Abstract
Background
The assay for ADAMTS13 activity helps clinicians to confirm the clinical diagnosis of idiopathic thrombotic thrombocytopenic purpura. The clinical value of testing for the antigen level of ADAMTS13 protein is, however, less clear.
Design and Methods
In this study, both ADAMTS13 antigen and activity levels were measured in 835 sequential samples from 40 consecutive patients who were followed for an average of 29 months throughout the course of acute episode plasma exchange treatment and clinical remission.
Results
During acute episodes, ADAMTS13 activity was severely deficient while ADAMTS13 antigen levels were more variable, ranging from severely deficient to as high as within the reference range. A severe depletion of ADAMTS13 antigen level during acute disease was, however, statistically associated with disease mortality (P=0.0322). For patients who achieved initial clinical responses, ADAMTS13 antigen levels appeared to be restored faster than ADAMTS13 activity to the normal range. Further analysis demonstrated that the ADAMTS13 antigen level at the time of initial clinical recovery was significantly higher in the patients who subsequently achieved a sustained clinical remission than in the group who soon after had an exacerbation (P=0.0187).
Conclusions
Our data suggest that evaluation of ADAMTS13 antigen levels during the course of therapy and follow-up may offer additional useful information for the management of patients with thrombotic thrombocytopenic purpura.
Keywords: thrombotic thrombocytopenic purpura, ADAMTS13, prognosis, biomarker
Introduction
Thrombotic thrombocytopenic purpura (TTP) is a devastating hematologic disorder frequently associated with multiple organ failure and sometimes death.1–3 Clinical biomarkers are needed to evaluate TTP patients more precisely and to provide the appropriate treatment accordingly. Deficiency of ADAMTS13 (A Disintegrin And Metalloprotease with ThromboSpondin type 1 repeats) protease is a known risk factor in the pathogenesis of TTP.4,5 ADAMTS13 is a physiological metalloprotease that cleaves von Willebrand factor, preventing formation of ultra-large multimers of this factor and formation of platelet aggregates.6–9 In adult patients, the vast majority of TTP cases are due to idiopathic production of an ADAMTS13 autoantibody that impairs ADAMTS13 functions and results in an acquired deficiency of ADAMTS13 activity.6,8 Several assays have been developed which rapidly detect ADAMTS13 activity and help to confirm a clinical suspicion of TTP.10–14 An enzyme-linked immunosorbent assay (ELISA)-based test has also been used to measure ADAMTS13 antigen levels.15,16 While ADAMTS13 activity measured at the onset of TTP is known to help to confirm the diagnosis, the clinical value of ADAMTS13 antigen measurements in the evaluation of TTP disease severity, responses to therapy, and clinical outcomes has not been well studied in a large cohort of TTP patients with longitudinal follow-up.
In the present study, we utilized resources generated by a TTP cohort from the Ohio State University TTP Research Program. Forty consecutive patients with idiopathic TTP were analyzed. These patients have been closely followed prospectively, and specimens were collected longitudinally at regular intervals. Overall, 835 sequential samples collected throughout the course of disease were analyzed in this study to evaluate the clinical usefulness of serial measurements of ADAMTS13 antigen in the ongoing management of patients with idiopathic TTP.
Design and Methods
Clinical characterization of the patients with thrombotic thrombocytopenic purpura
In this study, we only selected idiopathic cases of TTP that were caused by a deficiency of ADAMTS13 function: confounding influences are minimized by focusing on a homogenous population. All the cases of ADAMTS13 deficiency in our cohort were acquired cases because all patients were positive for ADAMTS13 autoantibodies, with antibody levels at least two times the upper limit of the normal range at the time of presentation. We excluded any categories of TTP that typically lack ADAMTS13 deficiency, including TTP associated with transplantation, malignancy and human immunodeficiency virus infection. A total of 40 subjects qualified for this study. All patients started plasma exchange treatment as soon as the diagnosis of TTP was made clinically. Plasma exchange was performed daily with one plasma volume exchanged using fresh-frozen plasma as the replacement fluid until clinical response criteria were met. Patients then had two more exchanges performed every other day, with the first day that the platelet count and lactate dehydrogenase level were normal counting as the first of the two exchanges. Among these study subjects, 32 patients received either prednisone or cyclosporine as an adjunct to plasma exchange at their physicians’ discretion. One patient received immunosuppressive therapy with vincristine in addition to plasma exchange.
For the purposes of this study, clinical response was objectively defined in the study protocol as the achievement of a normal platelet count (>150×109/L) and a normal lactate dehydrogenase concentration (<190 U/L). Additionally, patients had to demonstrate stabilization or recovery of neurological deficits present at presentation, without the development of any new deficits. Renal function also had to be stable or improved compared to that at initial presentation. TTP was said to be exacerbated in patients who achieved an initial clinical response but who, within 30 days of the last plasma exchange, had a return of TTP that required additional plasma exchange therapy. A full clinical response was defined as meeting the criteria for a clinical response and maintaining this normal status for at least 30 days after the last exchange procedure.
Protocols for specimen collection/processing
All participating patients or their legal guardians (if a patient was unable to give consent) signed informed consent under a protocol approved by the Ohio State University Institutional Review Board. The samples were collected before plasma exchange treatment at the initial visit for an acute episode of TTP at the Ohio State University and then weekly (prior to the plasma exchange treatment on the day of collection) until clinical response was achieved. After discharge from hospital, samples were collected during follow-up visits scheduled weekly during the first month and then at approximately 3-month intervals. When disease recurred, each patient was treated immediately as having a new disease event with samples collected longitudinally as before. For each collection, 10 mL of blood were drawn into sodium citrate. The blood was centrifuged at 500 g for 10 min. Plasma was then separated and spun at 3,200 g for 30 min to deplete residual platelets. Finally, the platelet-depleted plasma was divided into 20 aliquots (125 – 200 μL each), stored in a freezer at −80°C, and thawed only once for experiments.
Measurement of ADAMTS13 biomarkers
ADAMTS13 antigen level was determined using an ELISA kit developed by American Diagnostica Inc. (IMUBIND® ADAMTS13 ELISA).17,18 In this ELISA kit, the plate is coated with monoclonal anti-ADAMTS13 antibody that captures ADAMTS13 antigen in plasma. The ADAMTS13 antigen is subsequently detected by a second polyclonal rabbit antihuman ADAMTS13 antibody conjugated with horseradish peroxidase. By using a group of healthy donors (n=48), we established the reference range to be 657–2368 ng/mL (mean±2 SD). In order to determine whether the ADAMTS13 antigen ELISA kit allowed for adequate detection of antigen/antibody complexes, we selected seven samples from four patients in the study who exhibited an ADAMTS13 activity of less than 0.5% but had a high readout for the antigen measurement. These samples most likely contained a very high percentage of antigen/antibody complexes with almost no free antigen. These samples were tested by the ADAMTS13 antigen kit using both anti-ADAMTS13 and human IgG antibodies as the detection antibodies. The readouts resulting from the human IgG detection antibody correlated very well with the ADAMTS13 antigen results (r = 0.95). These data indicate that antigen results in the samples are in fact measuring ADAMTS13 complexed with IgG antibody. Furthermore, we performed a standard recovery study to evaluate the analytical specificity of this ELISA kit. In this experiment, we made serial dilutions of two of the above seven samples, and then assayed the samples using both detection antibodies mentioned above. Both detection antibodies again recovered the analytes in a dose-dependent manner in the diluted specimens (data not shown). These data further demonstrate that the ADAMTS13 antigen ELISA kit did indeed accurately measure the amount of the ADAMTS13 antigen/antibody complexes added to the sample. Our results are consistent with the evaluation of ADAMTS13 antigen ELISA kits developed by other laboratories.15,16
In addition to the controls provided in this commercial kit, in each run we included two levels of external controls that were patients’ samples with known results. We first assayed these control samples repeatedly over a period of time to establish the criteria for acceptability of control materials. Afterwards, the external controls were included each day patients’ specimens were assayed. The results were only reported when all the controls were within the acceptable ranges. All the antigen measurements in this study were performed in triplicate and means were used for analysis.
ADAMTS13 activity was determined using a surface enhanced laser desorption/ionization time-of-flight (SELDI-TOF) mass spectrometer (PCS4000), as reported previously.10,19 All samples with ADAMTS13 activity less than 5% were retested using the modified protocol that determines ADAMTS13 activity precisely to levels as low as 0.5%.19 ADAMTS13 autoantibody (IgG) was quantified using a commercially available ELISA kit from American Diagnostica Inc. (IMUBIND® ADAMTS13 Autoantibody ELISA), as described previously.17,20
Statistical methods
For the statistical analysis, all samples with an antigen level less than 25 ng/mL were given a value of 25. The samples with an ADAMTS13 activity level less than 0.5% were assigned a value of 0.5%. Since antigen and activity levels are skewed with some large values, they were log-transformed before any analysis to achieve approximate normality. Logistic regression modeling was used to investigate whether ADAMTS13 activity and antigen are predictive of mortality. Gender, age, and ethnicity were used as covariates in the model. Similarly, logistic regression modeling was used to evaluate whether ADAMTS13 antigen level at the time of initial clinical response could predict the outcome of achieving a full therapeutic response. The same set of covariates was included in the analysis.
Results
The demographic and clinical characteristics of the 40 TTP patients who met the study enrollment criteria are presented in Table 1. The earliest episode in which a pre-plasma exchange plasma sample was banked for laboratory investigation was chosen for each study subject. Information on the type of episode (initial or relapsed) or episode number (ordinally marked as relapse1 or relapse2, etc.) is given for each study subject. The 40 episodes investigated in this study comprised 26 initial episodes, 6 episodes of relapse1, 4 of relapse2, 1 of relapse3, 2 of relapse4 and 1 of relapse5. The cohort had a male-to-female ratio of 11:29 and an age distribution from 16 to 67 years old. Twenty-six of the subjects were Caucasian and 14 were African-American. Of these patients, four died during their acute disease, and two were not followed up continuously because of relocations. The laboratory data in the table correspond to the TTP episode selected for each study subject. All of the survivors were followed up closely for a period from 1 month up to as long as 55 months, with an average individual follow-up period of 29 months. During these periods of close follow-up, more than 90% of the samples were obtained at the sequential intervals described in the Design and Methods section.
Table 1.
Summary of the characteristics of the TTP patients in this study.
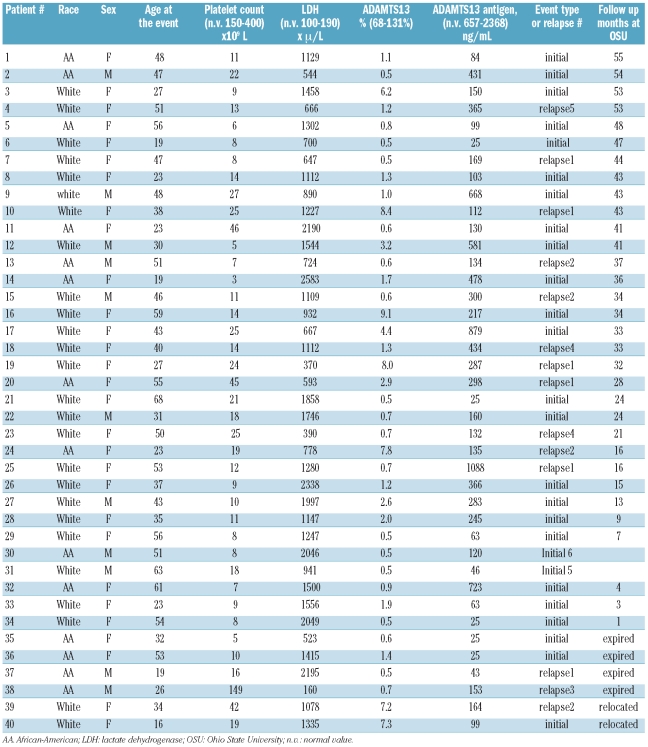
At presentation of acute disease, ADAMTS13 activity was severely deficient in all subjects. In contrast, ADAMTS13 antigen levels varied broadly from severely deficient to values within the normal range (<25 to 1088 ng/mL). When all 835 longitudinal samples were analyzed for association between ADAMTS13 antigen and activity levels, the agreement rate was not very strong, with a correlation coefficient (r) of 0.53 (Figure 1). When the samples were divided into four groups according to clinical stage, the measured ADAMTS13 antigen levels again displayed a poor correlation with the corresponding ADAMTS13 activity levels in all clinical periods: at presentation (r=0.23), during acute disease (r=0.35), at initial clinical response (r=0.31), and in sustained remission (r=0.28).
Figure 1.
Correlation between ADAMTS13 activity and antigen levels. ADAMTS13 activity data are expressed as percentage of activity and ADAMST13 antigen data as ng/mL. Both underwent common log-transformation before being plotted.
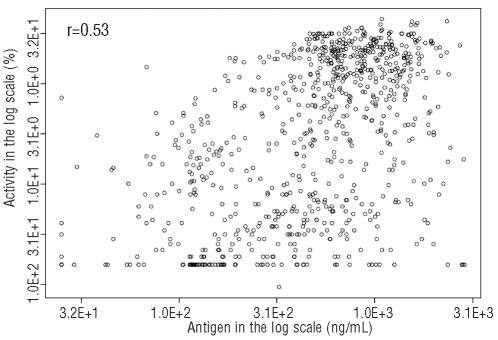
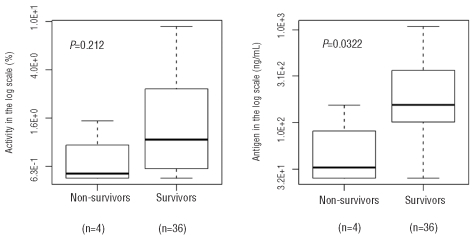
We examined whether ADAMTS13 antigen and activity levels at the time of acute disease were related to mortality. In order to reduce possible confounding variables, we only included one episode from each study subject: the earliest episode in which a pre-plasma exchange sample was banked for laboratory study. Of the 40 patients who presented acutely, four died while 36 patients achieved a full clinical response. Plasma samples collected prior to the start of plasma exchange therapy were used to evaluate whether low ADAMTS13 antigen and/or activity level is associated with TTP mortality. As shown in Figure 2, only ADAMTS13 antigen level was statistically lower in the patients who died than in the patients who survived (P=0.0322). A similar trend was also observed between higher mortality and lower ADAMTS13 activity although the relationship was not statistically significant. When all of the samples taken from patients during acute disease, including at presentation and during the plasmapheresis treatment, were compared depending on whether they were taken from subsequent survivors (n = 236) or non-survivors (n =13), low ADAMTS13 antigen was again significantly associated with mortality (data not shown). This suggests that a failure of adequate restoration of ADAMTS13 protein level by daily plasma exchange may be an indicator of poor prognosis. In our cohort, the four patients died from TTP events on days 2, 5, 6, and 7. In two of these patients, we were able to collect serial samples in the first week until the patients died. This allowed us to assess ADAMTS13 antigen levels longitudinally prior to their death. As shown in Figure 3, the ADAMTS13 antigen levels remained severely low until death despite daily plasma exchange therapy. In contrast, the average ADAMTS13 antigen levels in all samples (n=181) collected during acute plasma exchange treatment was 379±413 ng/mL (Figure 4). These data further indicate that when the ADAMTS13 protein remains depleted despite daily plasma exchange treatment, patients are at risk of a poor prognosis.
Figure 2.
Comparison of ADAMTS13 antigen and activity levels at the time of disease presentation between survivors and nonsurvivors.
Figure 3.
Serial ADAMTS13 antigen measurements from two non-survivors. Arrows represent the time of death. Both non-survivors died while receiving daily plasma exchange therapy.
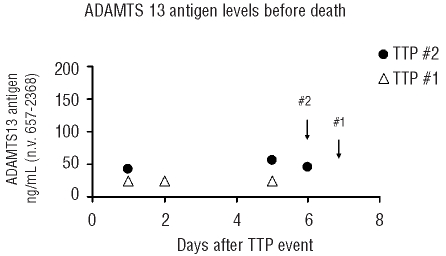
Figure 4.
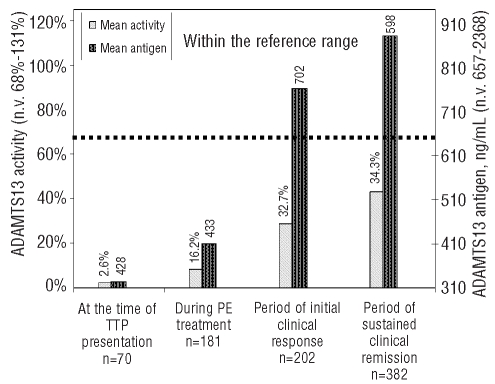
Measurement of ADAMTS13 antigen and activity throughout the entire clinical course of TTP. The bars represent the mean values of activity and antigen from each group. SD values are given on top of the bars. Longitudinal samples were divided into four groups: from presentation of the TTP event (including both initial and recurrent TTP) (n=70), from the period of acute disease while receiving daily plasmapheresis (n=181), from weekly samples in the first month after patients had achieved an initial clinical response with discontinuation of plasma exchange (PE) (n=202), and from quarterly samples obtained during the period of sustained clinical remission (n=382). The dotted line represents the low limit of normal for both ADAMTS13 activity and antigen reference ranges.
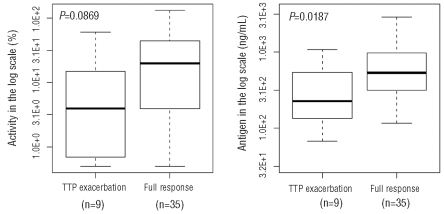
Next, the overall longitudinal performances of ADAMTS13 activity and antigen levels over the clinical course of TTP were compared. As shown in Figure 4, at the onset of disease, the average ADAMTS13 activity was significantly low at 2.4%, improved slightly to an average of 8.3% during plasma exchange treatment and increased to 28.8% during the initial clinical response. Subsequently, the mean ADAMTS13 activity level continued to increase and reached about 45% after achieving a sustained clinical remission although the value was still lower than the normal range of ADAMTS13 activity. ADAMTS13 antigen levels were restored faster than ADAMTS13 activity levels when patients recovered from acute TTP. For example, the mean ADAMTS13 antigen level was within the normal range at the time when initial clinical response was achieved and then rose slightly throughout the period of initial clinical response and the subsequent period of sustained clinical remission. These data again suggest that ADAMTS13 antigen and activity tests may not detect identical analytes in the samples. Since ADAMTS13 antigen measurement enables a more precise detection of how much total ADAMTS13 (including both free and complex proteins) is depleted or is recovered in vivo, we evaluated whether the ADAMTS13 antigen level at the time of initial clinical response could predict the achievement of a full therapeutic response. In our TTP sample repository, 74 samples were collected within 1 week after patients had achieved an initial clinical response and plasma exchange treatment had been discontinued. For 14 of these 74 samples, patients subsequently developed TTP exacerbations that required prolonged daily plasma exchange treatment. The other 60 samples came from patients who went on to achieve sustained clinical remissions without requiring further plasma exchange treatment. We compared these two groups with and without achievement of full clinical response. ADAMTS13 antigen levels in the group that required no further plasma exchange treatment were significantly higher than those in the group of patients who soon after developed an exacerbation of TTP (P=0.0187). In order to avoid potential confounding effects due to intrapatient correlation, we only considered the first occurrence for each study subject in each group (exacerbation versus full clinical response) for the data in Figure 5. As a result, there were samples taken during nine episodes in nine study subjects in the exacerbation group and samples taken during 35 episodes in 35 study subjects in the group achieving full clinical response. Again, ADAMTS13 antigen levels in the group of patients who achieved full clinical response were significantly higher than those in the group of patients who soon after had an exacerbation of TTP (P=0.0187).
Figure 5.
Comparison of ADAMTS13 antigen and activity levels at the time of achieving initial clinical responses. All samples were obtained in the first week after plasma exchange therapy was discontinued. Based on clinical outcomes, patients were divided into a group whose TTP exacerbated and a group who went on to achieve full clinical responses.
Discussion
TTP patients normally undergo daily plasma exchange therapy commencing immediately upon diagnosis. During treatment, patients are monitored frequently to assess their disease status and response to therapy. This close monitoring is critical to evaluate prognosis and to assess the need for adjustment of therapeutic regimens. Previous studies have indicated that elderly age, severe neurological manifestations, fever, and low hemoglobin level at presentation are poor prognostic indicators.21–23 However, none of these factors is specific for idiopathic TTP. Platelet count and lactate dehydrogenase level have been routinely used as laboratory parameters to monitor clinical responses of TTP to therapy. While these tests do provide sensitive measurements regarding the extent of thrombotic microangiopathy, intravascular hemolysis, and organ damage from tissue ischemia,24 the biomarkers are not specific for TTP. Many clinical conditions, including those that often coexist with TTP such as sepsis/infection, systemic lupus erythematosus, surgery or malignancy/chemotherapy, can cause low platelet counts and increased lactate dehydrogenase. Thus, a more specific objective measurement is needed to define the precise clinical course of TTP better.
Our correlation analyses demonstrated that ADAMTS13 activity level was not strongly associated with ADAMTS13 antigen level at the onset of TTP or when analyzed separately based on clinical stages. The results suggest that ADAMTS13 activity and antigen are not analogous to each other. The ADAMTS13 activity assay likely measures the free form of ADAMTS13, while the ADAMTS13 antigen assay detects the status of total ADAMTS13 protein that may include free protein, protein in complex with antibody inhibitor, and ADAMTS13 bound to other carrier proteins. Evaluation of total ADAMTS13 proteins conceivably may provide novel information for the assessment of TTP patients. Our data suggest that ADAMTS13 activity and antigen levels perform differently over the clinical course of TTP. Analyzing their performances separately may offer new perspectives on the pathobiology of TTP and provide new clinical utilities in the management of patients with TTP.
In comparison to the ADAMTS13 activity assay, the test for ADAMTS13 antigen is unable to correctly classify all cases of TTP with ADAMTS13 deficiency at the time of acute disease (Table 1). These data support the use of the test for ADAMTS13 activity as the method of choice for the diagnosis of TTP. However, as seen in Figure 2, the evaluation of total ADAMTS13 protein measured as ADAMTS13 antigen provides useful information about disease prognosis. In our statistical model of logistic regression analysis, the random effect was already taken into consideration. When a small sample (n=4) of patients who died gave rise to a statistically lower ADAMTS13 antigen level than that of a group of 36 surviving patients, this suggested that ADAMST13 antigen may be a strong risk factor associated with mortality during acute TTP. Considering the fact that this study is a first investigation based on a relatively small sample size, a confirmatory study involving multiple centers may be needed to establish more firmly the prognostic value of the test for ADAMTS13 antigen in the care of patients with TTP. A specific prognostic indicator in TTP would be clinically very significant. When ADAMTS13 antigen level is low and not adequately restored by routine plasmapheresis treatment, the clinician may be alerted to a potentially poor prognosis and be advised to provide more aggressive therapies, such as increasing the plasma exchange volume or frequency. Other options include adding an adjuvant treatment modality such as rituximab, cyclosporine, other forms of immunosuppressive therapy, or von Willebrand factor inhibitors.
The discovery regarding the association of ADAMTS13 antigen level and status of clinical recovery at the time of initial clinical response also has a novel clinical implication for the management of TTP. Patients with idiopathic TTP are known for their propensity to develop TTP exacerbations. Upon initial response, a significant fraction of patients will experience a return of TTP shortly after discontinuing plasma exchange. These recurring cases in turn may require a much longer course of plasma exchange treatment. Thus, it is critical at the time of initial clinical response to determine whether the patient is fully recovered from TTP or if the patient still has evidence of ongoing disease activity. Our study indicates that an adequate recovery of total ADAMTS13 proteins at the time of initial clinical response is associated with a sustained clinical recovery. This new information may enable clinicians to manage TTP patients more judiciously at the time of initial clinical response. With a full restoration of ADAMTS13 antigen, patients may be safely discharged and followed routinely as an outpatient. Without an adequate recovery of ADAMTS13 antigen, patients may need more close monitoring, and perhaps a catheter should be maintained for further plasma exchange treatment. Alternatively, the patients at risk may receive prophylactic interventions such as the initiation of adjunctive immunosuppressive therapy.25,26
The performances of ADAMTS13 antigen and activity were compared in a well-characterized idiopathic TTP cohort over different phases of the disease. Through this longitudinal analysis, we concluded that deficiency of ADAMTS13 activity provides more useful clinical information for the diagnosis of idiopathic TTP at the time of acute disease. However, ADAMTS13 antigen measurements may provide additional data to assess the patient’s disease status. A severe and persistent depletion of ADAMTS13 antigen at the time of acute presentation appears to be associated with an increased risk of disease mortality, while adequate recovery of ADAMTS13 antigen at the time of initial clinical response corresponds with a sustained course of clinical recovery. This report describes for the first time the potential value of ADAMTS13 antigen in the clinical evaluation of TTP patients. Future studies involving more patients from multiple centers are needed to delineate the prognostic utility of ADAMTS13 antigen definitively.
Footnotes
Funding: this work was supported in part by grants from the National Institutes of Health K08HL03279, and Ohio Biomedical Research and Technology Transfer Commission.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.George JN. Clinical practice. Thrombotic thrombocytopenic purpura. N Engl J Med. 2006;354(18):1927–35. doi: 10.1056/NEJMcp053024. [DOI] [PubMed] [Google Scholar]
- 2.Peyvandi F. The role of ADAMTS13 in the new pathogenesis of TTP. Hematology. 2005;10(Suppl 1):47–8. doi: 10.1080/10245330512331389890. [DOI] [PubMed] [Google Scholar]
- 3.Sadler JE. Thrombotic thrombocytopenic purpura: a moving target. Hematology Am Soc Hematol Educ Program. 2006:415–20. doi: 10.1182/asheducation-2006.1.415. [DOI] [PubMed] [Google Scholar]
- 4.Levy GG, Nichols WC, Lian EC, Foroud T, McClintick JN, McGee BM, et al. Mutations in a member of the ADAMTS gene family cause thrombotic thrombocytopenic purpura.[comment] Nature. 2001;413(6855):488–94. doi: 10.1038/35097008. [DOI] [PubMed] [Google Scholar]
- 5.Motto DG, Chauhan AK, Zhu G, Homeister J, Lamb CB, Desch KC, et al. Shigatoxin triggers thrombotic thrombocytopenic purpura in genetically susceptible ADAMTS13-deficient mice. J Clin Invest. 2005;115(10):2752–61. doi: 10.1172/JCI26007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Furlan M, Robles R, Galbusera M, Remuzzi G, Kyrle PA, Brenner B, et al. von Willebrand factor-cleaving protease in thrombotic thrombocytopenic purpura and the hemolytic-uremic syndrome. N Engl J Med. 1998;339(22):1578–84. doi: 10.1056/NEJM199811263392202. [DOI] [PubMed] [Google Scholar]
- 7.Sadler JE. Biochemistry and genetics of von Willebrand factor. Annu Rev Biochem. 1998;67:395–424. doi: 10.1146/annurev.biochem.67.1.395. [DOI] [PubMed] [Google Scholar]
- 8.Tsai HM, Lian EC. Antibodies to von Willebrand factor-cleaving protease in acute thrombotic thrombocytopenic purpura. N Engl J Med. 1998;339(22):1585–94. doi: 10.1056/NEJM199811263392203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levy GG, Motto DG, Ginsburg D. ADAMTS13 turns 3. Blood. 2005;106(1):11–7. doi: 10.1182/blood-2004-10-4097. [DOI] [PubMed] [Google Scholar]
- 10.Jin M, Cataland SR, Bissell M, Wu HM. A rapid test for the diagnosis of thrombotic thrombocytopenic purpura using surface enhanced laser desorption/ionization time-of-flight (SELDI-TOF)-mass spectrometry. J Thromb Haemost. 2006;4(2):333–8. doi: 10.1111/j.1538-7836.2006.01758.x. [DOI] [PubMed] [Google Scholar]
- 11.Kokame K, Nobe Y, Kokubo Y, Okayama A, Miyata T. FRETS-VWF73, a first fluorogenic substrate for ADAMTS13 assay. Br J Haematol. 2005;129(1):93–100. doi: 10.1111/j.1365-2141.2005.05420.x. [DOI] [PubMed] [Google Scholar]
- 12.Whitelock JL, Nolasco L, Bernardo A, Moake J, Dong JF, Cruz MA. ADAMTS-13 activity in plasma is rapidly measured by a new ELISA method that uses recombinant VWF-A2 domain as substrate. J Thromb Haemost. 2004;2(3):485–91. doi: 10.1111/j.1538-7836.2004.00601.x. [DOI] [PubMed] [Google Scholar]
- 13.Zhou W, Tsai HM. An enzyme immunoassay of ADAMTS13 distinguishes patients with thrombotic thrombocytopenic purpura from normal individuals and carriers of ADAMTS13 mutations. Thromb Haemost. 2004;91(4):806–11. doi: 10.1160/TH03-11-0675. [DOI] [PubMed] [Google Scholar]
- 14.Groot E, Hulstein JJ, Rison CN, de Groot PG, Fijnheer R. FRETS-VWF73: a rapid and predictive tool for thrombotic thrombocytopenic purpura. J Thromb Haemost. 2006;4(3):698–9. doi: 10.1111/j.1538-7836.2005.01767.x. [DOI] [PubMed] [Google Scholar]
- 15.Rieger M, Ferrari S, Kremer Hovinga JA, Konetschny C, Herzog A, Koller L, et al. Relation between ADAMTS13 activity and ADAMTS13 antigen levels in healthy donors and patients with thrombotic microangiopathies (TMA) Thromb Haemost. 2006;95(2):212–20. doi: 10.1160/TH05-08-0550. [DOI] [PubMed] [Google Scholar]
- 16.Liu F, Feys HB, Dong N, Zhao Y, Ruan C. Alteration of ADAMTS13 antigen levels in patients with idiopathic thrombotic thrombocytopenic purpura, idiopathic thrombocytopenic purpura and systemic lupus erythematosus. Thromb Haemost. 2006;95(4):749–50. [PubMed] [Google Scholar]
- 17.Cataland SR, Jin M, Smith E, Stanek M, Wu HM. Full evaluation of an acquired case of thrombotic thrombocytopenic purpura following the surgical resection of glioblastoma multiforme. J Thromb Haemost. 2006;4(12):2733–7. doi: 10.1111/j.1538-7836.2006.02217.x. [DOI] [PubMed] [Google Scholar]
- 18.Shelat SG, Smith P, Ai J, Zheng XL. Inhibitory autoantibodies against ADAMTS-13 in patients with thrombotic thrombocytopenic purpura bind ADAMTS-13 protease and may accelerate its clearance in vivo. J Thromb Haemost. 2006;4(8):1707–17. doi: 10.1111/j.1538-7836.2006.02025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin M, Casper TC, Cataland SR, Kennedy MS, Lin S, Li YJ, et al. Relationship between ADAMTS13 activity in clinical remission and the risk of TTP relapse. Br J Haematol. 2008;141(5):651–8. doi: 10.1111/j.1365-2141.2008.07107.x. [DOI] [PubMed] [Google Scholar]
- 20.Cataland SR, Jin M, Lin S, Kennedy MS, Kraut EH, George JN, et al. Ciclosporin and plasma exchange in thrombotic thrombocytopenic purpura: long-term follow-up with serial analysis of ADAMTS13 activity. Br J Haematol. 2007;139(3):486–93. doi: 10.1111/j.1365-2141.2007.06819.x. [DOI] [PubMed] [Google Scholar]
- 21.Sarode R, Gottschall JL, Aster RH, McFarland JG. Thrombotic thrombocytopenic purpura: early and late responders. Am J Hematol. 1997;54(2):102–7. doi: 10.1002/(sici)1096-8652(199702)54:2<102::aid-ajh2>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 22.Tuncer HH, Oster RA, Huang ST, Marques MB. Predictors of response and relapse in a cohort of adults with thrombotic thrombocytopenic purpura-hemolytic uremic syndrome: a single-institution experience. Transfusion. 2007;47(1):107–14. doi: 10.1111/j.1537-2995.2007.01071.x. [DOI] [PubMed] [Google Scholar]
- 23.Wyllie BF, Garg AX, Macnab J, Rock GA, Clark WF. Thrombotic thrombocytopenic purpura/haemolytic uraemic syndrome: a new index predicting response to plasma exchange. Br J Haematol. 2006;132(2):204–9. doi: 10.1111/j.1365-2141.2005.05857.x. [DOI] [PubMed] [Google Scholar]
- 24.Cohen JA, Brecher ME, Bandarenko N. Cellular source of serum lactate dehydrogenase elevation in patients with thrombotic thrombocytopenic purpura. J Clin Apher. 1998;13(1):16–9. doi: 10.1002/(sici)1098-1101(1998)13:1<16::aid-jca3>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 25.Cataland SR, Jin M, Lin S, Kraut EH, George JN, Wu HM. Effect of prophylactic cyclosporine therapy on ADAMTS13 biomarkers in patients with idiopathic thrombotic thrombocytopenic purpura. Am J Hematol. 2008;83(12):911–5. doi: 10.1002/ajh.21281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fakhouri F, Vernant JP, Veyradier A, Wolf M, Kaplanski G, Binaut R, et al. Efficiency of curative and prophylactic treatment with rituximab in ADAMTS13-deficient thrombotic thrombocytopenic purpura: a study of 11 cases. Blood. 2005;106(6):1932–7. doi: 10.1182/blood-2005-03-0848. [DOI] [PubMed] [Google Scholar]