AML patients who were eligible for treatment and were uniformly treated according to the Spanish Pethema Co-operative Group protocol LAM99.18 Samples were taken anonymously. Normal bone marrow, peripheral blood, and 19 samples of normal tissues from the human total RNA Master Panel II (Clontech, Takara-BIO, CA, USA) were used. The characteristics of the 16 myeloid cell lines used (DSMZ, Braunschweig, Germany) are summarized in Online Supplementary Table S2. Cell lines were cultured according to the supplier’s recommendations.
Cytogenetic and mutation analysis
Cytogenetic and fluorescence in situ hybridization (FISH) analyses were performed as previously described5 using six BAC clones: RP11-390G14 (3q21), RP11-475N22 (GATA2), RP11-689D3 (RPN1), RP11-82C9 (EVI1), RP11-115B16 (MDS1), RP11-196F13 (TNFSF10), and a probe for chromosome 3 centromere. The PR domain of MDS1EVI1 was amplified by reverse transcriptase polymerase chain reaction (RT-PCR), followed by a semi-nested reaction with specific primers (Online Supplementary Table S3). Gene mutation analysis of FLT3 and NPM1 was performed as previously described.19–21 PCR products were purified and sequenced.
Quantitative real-time reverse transcriptase polymerase chain reaction
The RNA isolation and EVI1 quantitative real-time RT-PCR conditions are described in the Online Supplementary Design and Methods.
Analysis of the methylation status of the EVI1 and MDS1EVI1 promoter regions
DNA methylation profiling of healthy donor peripheral blood (n=4), bone marrow (n=4) and CD34+ cells of bone marrow (n=4) samples was performed using the HumanMethylation27 Beadchip (Illumina, Inc., San Diego, CA, USA), according to the manufacturer’s instructions.22 Further details are provided in the Online Supplementary Design and Methods. Methylation status of the CpG islands of EVI1 (islands 1 and 2) and MDS1EVI1 (islands 1 and 2) was analyzed by bisulfite sequencing PCR (Online Supplementary Table S3). DNA was modified with the CpGenomeTM DNA Modification Kit (CHEMICON, Millipore Corporation, MA, USA). For the treatment of the cell lines, several concentrations and time points were tested, and optimal results were obtained for 10×106 cells in 10 mL of medium, cultured with 4 μM of 5-aza-2′-deoxycytidine (5-Aza), and 50 nM of trichostatin A (TSA) for 4 days; controls were cultured with dimethylsulfoxide and glacial acetic acid.
Chromatin immunoprecipitation
HEL, TF1, OCI-AML2, NOMO-1 and MV4-11 cell lines were subjected to chromatin immunoprecipitation in order to assess the acetylation of H3 and H4, and the trimethylation of histone H3 lysine 4 and lysine 27 as previously described.23 Further details are provided in the Online Supplementary Design and Methods.
Western blot analysis
Protein isolation and western blot conditions are described in the Online Supplementary Design and Methods.
Definitions and statistical analysis
Overall survival was defined as the time from diagnosis to death due to any cause or end of follow-up; disease-free survival as the time from complete remission until relapse or death, whichever occurred first; and event-free survival as the time from diagnosis until first event, in which failure to achieve complete remission, relapse or death were considered events. Overall, disease-free and event-free survival rates were determined using the Kaplan-Meier method and survival comparisons were done with the log-rank test. Proportional hazards models were constructed to determine whether the groups of EVI1 expression were associated with outcome when adjusting for other prognostic variables. P values for the significance among the cytogenetic subgroups were calculated using a two-tailed χ2 test. Spearman’s rho correlation coefficient was used to calculate the correlations between the over-expression of the EVI1 5′-end variants. Statistical analyses were performed using SPSS version 15.0 (SPSS Inc., IL, USA).
Results
Expression pattern of the alternative forms of EVI1
High expression of different splice-forms of EVI1 has been implicated in the development of high-risk AML.9,10 In order to understand the mechanisms leading to EVI1 over-expression better, we first analyzed the EVI1 5′-end variants, including MDS1EVI1, in a panel of human tissues, in AML cases, and in 16 myeloid cell lines. In each tissue, expression levels of the EVI1 transcripts were similar, and all transcripts could be detected in normal bone marrow, although at low levels (Online Supplementary Figure S2). Next, we quantified the EVI1 5′-end variants in a series of AML patients selected as a representation of the heterogeneity of AML cases (Online Supplementary Table S4) and in the myeloid cell lines (Online Supplementary Table S2 and Online Supplementary Figure S3), and completed the analysis of the cell lines with investigation of the expression of EVI1 protein. Expression levels of EVI1 transcripts in both patients’ samples and cell lines correlated with each other in a statistically significant manner (Online Supplementary Table S5). Among the cell lines over-expressing EVI1, we found two groups: AML cell lines over-expressed transcripts -1A, -1B, -1C, and -1D, whereas cell lines with chronic myeloid leukemia in blast phase (CML-BP) had only EVI1-1B over-expression as a common feature. Western blot analysis detected the EVI1-FL isoform (145 kDa) in cell lines with over-expression of at least one EVI1 transcript (Online Supplementary Table S2 and Online Supplementary Figure S3). As an exception, MEG-01 (CML-BC) had over-expression of EVI1-1B and no EVI1-FL protein. Moreover, we found no association between the expression of any EVI1 transcript and the amount of protein (Online Supplementary Table S2 and Online Supplementary Figure S3). Seven cell lines had no basal expression of either EVI1 or MDS1EVI1 (Online Supplementary Table S2).
The fragility of the PR domain, which is a hotspot in both retroviral insertions and 3q rearrangements,24 prompted us to perform a mutation analysis of the PR domain in the cell lines. We found no mutations in this region; we did, however, detect a novel MDS1EVI1 alternative splice form in four cell lines. The analysis of the panel of normal human tissues demonstrated that this novel alternative splice form was not expressed in peripheral blood, but was present in most of the tissues tested (Online Supplementary Figure S4). This form would codify for a truncated protein of 38 amino acids; however, a second open reading frame is possible from the EVI1 ATG start codon in exon 3, which would codify for the Evi1-FL protein (NCBI Accession GQ352634) (Online Supplementary Figure S4).
Prevalence of EVI1 over-expression in patients with acute myeloid leukemia
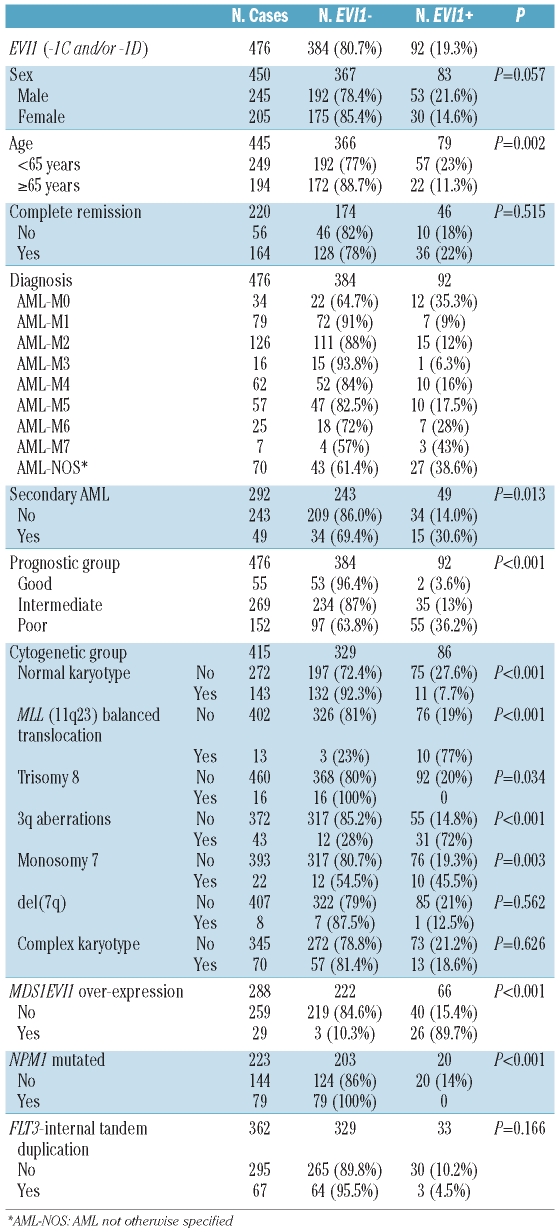
Since EVI1 alternative transcript forms correlated significantly, we investigated the expression of EVI1-1D, EVI1-1C, and MDS1EVI1 in a series of 476 AML patients (Table 1). EVI1 (-1C and/or -1D) was over-expressed in 92 out of the 476 patients (19.3%). Table 1 shows the prevalence of EVI1 over-expression, and its association with relevant clinical and molecular parameters. Statistical correlations for -1C and -1D were also calculated separately and yielded similar results (data not shown). The prevalence of EVI1 over-expression was significantly different among the cytogenetic prognostic groups (P<0.001). EVI1 over-expression was found in 72% of cases with 3q rearrangements, including all 25 cases with 3q26 (P<0.001). Other cytogenetic abnormalities associated with EVI1 over-expression were MLL translocations (P<0.001), and monosomy 7 (P=0.003), but not del(7q) (P=0.562). The prevalence of EVI1 over-expression in patients with a normal karyotype was 7.7%, and an inverse correlation was found between EVI1 over-expression and both trisomy 8 and NPM1 mutations; in fact, none of the patients with either trisomy 8 (16 cases) or NPM1 mutations (79 cases) had EVI1 over-expression.
Table 1.
Clinical and molecular characteristics of a series of 476 patients with AML and the association between EVI1 over-expression (-1C and/or -1D) and clinical and genetic parameters.
Prognostic impact of EVI1 expression in patients with acute myeloid leukemia
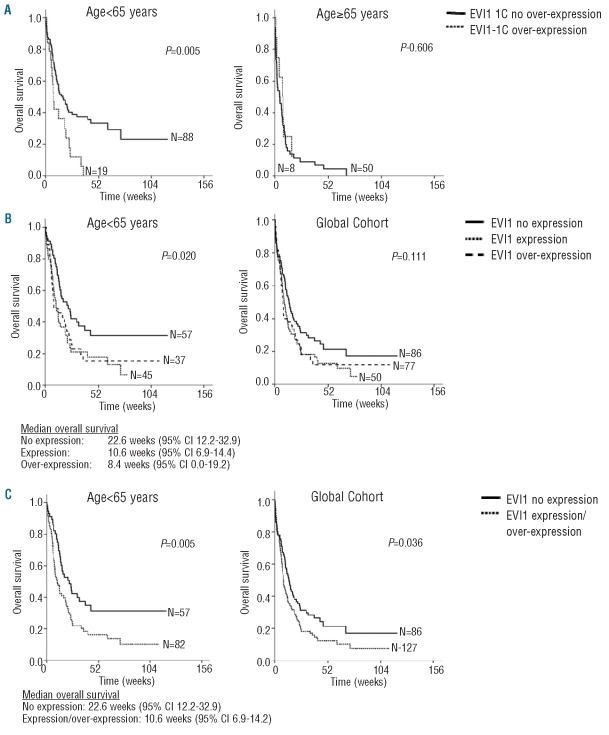
Clinical follow-up data of patients who received induction therapy and were uniformly treated were available for 213 patients (110 males and 103 females), with a median age at diagnosis of 58 years (range, 16–83 years). The median follow-up was 159 weeks, with a minimum of 24 weeks. The median overall survival of this cohort was 45.7 weeks (95% CI: 36.5–54.8). Kaplan-Meier analysis showed significant differences in well-recognized risk factors such as age and cytogenetic group (P<0.001). In a stratified analysis by age group, patients under 65 years old with EVI1-1C over-expression had significantly lower overall survival (P=0.005) and event-free survival (P=0.008) (Figure 1 and Online Supplementary Figure S5), while no significant differences were found in disease-free survival. However, we could not confirm the independent prognostic significance of EVI1-1C over-expression in a multivariate model (Online Supplementary Table S6). EVI1-1D over-expression had no significant impact on overall, disease-free or event-free survival. Among the whole cohort, the group of patients with EVI1 over-expression and no MDS1EVI1 expression had the worst outcome (P=0.017). When comparing patients with no basal expression, expression and over-expression of EVI1 in the group of patients under 65 years old, patients with no basal expression had a better overall survival (P=0.020) (Figure 1). Furthermore, patients with no basal expression of EVI1 had a better overall survival than patients with expression/over-expression in both the whole cohort (P=0.036) and in the group of patients less than 65 years (P=0.005) (Figure 1).
Figure 1.
Survival analysis of a series of patients with acute myeloid leukemia according to EVI1 expression status. (A) In Kaplan-Meier analysis stratified by age, patients <65 years and with EVI1-1C over-expression showed an inferior overall survival in comparison to patients with no EVI1-1C over-expression. (B) In Kaplan-Meier analysis, patients <65 years and no basal expression of EVI1 (-1C/-1D) had a better overall survival in comparison to patients with either expression or over-expression of EVI1, and a trend to better outcome in the global cohort. (C) In Kaplan-Meier analysis, patients <65 years with no basal expression of EVI1 (-1C/-1D) had a better overall survival than patients with EVI1 expression/over-expression. The same results were found in the global cohort.
EVI1 over-expression and 3q26 rearrangements
For a better understanding of the role of 3q rearrangements in the expression of EVI1, we characterized the 3q21q26 region by FISH, and quantified EVI1 expression in 16 myeloid cell lines and in 25 cases with myeloid neoplasias. The HEL and TF-1 cell lines had over-expression of EVI1 and several copies of probes located on 3q26; however, a similar pattern was found in NOMO-1 and OCI-AML2, with no EVI1 expression; moreover, OCI-AML2 had an inv(3)(q21q26) (Online Supplementary Table S2 and Online Supplementary Figure S6). In the patients’ samples, FISH analyses showed wide heterogeneity and complex 3q rearrangements. Cases were classified into four distinct groups: 3q21q26, 3q26, 3q21, and other 3q aberrations. Cases with either 3q21q26 (8 cases) or 3q26 (7 cases) breakpoints had EVI1 over-expression, except case 21872s, the only one with breakpoints located between the 689D3 (3q21; 128.4 Mb) and 82C9 (3q26; 168.8 Mb) probes. Cases with other 3q rearrangements and breakpoints located between these probes had no EVI1 over-expression either (Online Supplementary Table S8). Three cases with a single breakpoint on 3q21 had EVI1 overexpression (25704, 24316 and 14066s). The 3q26 break-points associated with EVI1 over-expression were mainly located centromeric to EVI1 in cases with inv(3), and telomeric to MDS1EVI1 in t(3;3) and other 3q26 rearrangements. Besides, 3q21 breakpoints associated with EVI1 over-expression were located centromeric to probe 390G14 (3/4 cases) (Online Supplementary Table S8).
Aberrant epigenetic pattern of EVI1 in acute myeloid leukemia
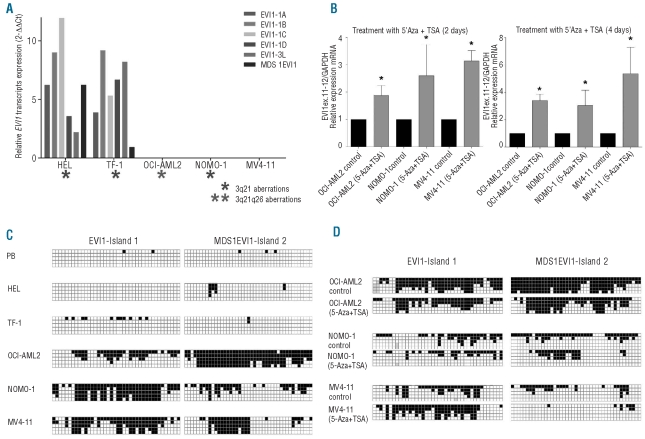
Results showing that EVI1 over-expression sometimes occurs irrespectively of 3q21q26 rearrangements, and the finding that normal basal expression of EVI1 and MDS1EVI1 was not detected in several patients’ samples and cell lines (including OCI-AML2, with 3q21q26) (Online Supplementary Table S2 and Online Supplementary Figure S3) prompted us to study whether EVI1 transcription could be regulated by epigenetic mechanisms. For the analysis, we selected five cell lines that represented the heterogeneity detected in patients’ samples: HEL and TF-1 had 3q aberrations and EVI1 over-expression; OCIAML2 and NOMO-1 had 3q and no EVI1 expression; and MV4-11 had neither 3q nor EVI1 expression (Figure 2A). Treatment of EVI1−/MDS1EVI1− cell lines with TSA in combination with the demethylating agent 5-Aza induced EVI1 expression (Figure 2B), confirming our hypothesis. The expression did not exceed the levels in peripheral blood or bone marrow. To assess whether the aberrant methylation status of the promoter region of the EVI1 locus was the epigenetic mechanism involved, we first analyzed the methylation status of the CpG islands predicted in the proximal promoter region of EVI1 and MDS1EVI1 in normal samples. High-resolution genome-wide methylation arrays from Illumina (Infinium HumanMethylation27 BeadChip, Illumina, CA, USA) showed the total absence of methylation in two probes of EVI1 and two of MDS1EVI1 in CD34+ progenitor cells (high EVI1 expression) and normal bone marrow and peripheral blood (very low EVI1 expression) (data not shown). These results indicate that aberrant hypomethylation of the promoter of EVI1 is not the mechanism of EVI1 over-expression; nevertheless, this could be the mechanism involved in the EVI1 gene silencing.
Figure 2.
Analysis of the epigenetic status of the EVI1 locus in five myeloid cell lines. (A) Quantification of the relative expression of the EVI1 splice variants, with bone marrow as the control sample. (B) Quantification of the relative expression of EVI1 (EVI1 11–12) after treatment with 5’Aza and TSA. Statistical significance was estimated using the non-parametric Wilcoxon’s matched pairs test; P<0.05 was considered significant (*). (C) Diagram of the methylation status of the EVI1-Island 1 and MDS1EVI1-Island 2 by direct sequencing after bisulfite treatment (white: non-methylated; black: methylated).(D) Diagram of the methylation status of the EVI1-Island 1 and MDS1EVI1-Island 2 after treatment with 5’Aza and TSA (white: non-methylated; black: methylated).
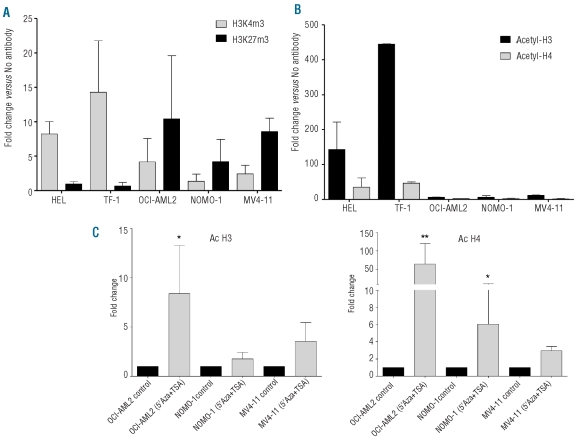
The methylation status of EVI1-island 1 and MDS1EVI1-island 2 showed concordance between EVI1 and MDS1EVI1 expression: the regions were hypermethylated in EVI1−/MDS1EVI1− cell lines (Figure 2C). However, we observed no significant changes in the methylation status of the EVI1-island 1 before or after treatment with TSA in combination with 5-Aza (Figure 2D). This result prompted us to analyze the trimethylation status of histone H3 lysine 4 (H3K4me3) and histone H3 lysine 27 (H3K27me3), and the acetylation of histone H3 and H4. Quantification of the amount of chromatin immunoprecipitated with anti-trimethyl Lys4 and Lys27 showed that HEL and TF-1 had enrichment of the active H3K4me3 pattern, while NOMO-1, MV4-11 and OCI-AML2 had the opposite signature, a mark of the repressive pattern H3K27me3 (Figure 3A). However, there was no difference in the histone methylation status of the cell lines with no expression of EVI1 after treatment with TSA and 5-Aza (Online Supplementary Figure S7). We also observed an enrichment of the acetylation of histones H3 and H4, especially H3, in HEL and TF-1 (Figure 3B), and chromatin immunoprecipitation analysis of the EVI1 promoter showed enrichment of acetylated histones H3 and H4 in treated cell lines (Figure 3C). The enrichment of the active marks both in cell lines with EVI1 over-expression and treated cell lines, strongly suggests that histone acetylation might play a role in EVI1 expression regulation. Regarding the MDS1EVI1 locus, we observed slight changes in the methylation status of the MDS1EVI1 promoter in MV4-11 after treatment with TSA and 5-Aza (Figure 2D); however, MDS1EVI1 gene expression was not induced after treatment, and we found no difference in either the histone methylation or acetylation pattern. Taken together, these results indicate that expression of EVI1 in AML is regulated at least in part by epigenetic mechanisms.
Figure 3.
Analysis of the epigenetic status of the histones of the EVI1 locus in five myeloid cell lines. (A) Quantitative real-time RT-PCR performed on fragmented chromatin, showing the enrichment of trimethylation of histone H3 lysine 4 (H3K4me3) and histone H3 lysine 27 (H3K27me3) on the EVI1 promoter. (B) Quantitative real-time RT-PCR performed on fragmented chromatin, showing the enrichment of acetylated histones H3 and H4 on the EVI1 promoter. The results were calculated using the ΔΔCt method. They were presented as the fold enrichment of chromatin DNA precipitated by the specific antibody versus chromatin DNA precipitated by no antibody, as control. (C) Quantitative real-time RT-PCR performed on fragmented chromatin, showing the enrichment of acetylated histones H3 and H4 on EVI1 promoter regions after treatment with 5’Aza and TSA. The results were calculated and presented as described above, and comparing with and without the treatment. Statistical significance was estimated using the non-parametric Wilcoxon’s matched pairs test; P<0.05 was considered significant (*), and P<0.01 strongly significant (**).
Discussion
EVI1 has been recognized as one of the most aggressive oncogenes associated with AML.2,3 Our results confirm that EVI1 over-expression is an adverse prognostic factor in AML patients, not always restricted to 3q26 aberrations. Notably, we found that the total absence of EVI1 expression might have a prognostic impact on the outcome of AML patients, and that this atypical pattern may be regulated by epigenetic mechanisms.
Our results confirm the prevalence of EVI1 over-expression and its adverse prognostic outcome in AML in an independent large cohort.8,10,17 For the first time, we included quantification and survival analysis of the EVI1-1C 5′-end variant, and found that in younger AML patients over-expression of this transcript was a poor prognostic marker with regards to both overall survival (P=0.005) and event-free survival (P=0.008) (Figure 1 and Online Supplementary Figure S5), suggesting that this variant could be a genetic marker in this subgroup. However, the correlation could not be confirmed in multivariate analysis. The significant impact of EVI1 over-expression on overall survival in a multivariate analysis was shown only in the two largest studies – by Lugthart et al. for EVI1-1A and EVI1-1B10 and by Groschel et al. for EVI1/MDS1EVI1 – which did not discriminate EVI1 from MDS1EVI1 (Online Supplementary Table S1);17 it is, therefore, possible that our sample size was not large enough to give statistically significant results. Of note, we found that younger AML patients with no EVI1 expression had a significantly better outcome than patients with either EVI1 expression or over-expression (Figure 1), although this could not be confirmed in multivariate analysis. To our knowledge, this is the first time this finding has been reported. Further studies in independent cohorts are needed to confirm the importance of this result.
We and others have shown the association between EVI1 over-expression and other specific cytogenetic aberrations such as MLL rearrangements and monosomy 7 (Table 1).8,10,17 Interestingly, it has been recently shown that the specific MLL-ENL fusion activates the transcription of Evi1 in undifferentiated hematopoietic cells.7 In addition, in mouse models, EVI1 over-expression induces a myelodysplastic syndrome that does not progress to AML,25 suggesting the necessity of cooperating mutations in the progression to AML. As demonstrated in gene therapy studies, in which enforced expression of EVI1 in human cells leads to genomic instability, monosomy 7, and clonal progression,24,26 our results support the putative role of monosomy 7 as a cooperating mutation in EVI1-positive AML. A similar cooperation has been reported in a murine model between RUNX1 mutation D171N and EVI1 in the AML transformation of myelodysplastic syndrome;27 however, we found no mutations of RUNX1 in a series of 46 cases with EVI1 over-expression (data not shown), suggesting that this mechanism is not frequent in human AML. Finally, we found an inverse correlation between EVI1 over-expression and NPM1 mutations,8,10,12 in agreement with the better outcome of patients with NPM1 mutations.28
To date, 3q rearrangements and MLL fusions are the only known mechanisms of EVI1 over expression. Quantitative real-time RT-PCR and FISH of 16 cell lines and a series of samples from patients with myeloid malignancies confirmed that EVI1 over-expression is associated with 3q26, although sometimes occurs irrespective of 3q rearrangements (Online Supplementary Table S8 and Online Supplementary Figure S6).9,10 Moreover, the prevalence of EVI1 over-expression among the patients with different categories of 3q abnormalities is similar to that found in another recent study.29 Interestingly, we showed that FISH breakpoints in cases with 3q26 and EVI1 over-expression were located telomeric to MDS1EVI1 (Online Supplementary Table S8), a hotspot locus for retroviral insertions,26 which suggests that disruption of this region is of the foremost importance in the regulation of EVI1 transcription. We also demonstrated that the EVI1 protein is present even if only one EVI1 transcript is over-expressed. As an exception, MEG-01 had over-expression of EVI1-1B and no EVI1-FL protein. In this cell line the protein levels might be low and, therefore, difficult to detect by western blot, although in the KU-812 cell line, also with low levels of EVI1 expression, the protein could be detected. Another explanation might be that the accumulation and degradation of the protein in these cell lines is different. Furthermore, in our study we identified a novel alternatively spliced MDS1EVI1 that, together with the previously described EVI1 transcripts, might codify to the same sized protein EVI1-FL. However, it is difficult to know whether all these transcripts are used or not because all cell lines with EVI1-FL protein express more than one transcript, and we did not find any association between any specific transcript and the protein. This highlights that the mechanism of EVI1 protein regulation is complex and still to be elucidated. Nevertheless, the fact that the EVI1 protein is present even if only one EVI1 transcript is over-expressed supports the importance of the detection of EVI1 expression status at diagnosis in AML patients, as indicated by the new World Health Organization classification.30 Moreover, AML cell lines over-expressed transcripts -1A, -1B, -1C, and -1D, whereas cell lines with CML-BP had only EVI1-1B over-expression as a common feature. This might indicate that the mechanisms of EVI1 over-expression may depend on the action of different transcription factors in the promoter of this gene, opening directions for future studies.
In order to investigate other mechanisms of EVI1 over-expression, we analyzed the role that epigenetic modifications could have in the regulation of the EVI1 gene. The loci showed no methylation in either CD34+ progenitor cells (high EVI1 expression) or normal bone marrow and peripheral blood samples (very low EVI1 expression). These results strongly suggest that DNA methylation modifications do not have a role in the normal regulation of EVI1 expression during the process of differentiation of hematopoietic cells, and that EVI1 promoter hypomethylation cannot be the mechanism of EVI1 over-expression. However, we detected the absence of normal basal expression of EVI1 and MDS1EVI1 in patients’ samples and cell lines, and several cell lines had 3q rearrangements and no EVI1 over-expression; we, therefore, hypothesized that epigenetic aberrations could play a role in the regulation of the expression of EVI1 in AML. We found an aberrant hypermethylation pattern in cell lines with no EVI1/MDS1EVI1 expression (Figure 2C), and treatment of these cell lines with TSA in combination with 5-Aza induced EVI1 expression (Figure 2B). However, there were no significant changes in the methylation status after the treatment, suggesting that other epigenetic mechanisms could be involved (Figure 2D). Our results showed that histone modifications could be a mechanism that contributes to silencing the normal basal expression of the EVI1 locus in the leukemic cells (Figure 3 A–B). An important observation in this study is the active pattern of H4 and especially of H3 in the HEL and TF-1 cell lines which over-express EVI1. Of note, treatment of cell lines with no EVI1 expression induced expression of this gene and increased acetylation of both histones H3 and H4 on the EVI1 promoter (Figure 3C). We also found that the AML cell lines with DNA methylation and no EVI1 expression displayed reduced H3K4me3. These data support the results of recent studies in which it was observed that in AML there is an inverse correlation between DNA methylation and the H3K4 trimethylation pattern compared with unmethylated samples.31–33 The epigenetic modifications H3K4me3 and H3K27me3 are of particular interest as these modifications are catalyzed by trithorax and polycomb-group proteins, respectively, which have key developmental functions. H3K4me3 methylation positively regulates transcription by recruiting nucleosome remodeling enzymes and histone acetylases, while H3K27me3 methylation negatively regulates transcription by promoting a compact chromatin structure. It has been described that the most highly conserved non-coding elements in mammalian genomes cluster within regions enriched for genes encoding developmentally important transcription factors, such as EVI1,34 suggesting that these transcription factors could have key epigenetic regulatory roles in development. Mapping histone methylation patterns in mouse embryonic stem cells showed that EVI1 has an open chromatin structure with a H3K4me3 pattern, as we observed in our EVI1-expressing cell lines, suggesting that this mechanism is involved in its regulation in early hematopoietic cells. Our results support the concept that the same mechanism could be involved in the leukemic cells.34 Taken together, the histone modifications could explain the atypical expression pattern of both cell lines and patients’ samples with no EVI1 expression. This is of special interest since patients with no basal expression of EVI1 tend to have a better overall survival rate in comparison with cases with either expression or over-expression (Figure 1). Nevertheless, prospective studies are needed to clarify the role of histone modifications in EVI1 regulation.
In summary, our results confirm that EVI1 over-expression is an adverse prognostic factor in AML, and corroborate the necessity of quantifying EVI1 and MDS1EVI1 expression during the diagnosis of AML in younger patients, mostly in cases with 3q aberrations, monosomy 7, MLL rearrangements, and in the subgroup with a normal karyotype and no NPM1 mutations. Notably, we found that the total absence of EVI1 expression may be associated with a better outcome in AML patients, and that this atypical pattern may be regulated by epigenetic mechanisms. Further studies are needed to elucidate the prevalence, prognostic impact, and the significance of no basal EVI1 expression in the leukemic transformation of AML.
Footnotes
Funding: this work was supported by grants from the Ministerio Educación y Ciencia (SAF2005/06425), Ministerio Ciencia e Innovación (PI081687), AECC, Departamento Salud del Gobierno de Navarra (14/2008), Generalitat de Catalunya (2009-SGR-1246), Fundación Cellex, SACYL (355/4/09), ISCIII-RTICC (RD06/0020/0078, RD06/0020/0101, RD06/0020/0006, RD06/0020/0031), and Fundación para la Investigación Médica Aplicada y UTE (Spain). We thank Marta García-Granero for the statistical analysis, and Eva Bandres and Marta Alonso for useful discussion.
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Mucenski ML, Taylor BA, Ihle JN, Hartley JW, Morse HC, 3rd, Jenkins NA, et al. Identification of a common ecotropic viral integration site, Evi-1, in the DNA of AKXD murine myeloid tumors. Mol Cell Biol. 1988;8(1):301–8. doi: 10.1128/mcb.8.1.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nucifora G, Laricchia-Robbio L, Senyuk V. EVI1 and hematopoietic disorders: history and perspectives. Gene. 2006;368:1–11. doi: 10.1016/j.gene.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 3.Wieser R. The oncogene and developmental regulator EVI1: expression, biochemical properties, and biological functions. Gene. 2007;396(2):346–57. doi: 10.1016/j.gene.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 4.Poppe B, Dastugue N, Vandesompele J, Cauwelier B, De Smet B, Yigit N, et al. EVI1 is consistently expressed as principal transcript in common and rare recurrent 3q26 rearrangements. Genes Chromosomes Cancer. 2006;45(4):349–56. doi: 10.1002/gcc.20295. [DOI] [PubMed] [Google Scholar]
- 5.Lahortiga I, Vazquez I, Agirre X, Larrayoz MJ, Vizmanos JL, Gozzetti A, et al. Molecular heterogeneity in AML/MDS patients with 3q21q26 rearrangements. Genes Chromosomes Cancer. 2004;40(3):179–89. doi: 10.1002/gcc.20033. [DOI] [PubMed] [Google Scholar]
- 6.Wieser R, Volz A, Schnittger S, Jager U, Gruner H, Meran JG, et al. Mapping of leukaemia-associated breakpoints in chromosome band 3q21 using a newly established PAC contig. Br J Haematol. 2000;110(2):343–50. doi: 10.1046/j.1365-2141.2000.02192.x. [DOI] [PubMed] [Google Scholar]
- 7.Arai S, Yoshimi A, Shimabe M, Ichikawa M, Nakagawa M, Imai Y, et al. Evi-1 is a transcriptional target of MLL oncoproteins in hematopoietic stem cells. Blood. 2011;117(23):6304–14. doi: 10.1182/blood-2009-07-234310. [DOI] [PubMed] [Google Scholar]
- 8.Barjesteh van Waalwijk van Doorn-Khosrovani S, Erpelinck C, van Putten WL, Valk PJ, van der Poel-van de Luytgaarde S, Hack R, et al. High EVI1 expression predicts poor survival in acute myeloid leukemia: a study of 319 de novo AML patients. Blood. 2003;101(3):837–45. doi: 10.1182/blood-2002-05-1459. [DOI] [PubMed] [Google Scholar]
- 9.Haas K, Kundi M, Sperr WR, Esterbauer H, Ludwig WD, Ratei R, et al. Expression and prognostic significance of different mRNA 5′-end variants of the oncogene EVI1 in 266 patients with de novo AML: EVI1 and MDS1/EVI1 overexpression both predict short remission duration. Genes Chromosomes Cancer. 2008;47(4):288–98. doi: 10.1002/gcc.20532. [DOI] [PubMed] [Google Scholar]
- 10.Lugthart S, van Drunen E, van Norden Y, van Hoven A, Erpelinck CA, Valk PJ, et al. High EVI1 levels predict adverse outcome in acute myeloid leukemia: prevalence of EVI1 overexpression and chromosome 3q26 abnormalities underestimated. Blood. 2008;111(8):4329–37. doi: 10.1182/blood-2007-10-119230. [DOI] [PubMed] [Google Scholar]
- 11.Valk PJ, Verhaak RG, Beijen MA, Erpelinck CA, Barjesteh van Waalwijk van Doorn-Khosrovani S, Boer JM, et al. Prognostically useful gene-expression profiles in acute myeloid leukemia. N Engl J Med. 2004;350(16):1617–28. doi: 10.1056/NEJMoa040465. [DOI] [PubMed] [Google Scholar]
- 12.Santamaria CM, Chillon MC, Garcia-Sanz R, Perez C, Caballero MD, Ramos F, et al. Molecular stratification model for prognosis in cytogenetically normal acute myeloid leukemia. Blood. 2009;114(1):148–52. doi: 10.1182/blood-2008-11-187724. [DOI] [PubMed] [Google Scholar]
- 13.Daghistani M, Marin D, Khorashad JS, Wang L, May PC, Paliompeis C, et al. EVI-1 oncogene expression predicts survival in chronic-phase CML patients resistant to imatinib treated with second-generation tyrosine kinase inhibitors. Blood. 2010;116(26):6014–7. doi: 10.1182/blood-2010-01-264234. [DOI] [PubMed] [Google Scholar]
- 14.Aytekin M, Vinatzer U, Musteanu M, Raynaud S, Wieser R. Regulation of the expression of the oncogene EVI1 through the use of alternative mRNA 5′-ends. Gene. 2005;356:160–8. doi: 10.1016/j.gene.2005.04.032. [DOI] [PubMed] [Google Scholar]
- 15.Morishita K. Leukemogenesis of the EVI1/MEL1 gene family. Int J Hematol. 2007;85(4):279–86. doi: 10.1532/IJH97.06174. [DOI] [PubMed] [Google Scholar]
- 16.Fears S, Mathieu C, Zeleznik-Le N, Huang S, Rowley JD, Nucifora G. Intergenic splicing of MDS1 and EVI1 occurs in normal tissues as well as in myeloid leukemia and produces a new member of the PR domain family. Proc Natl Acad Sci USA. 1996;93(4):1642–7. doi: 10.1073/pnas.93.4.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Groschel S, Lugthart S, Schlenk RF, Valk PJ, Eiwen K, Goudswaard C, et al. High EVI1 expression predicts outcome in younger adult patients with acute myeloid leukemia and is associated with distinct cytogenetic abnormalities. J Clin Oncol. 2010;28(12):2101–7. doi: 10.1200/JCO.2009.26.0646. [DOI] [PubMed] [Google Scholar]
- 18.Suarez L, Vidriales MB, Moreno MJ, Lopez A, Garcia-Larana J, Perez-Lopez C, et al. Differences in anti-apoptotic and multidrug resistance phenotypes in elderly and young acute myeloid leukemia patients are related to the maturation of blast cells. Haematologica. 2005;90(1):54–9. [PubMed] [Google Scholar]
- 19.Nakao M, Yokota S, Iwai T, Kaneko H, Horiike S, Kashima K, et al. Internal tandem duplication of the flt3 gene found in acute myeloid leukemia. Leukemia. 1996;10(12):1911–8. [PubMed] [Google Scholar]
- 20.Yamamoto Y, Kiyoi H, Nakano Y, Suzuki R, Kodera Y, Miyawaki S, et al. Activating mutation of D835 within the activation loop of FLT3 in human hematologic malignancies. Blood. 2001;97(8):2434–9. doi: 10.1182/blood.v97.8.2434. [DOI] [PubMed] [Google Scholar]
- 21.Falini B, Nicoletti I, Bolli N, Martelli MP, Liso A, Gorello P, et al. Translocations and mutations involving the nucleophosmin (NPM1) gene in lymphomas and leukemias. Haematologica. 2007;92(4):519–32. doi: 10.3324/haematol.11007. [DOI] [PubMed] [Google Scholar]
- 22.Bibikova M, Fan JB. Genome-wide DNA methylation profiling. Wiley Interdiscip Rev Syst Biol Med. 2010;2(2):210–23. doi: 10.1002/wsbm.35. [DOI] [PubMed] [Google Scholar]
- 23.Jose-Eneriz ES, Roman-Gomez J, Cordeu L, Ballestar E, Garate L, Andreu EJ, et al. BCR-ABL1-induced expression of HSPA8 promotes cell survival in chronic myeloid leukaemia. Br J Haematol. 2008;142(4):571–82. doi: 10.1111/j.1365-2141.2008.07221.x. [DOI] [PubMed] [Google Scholar]
- 24.Ott MG, Schmidt M, Schwarzwaelder K, Stein S, Siler U, Koehl U, et al. Correction of X-linked chronic granulomatous disease by gene therapy, augmented by insertional activation of MDS1-EVI1, PRDM16 or SETBP1. Nat Med. 2006;12(4):401–9. doi: 10.1038/nm1393. [DOI] [PubMed] [Google Scholar]
- 25.Buonamici S, Li D, Chi Y, Zhao R, Wang X, Brace L, et al. EVI1 induces myelodysplastic syndrome in mice. J Clin Invest. 2004;114(5):713–9. doi: 10.1172/JCI21716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stein S, Ott MG, Schultze-Strasser S, Jauch A, Burwinkel B, Kinner A, et al. Genomic instability and myelodysplasia with monosomy 7 consequent to EVI1 activation after gene therapy for chronic granulomatous disease. Nat Med. 2010;16(2):198–204. doi: 10.1038/nm.2088. [DOI] [PubMed] [Google Scholar]
- 27.Watanabe-Okochi N, Kitaura J, Ono R, Harada H, Harada Y, Komeno Y, et al. AML1 mutations induced MDS and MDS/AML in a mouse BMT model. Blood. 2008;111(8):4297–308. doi: 10.1182/blood-2007-01-068346. [DOI] [PubMed] [Google Scholar]
- 28.Lowenberg B. Diagnosis and prognosis in acute myeloid leukemia--the art of distinction. N Engl J Med. 2008;358(18):1960–2. doi: 10.1056/NEJMe0802379. [DOI] [PubMed] [Google Scholar]
- 29.Lugthart S, Groschel S, Beverloo HB, Kayser S, Valk PJ, van Zelderen-Bhola SL, et al. Clinical, molecular, and prognostic significance of WHO type inv(3)(q21q26.2)/t(3;3)(q21;q26.2) and various other 3q abnormalities in acute myeloid leukemia. J Clin Oncol. 2010;28(24):3890–8. doi: 10.1200/JCO.2010.29.2771. [DOI] [PubMed] [Google Scholar]
- 30.Lowenberg B. Acute myeloid leukemia: the challenge of capturing disease variety. Hematology Am Soc Hematol Educ Program. 2008:1–11. doi: 10.1182/asheducation-2008.1.1. [DOI] [PubMed] [Google Scholar]
- 31.Mohn F, Weber M, Rebhan M, Roloff TC, Richter J, Stadler MB, et al. Lineage-specific polycomb targets and de novo DNA methylation define restriction and potential of neuronal progenitors. Mol Cell. 2008;30(6):755–66. doi: 10.1016/j.molcel.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 32.Weber M, Hellmann I, Stadler MB, Ramos L, Paabo S, Rebhan M, et al. Distribution, silencing potential and evolutionary impact of promoter DNA methylation in the human genome. Nat Genet. 2007;39(4):457–66. doi: 10.1038/ng1990. [DOI] [PubMed] [Google Scholar]
- 33.Paul TA, Bies J, Small D, Wolff L. Signatures of polycomb repression and reduced H3K4 trimethylation are associated with p15INK4b DNA methylation in AML. Blood. 2010;115(15):3098–108. doi: 10.1182/blood-2009-07-233858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125(2):315–26. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]