Abstract
Background
Several studies of pediatric acute myeloid leukemia have described the various type-I or type-II aberrations and their relationship with clinical outcome. However, there has been no recent comprehensive overview of these genetic aberrations in one large pediatric acute myeloid leukemia cohort.
Design and Methods
We studied the different genetic aberrations, their associations and their impact on prognosis in a large pediatric acute myeloid leukemia series (n=506). Karyotypes were studied, and hotspot regions of NPM1, CEPBA, MLL, WT1, FLT3, N-RAS, K-RAS, PTPN11 and KIT were screened for mutations of available samples. The mutational status of all type-I and type-II aberrations was available in 330 and 263 cases, respectively. Survival analysis was performed in a subset (n=385) treated on consecutive acute myeloid leukemia Berlin-Frankfurt-Munster Study Group and Dutch Childhood Oncology Group treatment protocols.
Results
Genetic aberrations were associated with specific clinical characteristics, e.g. significantly higher diagnostic white blood cell counts in MLL-rearranged, WT1-mutated and FLT3-ITD-positive acute myeloid leukemia. Furthermore, there was a significant difference in the distribution of these aberrations between children below and above the age of two years. Non-random associations, e.g. KIT mutations with core-binding factor acute myeloid leukemia, and FLT3-ITD with t(15;17)(q22;q21), NPM1- and WT1-mutated acute myeloid leukemia, respectively, were observed. Multivariate analysis revealed a ‘favorable karyotype’, i.e. t(15;17)(q22;q21), t(8;21)(q22;q22) and inv(16)(p13q22)/t(16;16)(p13;q22). NPM1 and CEBPA double mutations were independent factors for favorable event-free survival. WT1 mutations combined with FLT3-ITD showed the worst outcome for 5-year overall survival (22±14%) and 5-year event-free survival (20±13%), although it was not an independent factor in multivariate analysis.
Conclusions
Integrative analysis of type-I and type-II aberrations provides an insight into the frequencies, non-random associations and prognostic impact of the various aberrations, reflecting the heterogeneity of pediatric acute myeloid leukemia. These aberrations are likely to guide the stratification of pediatric acute myeloid leukemia and may direct the development of targeted therapies.
Keywords: pediatric AML, type I/II aberrations, mutation, prognostic factor
Introduction
Acute myeloid leukemia (AML) accounts for 15–20% of pediatric leukemias.1 Despite intensification of chemotherapy over the last decades, only approximately 60–70% of children with AML are cured.2 AML is not a single disease entity, and its heterogeneity is reflected by differences in morphology, immunophenotype, as well as cytogenetic and molecular aberrations.3 Moreover, recurrent (cyto)genetic aberrations are important prognostic factors in pediatric AML and an increasing number of study groups are using them for risk group stratification.4–5
Gilliland et al. hypothesized that the development of AML requires at least two types of genetic events.6 Type-I aberrations occur as mutations in hotspots of specific genes involved in signal transduction pathways (FLT3, KIT, NRAS, KRAS and PTPN11) which lead to uncontrolled proliferation and/or survival of leukemic cells. Type-II aberrations are often chromosomal rearrangements of transcription factors resulting in the translation of fusion proteins leading to impaired differentiation of the leukemic cells, including PML-RARα [t(15;17)(q22;q21)], AML1-ETO [t(8;21)(q22;q22)], CBFB-MYH11 [inv(16)(p13q22)/t(16;16)(p13;q22)] and 11q23/MLL-rearrangements. This hypothesis was further strengthened by observations from mouse models that one aberration is not sufficient to induce leukemia, but that cooperative events are needed to develop frank leukemia. For example, knock-in of FLT3-ITD leads to the development of a myeloproliferative disorder but lacks the maturation arrest typical of acute leukemia,7 whereas co-expression with inv(16)(p13q22) or t(15;17)(q22;q21) resulted in AML.8–9
In pediatric AML, the individual type-I or type-II aberrations and their relationship with clinical outcome have been described in several studies.4–5, 10–16 However, there has been no comprehensive overview of the associations and the prognostic impact of type-I and type-II aberrations in one large cohort of pediatric AML patients. Furthermore, over the last decade, novel molecular genetic aberrations in pediatric AML, such as mutations in the CEBPA, NPM1 and WT1 genes, as well as partial tandem duplications in the MLL gene (MLL-PTD), have been identified.11,13,17 The prognostic impact of these newly identified aberrations all together in one large pediatric AML series has not so far been reported. Identifying prognostic factors in pediatric AML may lead to improved risk-group stratification, and may, therefore, have a direct impact on current and future treatment protocols. Secondly, specific leukemogenic aberrations may guide the development of targeted therapy approaches for selected patient groups.
We, therefore, performed a study on type-I and type-II aberrations in the largest pediatric AML series so far, which also focused on their association with clinical characteristics and outcome.
Design and Methods
Study cohort
This study included 506 pediatric patients with de novo AML, the data for whom were provided by the Dutch Childhood Oncology Group (DCOG), the AML ‘Berlin-Frankfurt-Münster’ Study Group (AML-BFM-SG), the Czech Pediatric Hematology (CPH) group, and the St. Louis Hospital in Paris, France. Institutional review board approval for these studies and informed consent was obtained according to local laws and regulations. Each study group performed a central review of the morphology according to the WHO/FAB classification.18 Clinical and cell-biological data, including cytogenetic results, were obtained from these study groups and institutes.
Survival analysis was restricted to a subset of 385 AML patients who received relatively homogenous treatment according to DCOG/AML-BFM 87, DCOG 92-94/AML-BFM 93, AML-BFM 98, AML-BFM 04 and MRC-12/15 protocols. Details of these treatment protocols and overall outcome data have already been published.19–24 Treatment consisted of 4 to 5 blocks of intensive chemotherapy, using a standard cytarabine and anthracycline backbone. Stem cell transplantation in first complete remission was only performed in a small number of selected high-risk patients.
Definition of gene mutations as type-I and type-II aberrations
Screening of gene mutations was carried out according to the availability of material. Mutations were determined in the hotspot regions of NPM1 (n=337), CEPBA (n=282), MLL (i.e. partial tandem duplications - PTD; n=309), WT1 (n=330), FLT3 (i.e. internal tandem duplications - ITD; n=372) and tyrosine kinase domain mutations (TKD; n=330), N-RAS and K-RAS (n=353), PTPN11 (n=350) and KIT (n=368), as previously described.13–14,25–29 This resulted in screening of all type-I aberrations in 330 cases and all type-II aberrations in 263 cases. A complete list of screened regions per gene, primers and PCR conditions is provided in the Online Supplementary Table S1. The ‘fusion gene’ type-II aberrations, i.e. MLL-rearrangements, t(8;21)(q22;q22), inv(16)(p13q22)/t(16;16)(p13;q22), t(15;17)(q22;q21), were mutually exclusive with NPM1 mutations, CEBPA double mutations and MLL-PTD aberrations, which suggests that these latter mutations can be considered as type-II aberrations. This is further strengthened by evidence that these aberrations result in a maturation arrest; e.g. targeted disruption of C/EBPα results in a selective early block of granulocyte differentiation,30 while NPM1 mutations and MLL-PTD disrupt the controlled expression of HOX-genes resulting in impaired differentiation of the hematopoietic cells.31–33 Hence NPM1 mutations, CEBPA double mutations, and MLL-PTD aberrations were considered to be type-II aberrations, whereas mutations in FLT3, N/K-RAS, PTPN11 and KIT were considered to be type-I aberrations. As the leukemogenic mechanism of WT1 mutations still needs to be clarified,34 these mutations were arbitrarily categorized as type-I aberrations for the purposes of this study because they overlapped with the type-II defined subtypes.
Further information concerning DNA and RNA isolation, cytogenetic analysis, definition of cytogenetic groups and statistical analysis is available in the Online Supplementary Appendix.
Results
Study cohort
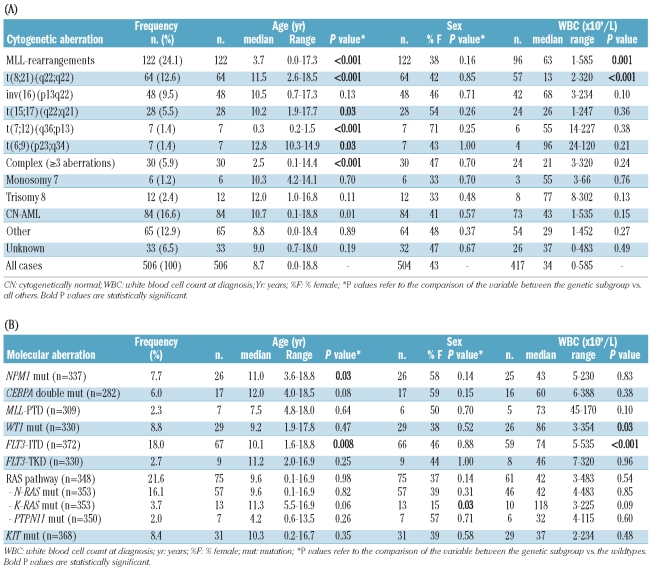
Characteristics of the study cohort are presented in Table 1. Sex distribution was 57% male versus 43% female. Median age was 8.7 years (range 0–18 years), and the distribution according to the age categories under two years, 2–9 years and ten years and over was 18%, 38% and 44%, respectively. The median white blood cell count (WBC) at diagnosis was 34×109/L (range 0–585×109/L). FAB-M2, -M4 and -M5 were the most common morphological subtypes in this cohort; these were 23%, 24% and 24%, respectively. Distribution according to sex, age, WBC and FAB-morphology was comparable with the AML-BFM trials, i.e. the AML-BFM 93 (n=471) and 98 (n=473) trials, indicating that our cohort is representative for pediatric AML (Online Supplementary Tables S2 and S3). The 385 pediatric AML cases included in the survival analysis had a 5-year probability of event-free survival (pEFS) and overall survival (pOS) of 42±3% and 60±3%, respectively. These survival rates are in a similar range to those of previously published studies.35
Table 1.
Overview of baseline clinical characteristics per cytogenetic (A) and molecular (B) aberration.
Characteristics of pediatric AML with specific cytogenetic subtypes
Patients were assigned to the following cytogenetic groups: MLL-rearranged AML (24%; 122/506), t(8;21)(q22;q22) (13%; 64/506), inv(16)(p13q22)/t(16;16) (p13;q22) (10%; 48/506), t(15;17)(q22;q21) (6%; 28/506), t(7;12)(q36;p13) (1%; 7/506), t(6;9)(p23;q34) (1%; 7/506), complex karyotype (6%; 30/506), monosomy 7 (1%; 6/506), trisomy 8 (2%; 12/506), CN-AML (17%; 84/506), and ‘other karyotype’ (13%; 65/506) (Table 1A). In 7% (33/506) of the cases, conventional karyotyping failed, and neither RT-PCR nor FISH led to classification of these patients; these cases were, therefore, assigned to the ‘unknown’ cytogenetic group. An overview of the cytogenetic group assignment and the mutational status of the investigated genes of all individual patients is provided in the Online Supplementary Table S4.
No difference in sex distribution was seen between the different cytogenetic groups (Table 1A). Patients with t(8;21)(q22;q22) presented with a significantly lower WBC (median 13×109/L; P<0.001), and MLL-rearranged AML patients with a significantly higher WBC (median 63×109/L; P=0.001) compared with the other cytogenetic groups (Table 1A). The median ages of children with MLL-rearranged AML (3.7 years; P<0.001), with t(7;12)(q36;p13) (0.3 years; P<0.001) and with a complex karyotype (2.5 years; P<0.001) were all significantly lower compared with the other cytogenetic groups. In contrast, children with t(8;21)(q22;q22) (11.5 years; P<0.001), t(15;17)(q22;q21) (10.2 years; P=0.03), and t(6;9)(p23;q34) (12.8 years; P=0.03) were significantly older compared with the other cytogenetic groups (Table 1A).
Characteristics of pediatric AML patients with type-II gene mutations
The following frequencies of gene mutations considered as type-II aberrations were found: NPM1 (8%; 26/337), CEBPA double mutations (6%; 17/282) and MLL-PTD (2%; 7/309) (Table 1B). These aberrations were mainly present in patients with CN-AML, and were mutually exclusive with all other type-II aberrations. No differences were found in WBC or sex between patients carrying any of these aberrations and those without the indicated type-II aberration. Patients with NPM1-mutated AML were significantly older (median 11.0 years; P=0.03) compared to their wild-type counterparts (Table 1B).
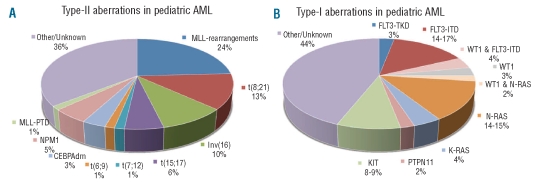
In 180/506 (36%) cases, neither one of the type-II gene mutations in NPM1, CEBPA and MLL, nor one of the ‘fusion gene’ type-II aberrations, i.e. MLL-rearrangements, t(8;21)(q22;q22), inv(16)(p13q22)/t(16;16)(p13;q22), t(15;17)(q22;q21), t(7;12)(q36;p13) and t(6;9)(p23;q34), were identified (Figure 1A). This percentage, however, is probably slightly lower (estimated ~33%) as we could only screen 118 of these 180 cases for NPM1, and 101 of the 180 cases for CEBPA and MLL-PTD mutations (Online Supplementary Table S5).
Figure 1.
Distribution of the different type-I and type-II aberrations in pediatric AML. The heterogeneity of pediatric AML is reflected by the presence of the different type-I and type-II genetic aberrations. However, in a large number of cases the type-II (A) or type-I (B) aberrations have not yet been identified.
Characteristics of pediatric AML patients with type-I aberrations
For the classical type-I aberrations, i.e. mutations in FLT3, N- and K-RAS, PTPN11 and KIT, the following frequencies were found: FLT3-ITD (18%; 67/372), FLT3-TKD (3%; 9/330), N-RAS (16%; 57/353), K-RAS (4%; 13/353), PTPN11 (2%; 7/350) and KIT (8%; 31/368) (Table 1B). WT1 mutations, which in this study were arbitrarily categorized as type-I aberrations, were found in 9% (29/330). Together, we identified type-I aberrations in 185/330 (56%) cases (Figure 1B). As far as sex distribution of the different type-I aberrations is concerned, K-RAS mutations were significantly associated with male sex (85% vs. 54% of K-RAS wild-type cases; P=0.03). FLT3-ITD-positive and WT1-mutated AML cases had a significantly higher WBC (median 74×109/L; P<0.001, and 86×109/L; P=0.03, respectively) compared to their wild-type counterparts. Patients with FLT3-ITD-positive AML had a significantly higher median age (10.1 years; P=0.008) compared to their wild-type counterparts (Table 1B).
Non-random associations between type-I and type-II aberrations in pediatric AML
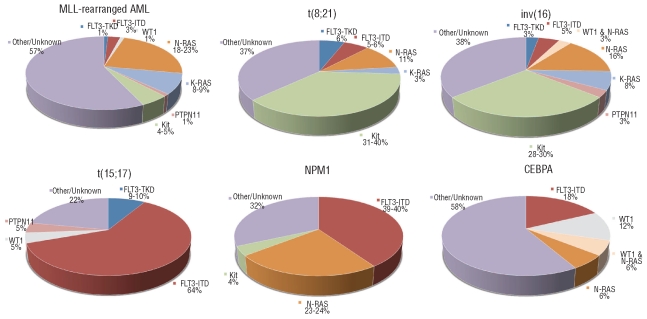
An overview of the associations between the type-I and type-II aberrations is presented in the Online Supplementary Table S5. Although FLT3-ITD mutations were identified in almost all type-II defined subtypes, the majority (42%) were restricted to cases with t(15;17)(q22;q21), MLL-PTD and NPM1 mutations, in which 64%, 57% and 39%, respectively, harbored an FLT3-ITD (Figure 2). It is interesting to note that FLT3-ITD was also simultaneously present in 41% of the WT1-mutated cases. For FLT3-TKD mutations, which were far less frequent in pediatric AML, the highest frequencies were found in the MLL-PTD (17%) and t(15;17)(q22;q21) (10%) subtypes.
Figure 2.
Type-I aberrations per type-II defined subtype. Distribution of the different type-I aberrations according to the different type-II defined subtypes including more than 10 cases, i.e. MLL-rearrangements, t(8;21), inv(16), t(15;17), NPM1-mutated and CEBPA double mutated AML.
KIT mutations clearly associated with core-binding factor AML (CBF-AML), i.e. t(8;21)(q22;q22) and inv(16)(p13q22)/t(16;16)(p13;q22) (P<0.001). They were observed in 31% of t(8;21)(q22;q22) cases and in 28% of the inv(16)(p13q22)/t(16;16)(p13;q22) cases (Figure 2).
The N-RAS, K-RAS and PTPN11 gene mutations, combined together as RAS-pathway activating mutations, showed an equal distribution among the patients with different type-II aberrations. The exception was the t(15;17)(q22;q21) subtype, in which no RAS-pathway mutations were observed, except for only one PTPN11 mutation. Interestingly, K-RAS mutations, which were four times less frequent than N-RAS mutations, were more frequently seen in MLL-rearranged AML and in CBF-AML, while N-RAS mutations were most prevalent in t(6;9)(p23;q34) and NPM1-mutated AML. In MLL-rearranged AML, 43% carried one of the investigated type-I aberrations, of which the majority were RAS-pathway aberrations (33%; Figure 2).
WT1 mutations were mainly present in t(6;9)(p23;q34)-AML (33%), CEBPA double-mutant AML (18%) and the subtype with ‘other/unknown’ type-II aberrations (20%).
The distribution of genetic aberrations is highly correlated with age in pediatric AML
We investigated the frequency of genetic subtypes, according to the age categories 0–2 years, 2–5 years, 5–10 years, 10–15 years, and 15 years and older (Online Supplementary Figure S1A-B). The largest differences in genetic aberrations were detected between children under two years of age and children two years of age and over. We, therefore, focused further on these 2 age groups (Online Supplementary Figure S1C-D). In children under two years of age, significantly higher frequencies of MLL-rearrangements and complex karyotypes were observed when compared with children two years of age and over: 51% versus 18%; P<0.001, and 13% versus 4%; P=0.001, respectively. Furthermore, the youngest age category included all 7 patients with a t(7;12)(q36;p13) (P<0.001). In contrast, in this age category t(8;21)(q22;q22) was not observed, but this translocation was found in 15% (P<0.001) of children two years of age and over. The t(15;17)(q22;q21) was only present in a single case under two year of ages (1.9 years; 1%) but occurred significantly more often in children two years of age and over (7%; P=0.04). Furthermore, in children two years and over, a higher frequency of CN-AML was found compared with children under two years of age (respectively 19% vs. 6%, P=0.002) (Online Supplementary Figure S1C). NPM1, CEBPA and MLL-PTD aberrations were not detected in any of the patients under two years of age. In both age categories (<2 years and ≥2 years) the percentage of ‘other/unknown’ type-II aberrations was about one-third that of the AML, but there was a clear difference in the distribution of the different type-II aberrations (Online Supplementary Figure S2A).
In children two years of age and over, a significantly higher frequency of FLT3-ITD was found (21% vs. 3% in children <2 years; P=0.001), and a trend was observed for a higher frequency of WT1 mutations in patients two years of age and over (10% vs. 2% in children <2 years; P=0.06). Furthermore, all FLT3-TKD (n=9) and K-RAS mutations (n=13) were found in children two years of age and over, although this did not reach statistical significance. In contrast, both age categories included almost similar frequencies of N-RAS (15–20%), PTPN11 (2–3%) and KIT mutations (5–10%) (Online Supplementary Figures S1D and S2B). When RAS-pathway aberrations were taken together, this pathway was affected at a similar frequency in both age categories (22% vs. 21% in children < 2 years and ≥2 years of age, respectively). In children under two years of age, 67% of the cases did not harbor one of the investigated type-I aberrations (i.e. FLT3-ITD, FLT3-TKD, NRAS, KRAS, PTPN11, KIT or WT1) versus only 40% in children two years of age and over (P<0.001). The difference between these age categories could largely be explained by the frequency of FLT3-ITD, which was only sporadically found in children under two years of age (Online Supplementary Figure S2B).
Clinical outcome of pediatric AML according to type-I and type-II aberrations
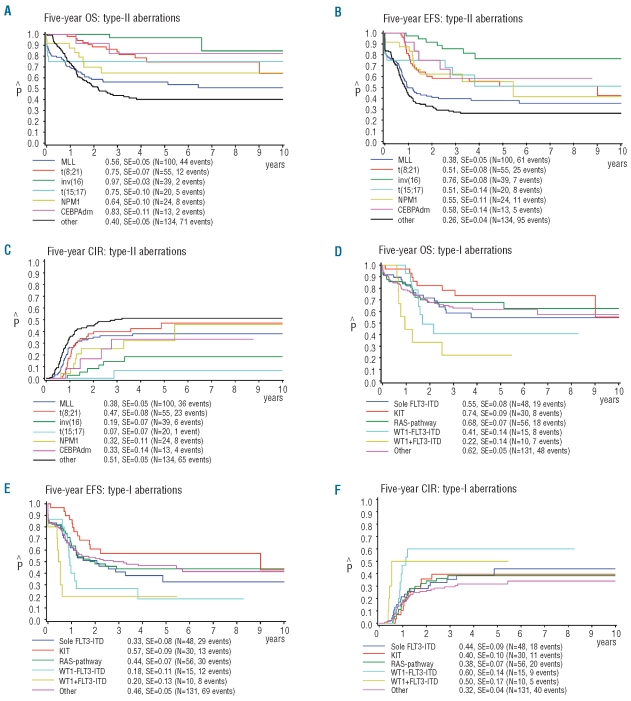
Survival analysis was only performed for the type-I and type-II defined subtypes containing more than 10 cases. For the type-II aberrations, this included MLL-rearrangements, t(8;21)(q22;q22), inv(16)(p13q22)/t(16;16) (p13;q22), t(15;17)(q22;q21), as well as NPM1- and CEBPA-double mutated cases. All other cases were grouped together and considered ‘other/unknown’ type-II aberrations (Table 2A). The Kaplan-Meier curves showed large differences between the different type-II aberrations for 5-year pOS, 5-year pEFS, and 5-year CIR (Figure 3A-C). Patients carrying an inv(16)(p13q22)/t(16;16)(p13;q22)-AML showed the most favorable outcome with a 5-year pOS, 5-year pEFS, and 5-year CIR of 97±3%, 76±8% and 19±7%, respectively. MLL-rearranged AML and the group with ‘other/unknown’ type-II aberrations showed the worst outcome with 5-year pOS of 56±5% and 40±5%, respectively, 5-year pEFS of 38±5% and 26±4%, respectively, and 5-year CIR of 38±5% and 51±5%, respectively. Interestingly, cases with a t(8;21)(q22;q22) had a relatively high 5-year CIR of 47±8%; this seemed to be related to concurrent KIT mutations, although numbers were too small to draw definitive conclusions. Cases with a t(15;17)(q22;q21) only had a 5-year CIR of 7±7%. For survival analysis of the type-I aberrations, cases with various RAS-pathway aberrations were combined. WT1-mutated AML cases were analyzed according to their FLT3-ITD status. All other cases were grouped together as ‘other/unknown’ type-I cases for the analysis. The Kaplan-Meier curves showed differences in 5-year pOS, 5-year pEFS, and 5-year CIR for the different type-I aberrations (Figure 3D-F). Cases with a combined WT1 mutation and FLT3-ITD showed the worst prognosis with 5-year pOS of 22±14%, 5-year pEFS of 20±13%, and 5-year CIR of 50±17%.
Table 2A.
Univariate analysis for survival parameters of pediatric AML.
Figure 3.
Survival analysis of the type-I and type-II aberrations in pediatric AML. Kaplan-Meier estimates for (A+D) pOS, (B+E) pEFS and (C+F) CIR for the different type-II and type-I aberrations, respectively.
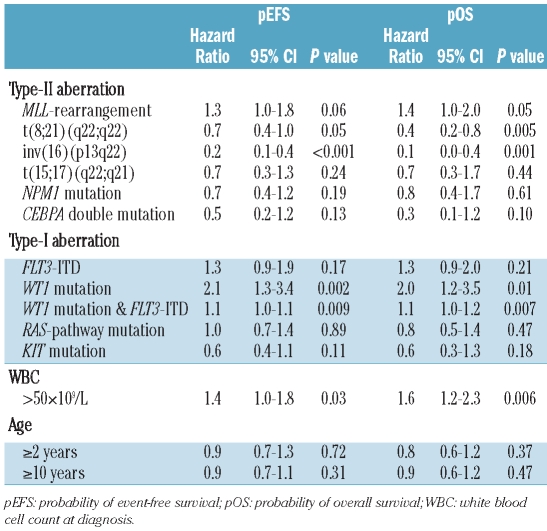
Independent prognostic factors in pediatric AML
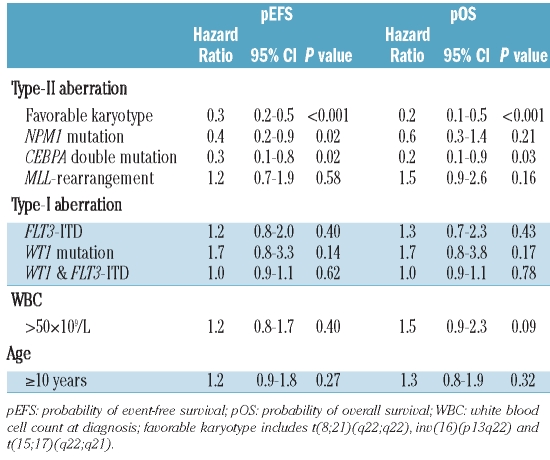
In order to reduce the number of variables in a multivariate Cox’s proportional hazard model, CBF-AML and t(15;17)(q22;q21) were grouped together as the variable ‘favorable karyotype’ (Table 2B). The following variables, which were significant in univariate analyses and/or commonly used in pediatric AML (WBC>50×109/L and age >10 years), entered the model: ‘favorable karyotype’, MLL-rearrangements, NPM1 mutations, CEBPA double mutations, FLT3-ITD, WT1 mutations, the combination of WT1 mutation plus an FLT3-ITD, WBC and age over ten years. This model identified favorable karyotype (hazard ratio (HR) 0.3, P<0.001), NPM1 mutations (HR 0.4, P=0.02) and CEBPA double mutations (HR 0.3, P=0.02) as independent prognostic factors for pEFS. For pOS, favorable karyotype was an independent prognostic factor (HR 0.2, P<0.001). Furthermore, CEBPA double mutations (HR 0.2, P=0.03) independently predicted favorable pOS.
Table 2B.
Multivariate analysis for survival parameters of pediatric AML.
Discussion
Unraveling the genetics of pediatric AML provides a basis on which to improve risk group stratification. Furthermore, specific genetic aberrations could direct the development of targeted therapy approaches. The low incidence of pediatric AML makes it difficult to describe the relevance of these aberrations, and published data often focus on only one specific aberration. This study describes for the first time a large cohort of pediatric AML cases characterized for various cytogenetic and molecular genetic aberrations, allowing the comprehensive study of non-random associations, and their correlation with clinical characteristics and outcome.
We confirmed the non-random associations previously described between the different types of aberrations, e.g. KIT mutations with CBF-AML and FLT3-ITD with t(15;17)(q22;q21).36–37 Moreover, FLT3-ITD significantly associated with NPM1-mutated (39%) and with WT1-mutated AML (41%). It is interesting to note that the association of FLT3/ITD was not correlated with a specific type of NPM1 or WT1 mutation (data not shown). As AML is likely to result from a multistep pathogenesis, it is conceivable that FLT3/ITD and WT1 mutations are associated with additional aberrations, and it has recently been shown that their combination is frequently present in the rare subtype of adult AML harboring NUP98-rearrangments.38 Overall, the majority of type-I aberrations showed an unequal distribution over the different type-II defined subtypes. Although MLL-rearranged AML harbored one of the lowest frequencies of type-I aberrations (43%), mutations in the RAS-signaling pathway interestingly represented the vast majority in MLL-rearranged AML.
Striking differences in genetic subtypes were found between children younger and older than two years at diagnosis of AML. Very young children with AML were characterized by a high frequency of MLL-rearrangements (51%), as previously reported.5 Furthermore, they were characterized by a higher frequency of complex karyotypes, the exclusive presence of t(7;12)(q36;p13), and low frequencies or even total absence of t(8;21)(q22;q22), t(15;17)(q22;q21) and CN-AML. Moreover, the increasing incidence of CN-AML in childhood is continued into adulthood, in which CN-AML is present in approximately 45% of AML cases, whereas MLL-rearrangements are rare in adult AML.39 We did not observe any difference in outcome between the age categories under two years and over two years. A recent large German study showed in more detail that adolescents (13–21 years) had a slightly inferior outcome compared to younger children, but no difference was seen between infants (0–2 years) and young children (2–13 years).40 Although this does not suggest that different treatment strategies based on age are of any benefit, it is conceivable that the biological differences may lead to different treatment strategies for these age categories in the future.
Besides the different frequencies of several cytogenetic aberrations between pediatric AML and adult AML (see above), type-II gene mutations also displayed different frequencies within pediatric AML as well as between pediatric and adult AML. NPM1 mutations, CEBPA double mutations and MLL-PTD did not occur in children below the age of two years. In line with this observation, NPM1 mutations and MLL-PTD are less frequent in pediatric compared to adult AML (5–8% and 1–3% vs. 35% and 3–6%, respectively). In contrast, CEBPA double mutations display relatively similar frequencies (3–6% vs. 4–10%) between children and adults. Regarding type-I aberrations, pediatric AML cases harbor less frequently FLT3-ITD and FLT3-TKD, but RAS-pathway aberrations (PTPN11, N-RAS and K-RAS mutations) and KIT mutations have comparable frequencies.41 WT1 mutations seem to occur at a higher frequency in pediatric versus adult AML: 8–12% versus approximately 5–7%, respectively.
Different type-I and type-II aberrations clearly had an impact on clinical outcome. In addition to the established favorable prognostic cytogenetic group including t(8;21)(q22;q22), inv(16)(p13q22)/t(16;16)(p13;q22), and t(15;17)(q22;q21), the type-II gene mutations NPM1 and CEBPA double mutations were of independent prognostic relevance in pediatric AML when all prognostic factors were considered. From this, the established favorable cytogenetic risk group in pediatric AML can be extended with the molecular aberrations NPM1 and CEBPA double mutations, and will now include approximately 35–40% of pediatric AML cases. MLL-rearrangements did not have any impact on clinical outcome, which is in agreement with our recent report that not MLL-rearrangements per se, but the specific MLL-translocation partners independently predict prognosis.42 Regarding the type-I aberrations, WT1 mutations and the combination of a WT1 mutation and FLT3/ITD characterized poor prognostic subgroups in univariate analyses. These aberrations could not be shown to have independent prognostic significance in multivariate analyses, which might be influenced by the small numbers. We previously showed that this group with combined FLT3-ITD/WT1 mutation had a dismal 5-year survival of 21%,13 and this was confirmed by a large pediatric AML study from the COG.12 FLT3-ITD did not have any prognostic value in our study, which might be influenced by the mutant/wild-type ratio. This has been shown to have a big influence on the prognostic impact,16 or by its association with other favorable aberrations such as t(15;17)(q22;q21) and NPM1 mutations. The investigation of the impact of the different type-I aberrations within specific subtypes of AML was restricted by small numbers, although KIT mutations seemed to be associated with the relatively high relapse rate in t(8;21)(q22;q22). However, in a large COG series, Pollard et al. recently showed that KIT mutations lacked prognostic significance in pediatric CBF-AML in contrast to adult CBF-AM.43–44 This shows that further risk stratification in pediatric AML based on genetic aberrations has to be further validated by prospective pediatric studies.
Our study has implications for diagnostics in pediatric AML, and based on their frequency, impact on outcome, and possible target for therapy, we would currently suggest to screen for the fusion genes t(8;21)(q22;q22), inv(16)(p13q22)/t(16;16)(p13;q22), t(15;17)(q22;q21), and MLL-rearrangements [specifically t(6;11)(q27;q23), t(10;11)(p11.2/p12;q23) and t(1;11) (q21;q23)],42 and for NPM1, CEBPA, WT1 and FLT3/ITD mutations, as well as KIT mutations in CBF-AML.
Current pediatric AML treatment protocols consist of very intensive chemotherapy regimens which induce considerable toxicity. To further improve outcome in pediatric AML, new treatment strategies are needed. Different compounds targeting type-I aberrations are currently under development. The poor prognostic group combining an FLT3/ITD and a WT1 mutation may potentially benefit from simultaneously targeting these aberrations. Activated FLT3 can be targeted by compounds such as midostaurin, lestaurtanib, sorafenib, and other multi-targeted tyrosine kinase inhibitors.45–46 However, so far monotherapy with these agents in adult AML has shown limited clinical activity.47–48 In combination with chemotherapy, upregulation of FLT3-ligand might be a newly identified resistance mechanism.49 Moreover, a recent randomized placebo-controlled trial of sorafenib did not show any benefit in patients in the experimental arm.50 Therefore, we still need to see whether this strategy turns out to be successful. Compounds targeting WT1 mutations are currently not available. Still, high expression of the WT1 gene is found in most AML cases, and all WT1-mutated cases show high WT1 expression.51 Immunotherapy using a WT1-peptide vaccine is being developed, and a phase II trial in adult AML showed promising results.52 In general, due to the different cooperating genetic events in AML, monotherapy, as with imatinib in CML (where a single fusion gene drives the disease), does not seem to be feasible,45 and combinations of inhibitors may be required to efficiently kill the leukemic cells.
Intriguingly, in approximately 44% and 33–36% of pediatric AML cases, respectively, none of the investigated type-I or type-II aberrations were present. It needs to be mentioned that we may have missed mutations outside the screened hotspot regions, although from previous studies we expect them to be relatively rare. Furthermore, RUNX1 mutations were not determined but a recent report suggests they are infrequent events in pediatric AML.53 Over the last decade efforts have been made to identify the remaining genetic aberrations with high-throughput screening techniques, e.g. by genome-wide copy number analyses (using high resolution array-CGH and SNP-arrays), and by re-sequencing candidate genes such as all kinase-coding genes.25, 54–56 Although the former led to the discovery of ASXL1 and TET2 mutations, it also revealed that AML harbored only a small number of genomic alterations compared with other cancers.56 High-throughput sequencing of the first whole genomes of adult AML identified mutations in the metabolites IDH1 and IDH2, and recently in the DNA methyltransferase gene DNMT3A, which both seemed frequent in adult AML.57–58 Interestingly, in agreement with the hypothesis that AML results from a multistep pathogenesis, these aberrations might add an additional class of mutations as aberrant TET2, IDH1 and −2 and DNMT3A have been shown to affect the epigenetic landscape of AML. Recent studies, however, indicated that these mutations might be rare in pediatric AML,59–61 stressing the need for separate pediatric studies to discover the remaining genetic aberrations, including aberrations in miRNA-coding genes or in methylation of genes or their promoter regions.
In conclusion, the heterogeneity of pediatric AML is reflected by the presence of different age-dependent and clinically relevant genetic aberrations, allowing prognostically relevant groups to be identified. In addition, several non-random associations between genetic aberrations have been observed. The addition of these aberrations will help us to stratify pediatric AML and to direct further development towards targeted therapies.
Footnotes
Funding: BB was funded by the ‘Netherlands Organisation for Scientific Research’ (NWO) and the KOCR foundation. IH was funded by the KOCR foundation. HBB was partly funded by the Dutch Cancer Society (grant EMCR 2003-2871).
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Downing JR, Shannon KM. Acute leukemia: a pediatric perspective. Cancer Cell. 2002;2(6):437–45. doi: 10.1016/s1535-6108(02)00211-8. [DOI] [PubMed] [Google Scholar]
- 2.Kaspers GJ, Zwaan CM. Pediatric acute myeloid leukemia: towards high-quality cure of all patients. Haematologica. 2007;92(11):1519–32. doi: 10.3324/haematol.11203. [DOI] [PubMed] [Google Scholar]
- 3.Raimondi SC, Chang MN, Ravindranath Y, Behm FG, Gresik MV, Steuber CP, et al. Chromosomal abnormalities in 478 children with acute myeloid leukemia: clinical characteristics and treatment outcome in a cooperative pediatric oncology group study-POG 8821. Blood. 1999;94(11):3707–16. [PubMed] [Google Scholar]
- 4.Harrison CJ, Hills RK, Moorman AV, Grimwade DJ, Hann I, Webb DK, et al. Cytogenetics of childhood acute myeloid leukemia: United Kingdom Medical Research Council. Treatment trials AML 10 and 12. J Clin Oncol. 28(16):2674–81. doi: 10.1200/JCO.2009.24.8997. [DOI] [PubMed] [Google Scholar]
- 5.von Neuhoff C, Reinhardt D, Sander A, Zimmermann M, Bradtke J, Betts DR, et al. Prognostic impact of specific chromosomal aberrations in a large group of pediatric patients with acute myeloid leukemia treated uniformly according to trial AML-BFM 98. J Clin Oncol. 2010;28(16):2682–9. doi: 10.1200/JCO.2009.25.6321. [DOI] [PubMed] [Google Scholar]
- 6.Gilliland DG, Griffin JD. The roles of FLT3 in hematopoiesis and leukemia. Blood. 2002;100(5):1532–42. doi: 10.1182/blood-2002-02-0492. [DOI] [PubMed] [Google Scholar]
- 7.Li L, Piloto O, Nguyen HB, Greenberg K, Takamiya K, Racke F, et al. Knock-in of an internal tandem duplication mutation into murine FLT3 confers myeloproliferative disease in a mouse model. Blood. 2008;111(7):3849–58. doi: 10.1182/blood-2007-08-109942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim HG, Kojima K, Swindle CS, Cotta CV, Huo Y, Reddy V, et al. FLT3-ITD cooperates with inv(16) to promote progression to acute myeloid leukemia. Blood. 2008;111(3):1567–74. doi: 10.1182/blood-2006-06-030312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelly LM, Kutok JL, Williams IR, Boulton CL, Amaral SM, Curley DP, et al. PML/RARalpha and FLT3-ITD induce an APL-like disease in a mouse model. Proc Natl Acad Sci USA. 2002;99(12):8283–8. doi: 10.1073/pnas.122233699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goemans BF, Zwaan CM, Miller M, Zimmermann M, Harlow A, Meshinchi S, et al. Mutations in KIT and RAS are frequent events in pediatric core-binding factor acute myeloid leukemia. Leukemia. 2005;19(9):1536–42. doi: 10.1038/sj.leu.2403870. [DOI] [PubMed] [Google Scholar]
- 11.Ho PA, Alonzo TA, Gerbing RB, Pollard J, Stirewalt DL, Hurwitz C, et al. Prevalence and prognostic implications of CEBPA mutations in pediatric acute myeloid leukemia (AML): a report from the Children’s Oncology Group. Blood. 2009;113(26):6558–66. doi: 10.1182/blood-2008-10-184747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ho PA, Zeng R, Alonzo TA, Gerbing RB, Miller KL, Pollard JA, et al. Prevalence and prognostic implications of WT1 mutations in pediatric acute myeloid leukemia (AML): a report from the Children’s Oncology Group. Blood. 2010;116(5):702–10. doi: 10.1182/blood-2010-02-268953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hollink IH, van den Heuvel-Eibrink MM, Zimmermann M, Balgobind BV, Arentsen-Peters ST, Alders M, et al. Clinical relevance of Wilms tumor 1 gene mutations in childhood acute myeloid leukemia. Blood. 2009;113(23):5951–60. doi: 10.1182/blood-2008-09-177949. [DOI] [PubMed] [Google Scholar]
- 14.Hollink IH, Zwaan CM, Zimmermann M, Arentsen-Peters TC, Pieters R, Cloos J, et al. Favorable prognostic impact of NPM1 gene mutations in childhood acute myeloid leukemia, with emphasis on cytogenetically normal AML. Leukemia. 2009;23(2):262–70. doi: 10.1038/leu.2008.313. [DOI] [PubMed] [Google Scholar]
- 15.Meshinchi S, Woods WG, Stirewalt DL, Sweetser DA, Buckley JD, Tjoa TK, et al. Prevalence and prognostic significance of Flt3 internal tandem duplication in pediatric acute myeloid leukemia. Blood. 2001;97(1):89–94. doi: 10.1182/blood.v97.1.89. [DOI] [PubMed] [Google Scholar]
- 16.Zwaan CM, Meshinchi S, Radich JP, Veerman AJ, Huismans DR, Munske L, et al. FLT3 internal tandem duplication in 234 children with acute myeloid leukemia: prognostic significance and relation to cellular drug resistance. Blood. 2003;102(7):2387–94. doi: 10.1182/blood-2002-12-3627. [DOI] [PubMed] [Google Scholar]
- 17.Balgobind BV, Hollink IH, Reinhardt D, van Wering ER, de Graaf SS, Baruchel A, et al. Low frequency of MLL-partial tandem duplications in paediatric acute myeloid leukaemia using MLPA as a novel DNA screenings technique. Eur J Cancer. 2010;46(10):1892–9. doi: 10.1016/j.ejca.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 18.Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937–51. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 19.Creutzig U, Zimmermann M, Ritter J, Reinhardt D, Hermann J, Henze G, et al. Treatment strategies and long-term results in paediatric patients treated in four consecutive AML-BFM trials. Leukemia. 2005;19(12):2030–42. doi: 10.1038/sj.leu.2403920. [DOI] [PubMed] [Google Scholar]
- 20.Gibson BE, Wheatley K, Hann IM, Stevens RF, Webb D, Hills RK, et al. Treatment strategy and long-term results in paediatric patients treated in consecutive UK AML trials. Leukemia. 2005;19(12):2130–8. doi: 10.1038/sj.leu.2403924. [DOI] [PubMed] [Google Scholar]
- 21.Kardos G, Zwaan CM, Kaspers GJ, de-Graaf SS, de Bont ES, Postma A, et al. Treatment strategy and results in children treated on three Dutch Childhood Oncology Group acute myeloid leukemia trials. Leukemia. 2005;19(12):2063–71. doi: 10.1038/sj.leu.2403873. [DOI] [PubMed] [Google Scholar]
- 22.Creutzig U, Zimmermann M, Lehrnbecher T, Graf N, Hermann J, Niemeyer CM, et al. Less toxicity by optimizing chemotherapy, but not by addition of granulocyte colony-stimulating factor in children and adolescents with acute myeloid leukemia: results of AML-BFM 98. J Clin Oncol. 2006;24(27):4499–506. doi: 10.1200/JCO.2006.06.5037. [DOI] [PubMed] [Google Scholar]
- 23.Burnett AK, Hills RK, Milligan D, Kjeldsen L, Kell J, Russell NH, et al. Identification of patients with acute myeloblastic leukemia who benefit from the addition of gem-tuzumab ozogamicin: results of the MRC AML15 trial. J Clin Oncol. 2011;29(4):369–77. doi: 10.1200/JCO.2010.31.4310. [DOI] [PubMed] [Google Scholar]
- 24.Creutzig U, Martin Z, Dworzak M, Bourquin J-P, Neuhoff C, Sander A, et al. Excellent Outcome In Infants below One Year of Age with AML - Results of Studies AML-BFM -98 and -2004. ASH Annual Meeting Abstracts. 2010;116(21):17. [Google Scholar]
- 25.Balgobind BV, Van Vlierberghe P, van den Ouweland AM, Beverloo HB, Terlouw-Kromosoeto JN, van Wering ER, et al. Leukemia-associated NF1 inactivation in patients with pediatric T-ALL and AML lacking evidence for neurofibromatosis. Blood. 2008;111(8):4322–8. doi: 10.1182/blood-2007-06-095075. [DOI] [PubMed] [Google Scholar]
- 26.Barjesteh van Waalwijk van Doorn-Khosrovani S, Erpelinck C, Meijer J, van Oosterhoud S, van Putten WL, Valk PJ, et al. Biallelic mutations in the CEBPA gene and low CEBPA expression levels as prognostic markers in intermediate-risk AML. Hematol J. 2003;4(1):31–40. doi: 10.1038/sj.thj.6200216. [DOI] [PubMed] [Google Scholar]
- 27.Caligiuri MA, Strout MP, Schichman SA, Mrozek K, Arthur DC, Herzig GP, et al. Partial tandem duplication of ALL1 as a recurrent molecular defect in acute myeloid leukemia with trisomy 11. Cancer Research. 1996;56(6):1418–25. [PubMed] [Google Scholar]
- 28.Kiyoi H, Naoe T, Yokota S, Nakao M, Minami S, Kuriyama K, et al. Internal tandem duplication of FLT3 associated with leukocytosis in acute promyelocytic leukemia. Leukemia Study Group of the Ministry of Health and Welfare (Kohseisho) Leukemia. 1997;11(9):1447–52. doi: 10.1038/sj.leu.2400756. [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto Y, Kiyoi H, Nakano Y, Suzuki R, Kodera Y, Miyawaki S, et al. Activating mutation of D835 within the activation loop of FLT3 in human hematologic malignancies. Blood. 2001;97(8):2434–9. doi: 10.1182/blood.v97.8.2434. [DOI] [PubMed] [Google Scholar]
- 30.Zhang DE, Zhang P, Wang ND, Hetherington CJ, Darlington GJ, Tenen DG. Absence of granulocyte colony-stimulating factor signaling and neutrophil development in CCAAT enhancer binding protein alpha-deficient mice. Proc Natl Acad Sci USA. 1997;94(2):569–74. doi: 10.1073/pnas.94.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vassiliou GS, Cooper JL, Rad R, Li J, Rice S, Uren A, et al. Mutant nucleophosmin and cooperating pathways drive leukemia initiation and progression in mice. Nat Genet. 2011;43(5):470–5. doi: 10.1038/ng.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mullighan CG, Kennedy A, Zhou X, Radtke I, Phillips LA, Shurtleff SA, et al. Pediatric acute myeloid leukemia with NPM1 mutations is characterized by a gene expression profile with dysregulated HOX gene expression distinct from MLL-rearranged leukemias. Leukemia. 2007;21(9):2000–9. doi: 10.1038/sj.leu.2404808. [DOI] [PubMed] [Google Scholar]
- 33.Dorrance AM, Liu S, Yuan W, Becknell B, Arnoczky KJ, Guimond M, et al. Mll partial tandem duplication induces aberrant Hox expression in vivo via specific epigenetic alterations. J Clin Invest. 2006;116(10):2707–16. doi: 10.1172/JCI25546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang L, Han Y, Suarez Saiz F, Minden MD. A tumor suppressor and oncogene: the WT1 story. Leukemia. 2007;21(5):868–76. doi: 10.1038/sj.leu.2404624. [DOI] [PubMed] [Google Scholar]
- 35.Kaspers GJ, Creutzig U. Pediatric acute myeloid leukemia: international progress and future directions. Leukemia. 2005;19(12):2025–9. doi: 10.1038/sj.leu.2403958. [DOI] [PubMed] [Google Scholar]
- 36.Care RS, Valk PJ, Goodeve AC, Abu-Duhier FM, Geertsma-Kleinekoort WM, Wilson GA, et al. Incidence and prognosis of c-KIT and FLT3 mutations in core binding factor (CBF) acute myeloid leukaemias. Br J Haematol. 2003;121(5):775–7. doi: 10.1046/j.1365-2141.2003.04362.x. [DOI] [PubMed] [Google Scholar]
- 37.Thiede C, Steudel C, Mohr B, Schaich M, Schakel U, Platzbecker U, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood. 2002;99(12):4326–35. doi: 10.1182/blood.v99.12.4326. [DOI] [PubMed] [Google Scholar]
- 38.Taketani T, Taki T, Nakamura T, Kobayashi Y, Ito E, Fukuda S, et al. High frequencies of simultaneous FLT3-ITD, WT1 and KIT mutations in hematological malignancies with NUP98-fusion genes. Leukemia. 2010;24(11):1975–7. doi: 10.1038/leu.2010.207. [DOI] [PubMed] [Google Scholar]
- 39.Mrozek K, Heerema NA, Bloomfield CD. Cytogenetics in acute leukemia. Blood Rev. 2004;18(2):115–36. doi: 10.1016/S0268-960X(03)00040-7. [DOI] [PubMed] [Google Scholar]
- 40.Creutzig U, Buchner T, Sauerland MC, Zimmermann M, Reinhardt D, Dohner H, et al. Significance of age in acute myeloid leukemia patients younger than 30 years: a common analysis of the pediatric trials AML-BFM 93/98 and the adult trials AMLCG 92/99 and AMLSG HD93/98A. Cancer. 2008;112(3):562–71. doi: 10.1002/cncr.23220. [DOI] [PubMed] [Google Scholar]
- 41.Marcucci G, Haferlach T, Dohner H. Molecular genetics of adult acute myeloid leukemia: prognostic and therapeutic implications. J Clin Oncol. 2011;29(5):475–86. doi: 10.1200/JCO.2010.30.2554. [DOI] [PubMed] [Google Scholar]
- 42.Balgobind BV, Raimondi SC, Harbott J, Zimmermann M, Alonzo TA, Auvrignon A, et al. Novel prognostic subgroups in child-hood 11q23/MLL-rearranged acute myeloid leukemia: results of an international retrospective study. Blood. 2009;114(12):2489–96. doi: 10.1182/blood-2009-04-215152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pollard JA, Alonzo TA, Gerbing RB, Ho PA, Zeng R, Ravindranath Y, et al. Prevalence and prognostic significance of KIT mutations in pediatric patients with core binding factor AML enrolled on serial pediatric cooperative trials for de novo AML. Blood. 2010;115(12):2372–9. doi: 10.1182/blood-2009-09-241075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paschka P, Marcucci G, Ruppert AS, Mrozek K, Chen H, Kittles RA, et al. Adverse prognostic significance of KIT mutations in adult acute myeloid leukemia with inv(16) and t(8;21): a Cancer and Leukemia Group B Study. J Clin Oncol. 2006;24(24):3904–11. doi: 10.1200/JCO.2006.06.9500. [DOI] [PubMed] [Google Scholar]
- 45.Levis M, Allebach J, Tse KF, Zheng R, Baldwin BR, Smith BD, et al. A FLT3-targeted tyrosine kinase inhibitor is cytotoxic to leukemia cells in vitro and in vivo. Blood. 2002;99(11):3885–91. doi: 10.1182/blood.v99.11.3885. [DOI] [PubMed] [Google Scholar]
- 46.Weisberg E, Boulton C, Kelly LM, Manley P, Fabbro D, Meyer T, et al. Inhibition of mutant FLT3 receptors in leukemia cells by the small molecule tyrosine kinase inhibitor PKC412. Cancer Cell. 2002;1(5):433–43. doi: 10.1016/s1535-6108(02)00069-7. [DOI] [PubMed] [Google Scholar]
- 47.Pratz KW, Cho E, Levis MJ, Karp JE, Gore SD, McDevitt M, et al. A pharmacodynamic study of sorafenib in patients with relapsed and refractory acute leukemias. Leukemia. 2010;24(8):1437–44. doi: 10.1038/leu.2010.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stone RM, Fischer T, Paquette R, Schiller G, Schiffer CA, Ehninger G, et al. A Phase 1b Study of Midostaurin (PKC412) in Combination with Daunorubicin and Cytarabine Induction and High-Dose Cytarabine Consolidation in Patients Under Age 61 with Newly Diagnosed De Novo Acute Myeloid Leukemia: Overall Survival of Patients Whose Blasts Have FLT3 Mutations Is Similar to Those with Wild-Type FLT3. ASH Annual Meeting Abstracts. 2009;114(22):634. [Google Scholar]
- 49.Sato T, Yang X, Knapper S, White P, Smith BD, Galkin S, et al. FLT3 ligand impedes the efficacy of FLT3 inhibitors in vitro and in vivo. Blood. 2011;117(12):3286–93. doi: 10.1182/blood-2010-01-266742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Serve H, Wagner R, Sauerland C, Brunnberg U, Krug U, Schaich M, et al. Sorafenib In Combination with Standard Induction and Consolidation Therapy In Elderly AML Patients: Results From a Randomized, Placebo-Controlled Phase II Trial. ASH Annual Meeting Abstracts. 2010;116(21):333. [Google Scholar]
- 51.Hollink IH, van den Heuvel-Eibrink MM, Zimmermann M, Balgobind BV, Arentsen-Peters ST, Alders M, et al. No Prognostic Impact of the WT1 Gene Single Nucleotide Polymorphism rs16754 in Pediatric Acute Myeloid Leukemia. J Clin Oncol. 2010;28(28):e523–6. doi: 10.1200/JCO.2010.29.3860. [DOI] [PubMed] [Google Scholar]
- 52.Keilholz U, Letsch A, Busse A, Asemissen AM, Bauer S, Blau IW, et al. A clinical and immunologic phase 2 trial of Wilms tumor gene product 1 (WT1) peptide vaccination in patients with AML and MDS. Blood. 2009;113(26):6541–8. doi: 10.1182/blood-2009-02-202598. [DOI] [PubMed] [Google Scholar]
- 53.Migas A, Savva N, Mishkova O, Aleinikova OV. AML1/RUNX1 gene point mutations in childhood myeloid malignancies. Pediatr Blood Cancer. 2011;57(4):583–7. doi: 10.1002/pbc.22980. [DOI] [PubMed] [Google Scholar]
- 54.Loriaux MM, Levine RL, Tyner JW, Frohling S, Scholl C, Stoffregen EP, et al. High-throughput sequence analysis of the tyrosine kinome in acute myeloid leukemia. Blood. 2008;111(9):4788–96. doi: 10.1182/blood-2007-07-101394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tomasson MH, Xiang Z, Walgren R, Zhao Y, Kasai Y, Miner T, et al. Somatic mutations and germline sequence variants in the expressed tyrosine kinase genes of patients with de novo acute myeloid leukemia. Blood. 2008;111(9):4797–808. doi: 10.1182/blood-2007-09-113027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Radtke I, Mullighan CG, Ishii M, Su X, Cheng J, Ma J, et al. Genomic analysis reveals few genetic alterations in pediatric acute myeloid leukemia. Proc Natl Acad Sci USA. 2009;106(31):12944–9. doi: 10.1073/pnas.0903142106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Paschka P, Schlenk RF, Gaidzik VI, Habdank M, Kronke J, Bullinger L, et al. IDH1 and IDH2 mutations are frequent genetic alterations in acute myeloid leukemia and confer adverse prognosis in cytogenetically normal acute myeloid leukemia with NPM1 mutation without FLT3 internal tandem duplication. J Clin Oncol. 2010;28(22):3636–43. doi: 10.1200/JCO.2010.28.3762. [DOI] [PubMed] [Google Scholar]
- 58.Ley TJ, Ding L, Walter MJ, McLellan MD, Lamprecht T, Larson DE, et al. DNMT3A mutations in acute myeloid leukemia. N Engl J Med. 2010;363(25):2424–33. doi: 10.1056/NEJMoa1005143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ho PA, Kutny MA, Alonzo TA, Gerbing RB, Joaquin J, Raimondi SC, et al. Leukemic mutations in the methylation-associated genes DNMT3A and IDH2 are rare events in pediatric AML: A report from the Children’s Oncology Group. Pediatr Blood Cancer. 2011;57:204–9. doi: 10.1002/pbc.23179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Langemeijer SM, Jansen JH, Hooijer J, van Hoogen P, Stevens-Linders E, Massop M, et al. TET2 mutations in childhood leukemia. Leukemia. 2010;25(1):189–92. doi: 10.1038/leu.2010.243. [DOI] [PubMed] [Google Scholar]
- 61.Ho PA, Alonzo TA, Kopecky KJ, Miller KL, Kuhn J, Zeng R, et al. Molecular alterations of the IDH1 gene in AML: a Children’s Oncology Group and Southwest Oncology Group study. Leukemia. 2010;24(5):909–13. doi: 10.1038/leu.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]