Abstract
Interaction between neutrophils and other leukocytes plays a variety of important roles in regulating innate and adaptive immune responses. Recently, we have shown that neu-trophils amplify NK cell/6-sulfo LacNAc+ dendritic cells (slanDC)-mediated cytokine production, by potentiating IL-12p70 release by slanDC via CD18/ICAM-1 and directly co-stimulating IFNγ production by NK cells via ICAM-3. Herein, we have identified additional molecules involved in the interactions among neutrophils, NK cells and slanDC. More specifically, we provide evidence that: i) the cross-talk between neutrophils and NK cells is mediated by ICAM-3 and CD11d/CD18, respectively; ii) slanDC potentiate the production of IFNγ by NK cells via CD11a/CD18. Altogether, our studies shed more light on the role that adhesion molecules play within the neutrophil/NK cell/slanDC network. Our data also have potential implications in the pathogenesis of diseases driven by hyperactivated leukocytes, such as Sweet’s syndrome, in which a neutrophil/NK cell co-localization is frequently observed.
Keywords: CD11d, neutrophil, NK cell, slanDC, IFNγ, Sweet’s syndrome
Introduction
Cells belonging to the innate immune system are programmed to provide rapid and optimal responses when the host is challenged by environmental insults. While performing their specific activities, innate immune cells continuously interact to modulate each others’ activities and adapt their response to the specific characteristics of the insult. The role of neutrophils in shaping the developing immune response is increasingly being recognized, not only for their cross-talks with components of the innate immune system, such as monocytes and macrophages,1 dendritic cells (DC)2 and natural killer (NK) cells,3 but also with unpolarized/polarized T lymphocytes.4 Recently, we have discovered and have started characterizing a novel network within the innate immune response in which human neutrophils can directly cooperate with both NK cells and one of the major DC subsets present in the blood and tissues, known as 6-sulfo LacNAc+ DC (slanDC), to ultimately amplify the production of IFNγ by NK cells upon activation with LPS plus the IL-15/IL-18 combination.5 Accordingly, we showed that neutrophils promote the release of IL-12p70 by slanDC via a CD18/ICAM-1 interaction, and that such IL-12p70 stimulates NK cells to produce IFNγ: the latter, in turn, has been shown to further potentiate the interaction between neutrophils and slanDC and the release of slanDC-derived IL-12p70, thus creating a positive feedback loop. On the other hand, we also showed that neutrophils could directly co-stimulate the production of IFNγ by NK cells via ICAM-3,5 but its counter-receptor has not been identified. Originally, ICAM-3 was functionally identified as the third CD11a/CD18 (LFA-1) ligand, based on the inability of ICAM-1 and ICAM-2 neutralizing antibodies (Abs) to completely block LFA-1-dependent adhesion, and thereafter cloned.6, 7 Additional receptors for ICAM-3 have been subsequently identified, specifically CD11d/CD188 and dendritic cell-specific ICAM-3 grabbing non-integrin (DC-SIGN).9
Herein, we report that the specific counter-receptor for ICAM-3 in our system is likely represented by CD11d/CD18, and not by CD11a/CD18 or DC-SIGN. We also show that neutrophils contribute to this receptor-ligand interaction with ICAM-3, whereas NK cells contribute with CD11d/CD18. Finally, we show that these interactions might occur in Sweet’s syndrome disease, in which neutrophils and NK cells are frequently observed in close contact.
Design and Methods
Isolation and co-cultures of neutrophils, NK cells and DC
Highly purified neutrophils, NK cells and slanDC were isolated and cultured for 18 h as previously described,5 with or without 100 ng/mL LPS plus the IL-15/IL-18 combination (both at 10 ng/mL), in the presence of 10 μg/mL αCD11a/CD18 efalizumab, a humanized IgG1 antibody derived from the murine MHM24 clone10 which, in preliminary experiments, confirmed its ability to prevent the binding of CD11a to either ICAM-111 or ICAM-312 in a specific T-cell adhesion assay13, 10 μg/mL αCD18 or αCD18 F(ab’)2 fragments [clone IB4 (mouse IgG2a)], 10 μg/mL αDC-SIGN [clone 120507 (mouse IgG2b)14 or clone MR-1 (mouse IgG1)15], as well as corresponding isotype control antibodies. Monocyte-derived dendritic cells (moDC) were generated as previously described.16 All reagents used were of the highest available grade and were dissolved in pyrogen-free water for clinical use. Patients provided their informed consent before samples were taken. The study was approved by the Local Ethics Committee.
Neutrophil, NK cell and slanDC characterization
Analysis of cytokine production, cytofluorimetric analysis, laser confocal microscopy, and co-stimulation assays were performed exactly as previously described.5 The Abs specifically used in this study were: αDC-SIGN [clone 120507, mouse IgG2b from Abcam], αCD11a (clone HI111, mouse IgG1 from BD BioSciences), and αCD11d (rabbit IgG) Abs.
Immunohistochemistry
Formalin-fixed paraffin embedded skin sections were obtained from 4 cases of Sweet’s syndrome and immunostained for CD15 (clone MMA, mouse IgM, 1:50 dilution, Thermo Scientific), neutrophil elastase (clone NP57, mouse IgG1, 1:150 dilution, Dako), CD56 (clone 123C3.D5, mouse IgG1, 1:30 dilution, Thermo Scientific), CD3 (rabbit monoclonal, 1:100 dilution; Thermo Scientific) and IFNγ (clone 25718, mouse IgG2a, 1:250 dilution, R&D Systems). Upon appropriate antigen retrieval, reactivity was revealed using EnVision Mouse (Dako, Glostrup, Denmark), followed by diaminobenzidine. After completing the first immune reaction, the second was visualized using Mach 4-AP (Biocare Medical, Concord, CA, USA) and the reaction was developed with Ferangi Blue as chromogen (Biocare Medical). Digital images taken using the Olympus BX60 microscope were captured using a DP-70 Olympus digital camera and processed using Analysis Image Processing software (Olympus).
Statistical analysis
Bar graphs represent the means ±SEM of the number of experiments indicated in the figure legends. Statistical analysis was performed with GraphPad Prism version 5.0 (GraphPad Software, San Diego, CA, USA).
Results and Discussion
CD11a/CD18, but not DC-SIGN, mediates the release of IL-12p70 and IFNγ by NK cell/slanDC co-cultures
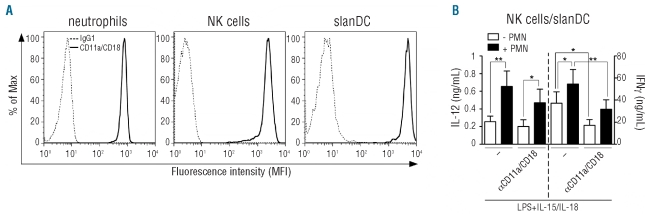
To identify the specific counter-receptors of ICAM-3 that mediate the production of IFNγ by activated neutrophil/NK cell/slanDC co-cultures5 we initially focused on CD11a that binds both ICAM-1 and ICAM-3,17 since it was found to be constitutively expressed at high levels in neutrophils, NK cells and slanDC (Figure 1A). αCD11a mAbs did not affect the release of IL-12p70 by NK cell/slanDC co-cultures, but significantly inhibited the production of IFNγ (Figure 1B). In contrast, a moderate but not statistically significant inhibition of the neutrophil-dependent potentiation of IL-12p70 (by about 30%) as well as IFNγ (by about 15%) release by NK cell/slanDC co-cultures was observed using αCD11a mAbs (Figure 1B). Corresponding isotype-matched control antibodies were without effect under any experimental conditions (data not shown). The results shown in Figure 1B recall our previous findings, again confirmed in parallel experiments (data not shown), demonstrating that, while diminishing the production of IFNγ, but not IL-12p70, by NK cell/slanDC co-cultures,5 αICAM-1 mAbs significantly inhibit the ability of neutrophils to potentiate the release of IL-12p70 and IFNγ by NK cell/slanDC co-cultures.5
Figure 1.
CD11a/CD18 is expressed by neutrophils, NK cells and slanDC and is involved in the release of IFNγ by NK cell/slanDC co-cultures. (A) Analysis for CD11a/CD18 expression by flow cytometry (as described in Design and Methods) in freshly isolated neutrophils, NK cells and slanDC. Cells were also stained with isotype control mAbs (gray histograms). One representative experiment out of 3 with similar results is shown. (B) NK cell/slanDC co-cultures were incubated with or without neutrophils and then treated with LPS plus IL-15/IL-18, in the absence or presence of αCD11a/CD18 mAbs (efalizumab). After 18 h, both extracellular IFNγ and IL-12p70 were measured by ELISA (n=3). P<0.05 (*) and P<0.01 (**) by one-way ANOVA of paired samples.
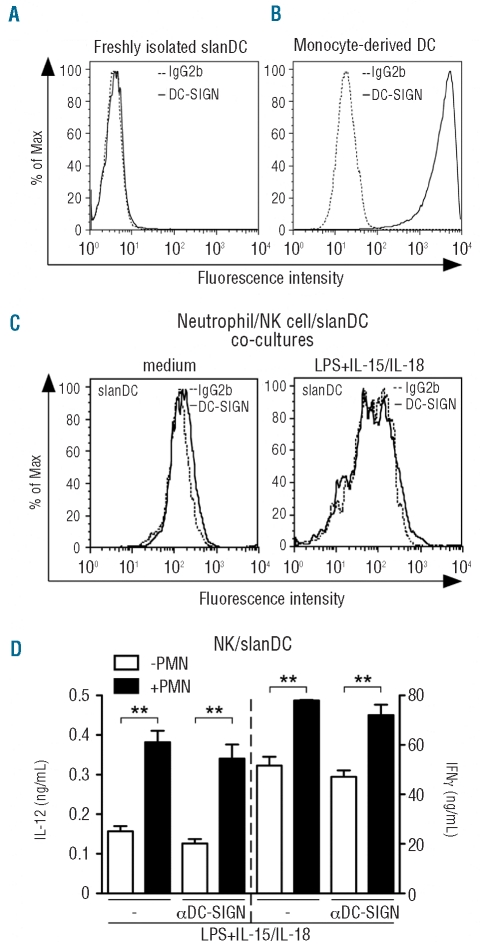
Subsequently, we focused on DC-SIGN. However, in line with previous results showing that only a small subset of peripheral blood DC, characterized by the expression of CD14, CD16 and CD1c and corresponding to 0.02% of total PBMC, expresses DC-SIGN,18 this could not be detected in freshly isolated slanDC (Figure 2A). By comparison, high levels of DC-SIGN were detected in moDC (Figure 2B), as expected.9 In addition, DC-SIGN was also undetectable in slanDC cultured for 18 h with NK cells, with or without LPS plus IL-15/IL18-stimulation, either in the absence (F. Calzetti, C. Costantini and M.A. Cassatella, unpublished data, September 2010) or the presence of neutrophils (Figure 2C). Consistent with the absence of DC-SIGN, two different neutralizing mAbs (clones 120507 and MR-1), as well as the corresponding isotype control antibodies (data not shown), did not inhibit the neutrophil-dependent potentiation of IL-12p70 and IFNγ in the neutrophil/NK cell/slanDC co-cultures (Figure 2D and data not shown).
Figure 2.
DC-SIGN does not mediate the production of IFNγ and IL-12 by NK cell/slanDC co-cultures in the presence or the absence of neutrophils. (A, C) Analysis for DC-SIGN expression by flow cytometry (as described in Design and Methods), in slanDC immediately after isolation (A) as well as after an 18 h co-culture with NK cells and neutrophils in the absence or the presence of LPS plus IL-15/IL-18. (C) One representative experiment out of 3 with similar results is shown. (B) DC-SIGN expression by moDC is shown as positive control. Cells were also stained with the corresponding isotype control mAbs (dashed lines). One representative experiment out of 3 with similar results is shown. (D) NK cell/slanDC co-cultures were incubated with or without neutrophils and then treated with LPS plus IL-15/IL-18, in the absence or presence of αDC-SIGN (clone MR-1) mAbs. After 18 h, both extracellular IFNγ and IL-12p70 were measured in culture supernatants (n=3). P<0.01 (**) by one-way ANOVA of paired samples.
Taken together, our data suggest that CD11a/CD18, that binds ICAM-1 with high affinity,17 likely mediates the effects of ICAM-1 within the neutrophil/NK cell/slanDC network, especially in the interaction between NK cells and slanDC. Finally, data suggest that a counter-receptor different from CD11a or DC-SIGN interacts with ICAM-3 in the neutrophil/NK cell/slanDC network.
ICAM-3 clusters and co-localizes with CD11d within the contact regions between neutrophils and NK cells
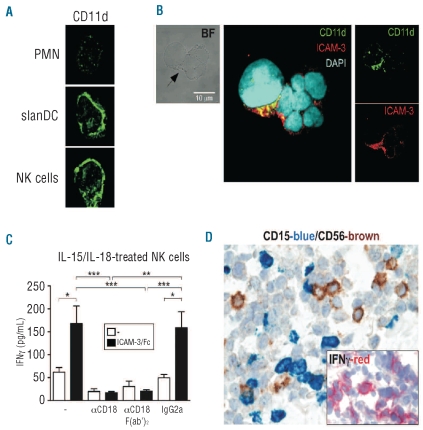
Since CD11a and DC-SIGN do not appear to interact with ICAM-3 (Figures 1 and 2), we subsequently evaluated whether CD11d, known to show a preferential binding to ICAM-3,8 could instead function as ICAM-3 counter-receptor in our system. Because of the absence of commercially available Abs displaying CD11d blocking properties, we performed confocal microscopy studies and found a strong constitutive expression of CD11d by slanDC and NK cells, while neutrophils were nearly negative (Figure 3A). ICAM-3 was instead homogeneously expressed in the three cell types.5
Figure 3.
CD11d/CD18 co-localizes within the contact regions between neutrophils and NK cells. (A) Neutrophil/NK cell/slanDC co-cultures were incubated for 2 h, harvested, cytocentrifuged and finally co-stained with αCD11d/CD18 Abs, prior to confocal microscopy analysis (as described in Design and Methods). (B) Neutrophil/NK cell/slanDC co-cultures were incubated with LPS plus IL-15/IL-18 for 15 h prior to confocal microscopy analysis. The contact regions between neutrophils and NK cells are shown by a bright field image (BF) (left panels) and indicated by an arrow. Overlay of fluorescent images (middle panels and right panels) demonstrates the co-localization of CD11d (green) and ICAM-3 (red) within the contact regions between neutrophils and NK cells. (A–B) Representative images from 3 experiments with similar results are shown. (C) ICAM-3/Fc was immobilized on plates prior to the addition of NK cells and subsequent incubation in the presence of IL-15/IL-18 with or without αCD18 (clone IB4), its F(ab’)2 fragments or mouse IgG2a isotype-matched control. Extracellular IFNγ was measured in cell-free supernatants after 18 h of culture (n=4). Symbols stand for: P<0.05 (*), P<0.01 (**) or P<0.001 (***) by one-way ANOVA of paired samples. (D) Sections are from cases of SS (n=4), immunostained for CD15, CD56 (original magnification: 600X) and IFNγ (inset, original magnification: 400X).
We have previously shown that neutrophils, NK cells and slanDC are frequently found in direct contact upon incubation with LPS plus IL-15/IL-18,5 and that ICAM-3 co-localizes with CD18 within the contact regions between neutrophils and NK cells.5 By using a previously established fluorescence staining procedure to unequivocally identify polymorphonuclear neutrophils, NK cells and slanDC,5 we found a co-localization between ICAM-3 and CD11d within the contact regions between neutrophils and NK cells (Figure 3B). Interestingly, an ICAM-3/CD11d co-localization was observed if neutrophils and NK cells were co-cultured in the absence of slanDC (data not shown), supporting the concept that neutrophils can directly function as accessory cells for NK cell activation. Since neutrophils appear to express very little CD11d (Figure 3A), it is likely that CD11d/CD18 on the NK cell side, and ICAM-3 on the neutrophil side, mediate the interaction between the two cell types. To unequivocally exclude the possibility that other receptors might be involved, we took advantage of an assay19 that we had recently adapted,5 showing that the release of IFNγ by NK cells is increased when either purified ICAM-3/Fc or αICAM-3 mAbs are immobilized on the culture plates prior to NK cell addition and their concurrent stimulation with IL-15 plus IL-18. By the same procedure, we found that the co-stimulatory effect of immobilized ICAM-3/Fc on the release of IFNγ by IL-15 plus IL-18-treated NK cells was completely prevented by αCD18, but not iso-type-matched control, mAbs (Figure 3C). A similar degree of inhibition was obtained using αCD18 F(ab’)2 fragments (Figure 3C), thus definitively excluding any potential non-specific involvement of FcγR-mediated effects. Taken together, our results strongly suggest that NK cells express a functional CD11d/CD18 receptor and that neutrophils potentiate the production of IFNγ by NK cells via an ICAM-3/CD11d interaction.
Neutrophils co-localize with NK cells in Sweet’s syndrome
Co-occurrence of NK cells and neutrophils was tested in Sweet’s syndrome (SS), an acute febrile neutrophilic dermatosis which can follow viral infections, autoimmune diseases and hematologic malignancies.20 SS is characterized by a dense dermal inflammatory infiltrate, mainly composed of mature neutrophils. By double staining tissue sections from our 4 cases of SS, we found that CD15+Neutrophil Elastase+ neutrophils were admixed with a variable number of CD56+ (CD3−) NK cells (Figure 3D and data not shown). Interestingly, we were able to detect numerous IFNγ-producing inflammatory dermal cells in the same areas (Figure 3D). By contrast, NK cells and neutrophils are regularly absent in normal skin (data not shown). These data suggest that a functional cross-talk between these two populations might occur in SS. Since neutrophil-survival cytokines, including IFNγ, have been associated with the etiology of the disease,21 the presence of NK cells in Sweet’s syndrome inflammatory infiltrate, likely producing IFNγ, provides new insights into the pathogenesis of this dermatosis and paves the way for novel therapeutic strategies.
Acknowledgments
The authors would like to thank Dr. C. Laudanna (Dept. of Pathology and Diagnostics, University of Verona, Italy), Dr. G. Girolomoni (Dept. of Medicine, University of Verona, Italy), Dr. Valentin Yakubenko (Dept. of Cell Biology, Cleveland Clinic, OH, USA) and Dr. A. Corbì (Centro de Investigaciones Biológicas, Consejo Superior de Investigaciones Científicas, Madrid, Spain) for providing us with the αCD18 (clone IB4), αCD11a (efalizumab), αCD11d and αDC-SIGN (clone MR-1) antibodies, respectively. We would also like to thank Dr. A. Montresor and Dr. S. Lonardi for their technical assistance.
Footnotes
Funding: this work was supported by grants from the Ministero dell’Istruzione, dell’Università e della Ricerca (PRIN), Fondazione Cariverona and Associazione Italiana per la Ricerca sul Cancro (AIRC 5839) to MAC. CC and NT were supported by AIRC fellowships.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Soehnlein O, Lindbom L. Phagocyte partnership during the onset and resolution of inflammation. Nat Rev Immunol. 2010;10(6):427–39. doi: 10.1038/nri2779. [DOI] [PubMed] [Google Scholar]
- 2.Boudaly S. Activation of dendritic cells by polymorphonuclear neutrophils. Front Biosci. 2009;14:1589–95. doi: 10.2741/3326. [DOI] [PubMed] [Google Scholar]
- 3.Costantini C, Cassatella MA. The defensive alliance between neutrophils and NK cells as a novel arm of innate immunity. J Leukoc Biol. 2011;89(2):221–33. doi: 10.1189/jlb.0510250. [DOI] [PubMed] [Google Scholar]
- 4.Muller I, Munder M, Kropf P, Hansch GM. Polymorphonuclear neutrophils and T lymphocytes: strange bedfellows or brothers in arms? Trends Immunol. 2009;30(11):522–30. doi: 10.1016/j.it.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 5.Costantini C, Calzetti F, Perbellini O, Micheletti A, Scarponi C, Lonardi S, et al. Human neutrophils interact with both 6-sulfo LacNAc+ DC and NK cells to amplify NK-derived IFN{gamma}: role of CD18, ICAM-1, and ICAM-3. Blood. 2011;117(5):1677–86. doi: 10.1182/blood-2010-06-287243. [DOI] [PubMed] [Google Scholar]
- 6.Fawcett J, Holness CL, Needham LA, Turley H, Gatter KC, Mason DY, et al. Molecular cloning of ICAM-3, a third ligand for LFA-1, constitutively expressed on resting leukocytes. Nature. 1992;360(6403):481–4. doi: 10.1038/360481a0. [DOI] [PubMed] [Google Scholar]
- 7.Vazeux R, Hoffman PA, Tomita JK, Dickinson ES, Jasman RL, St John T, et al. Cloning and characterization of a new intercellular adhesion molecule ICAM-R. Nature. 1992;360(6403):485–8. doi: 10.1038/360485a0. [DOI] [PubMed] [Google Scholar]
- 8.Van der Vieren M, Le Trong H, Wood CL, Moore PF, St John T, Staunton DE, et al. A novel leukointegrin, alpha d beta 2, binds preferentially to ICAM-3. Immunity. 1995;3(6):683–90. doi: 10.1016/1074-7613(95)90058-6. [DOI] [PubMed] [Google Scholar]
- 9.Geijtenbeek TB, Torensma R, van Vliet SJ, van Duijnhoven GC, Adema GJ, van Kooyk Y, et al. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell. 2000;100(5):575–85. doi: 10.1016/s0092-8674(00)80693-5. [DOI] [PubMed] [Google Scholar]
- 10.Werther WA, Gonzalez TN, O’Connor SJ, McCabe S, Chan B, Hotaling T, et al. Humanization of an anti-lymphocyte function-associated antigen (LFA)-1 monoclonal antibody and reengineering of the humanized antibody for binding to rhesus LFA-1. J Immunol. 1996;157(11):4986–95. [PubMed] [Google Scholar]
- 11.Li S, Wang H, Peng B, Zhang M, Zhang D, Hou S, et al. Efalizumab binding to the LFA-1 alphaL I domain blocks ICAM-1 binding via steric hindrance. Proc Natl Acad Sci USA. 2009;106(11):4349–54. doi: 10.1073/pnas.0810844106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hogg N, Stewart MP, Scarth SL, Newton R, Shaw JM, Law SK, et al. A novel leukocyte adhesion deficiency caused by expressed but nonfunctional beta2 integrins Mac-1 and LFA-1. J Clin Invest. 1999;103(1):97–106. doi: 10.1172/JCI3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bolomini-Vittori M, Montresor A, Giagulli C, Staunton D, Rossi B, Martinello M, et al. Regulation of conformer-specific activation of the integrin LFA-1 by a chemokine-triggered Rho signaling module. Nat Immunol. 2009;10(2):185–94. doi: 10.1038/ni.1691. [DOI] [PubMed] [Google Scholar]
- 14.Jameson B, Baribaud F, Pohlmann S, Ghavimi D, Mortari F, Doms RW, et al. Expression of DC-SIGN by dendritic cells of intestinal and genital mucosae in humans and rhesus macaques. J Virol. 2002;76(4):1866–75. doi: 10.1128/JVI.76.4.1866-1875.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Relloso M, Puig-Kroger A, Pello OM, Rodriguez-Fernandez JL, de la Rosa G, Longo N, et al. DC-SIGN (CD209) expression is IL-4 dependent and is negatively regulated by IFN, TGF-beta, and anti-inflammatory agents. J Immunol. 2002;168(6):2634–43. doi: 10.4049/jimmunol.168.6.2634. [DOI] [PubMed] [Google Scholar]
- 16.Tamassia N, Le Moigne V, Rossato M, Donini M, McCartney S, Calzetti F, et al. Activation of an immunoregulatory and antiviral gene expression program in poly(I:C)-transfected human neutrophils. J Immunol. 2008;181(9):6563–73. doi: 10.4049/jimmunol.181.9.6563. [DOI] [PubMed] [Google Scholar]
- 17.Luo BH, Carman CV, Springer TA. Structural basis of integrin regulation and signaling. Annu Rev Immunol. 2007;25:619–47. doi: 10.1146/annurev.immunol.25.022106.141618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Engering A, Van Vliet SJ, Geijtenbeek TB, Van Kooyk Y. Subset of DC-SIGN(+) dendritic cells in human blood transmits HIV-1 to T lymphocytes. Blood. 2002;100(5):1780–6. doi: 10.1182/blood-2001-12-0179. [DOI] [PubMed] [Google Scholar]
- 19.Bossy D, Buckley CD, Holness CL, Littler AJ, Murray N, Collins I, et al. Epitope mapping and functional properties of anti-intercellular adhesion molecule-3 (CD50) monoclonal antibodies. Eur J Immunol. 1995;25(2):459–65. doi: 10.1002/eji.1830250223. [DOI] [PubMed] [Google Scholar]
- 20.Buck T, Gonzalez LM, Lambert WC, Schwartz RA. Sweet’s syndrome with hematologic disorders: a review and reappraisal. Int J Dermatol. 2008;47(8):775–82. doi: 10.1111/j.1365-4632.2008.03859.x. [DOI] [PubMed] [Google Scholar]
- 21.Cohen PR. Sweet’s syndrome--a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34–61. doi: 10.1186/1750-1172-2-34. [DOI] [PMC free article] [PubMed] [Google Scholar]