Abstract
Myeloid sarcoma in acute myeloid leukemia has been clearly defined by the World Health Organization but studies regarding the prevalence and the prognostic impact of extramedullary acute myeloid leukemia have not been conducted. We performed 18Fluoro-deoxy-Glucose Positron Emission Tomography/Computed Tomography scans in 10 patients with de novo and relapsed acute myeloid leukemia and histologically proven extramedullary disease. The scans were able to detect the known extramedullary lesions in 9 out of 10 patients (90%). Furthermore, additional extramedullary sites were detected in 6 patients (60%). Thus, it is possible to identify known and clinically undetectable extramedullary manifestations of acute myeloid leukemia. Since most of these patients relapsed within a short period of time after initiation of therapy or had refractory disease, the detection of extramedullary disease with 18Fluoro-deoxy-Glucose Positron Emission Tomography/Computed Tomography might be helpful in the development of individual treatment algorithms for these high-risk patients. (ClinicalTrials.gov Identifier: NCT01278069).
Keywords: acute myeloid leukemia (AML), extramedullary AML, myeloid sarcoma, 18F-FDG-PET/CT
Introduction
Acute myeloid leukemia (AML) may present with extramedullary AML (EM-AML) at initial diagnosis or relapse. Myeloid sarcoma (MS) is defined as an extramedullary mass composed of myeloid blasts occurring at an anatomical site other than the bone marrow. Infiltrations occurring at any site in the body of leukemic patients are not classified as MS unless the mass destroys the tissue architecture.1 EM-AML is most frequently located in the skin but it can affect almost every part of the body.2–3 It occurs less commonly in patients with a transforming myelodysplastic syndrome (MDS) or myeloproliferative disorder (MPD).1 For AML patients who had undergone allogeneic hematopoietic stem cell transplantation (HSCT), retrospective data indicate relapse rates of 0.65% for extramedullary disease alone, compared to 30% in those with combined bone marrow and extramedullary disease.2,4–5 In the past, prognosis of MS or EM-AML has been a controversial topic. While an underlying MDS or MPD is thought to have a negative prognostic impact, de novo MS seems to be responsive to radiotherapy and/or chemotherapy.1,3 Recent data indicate that patients with EM-AML treated with autologous or allogeneic HSCT have a better overall survival (OS) compared to patients treated with chemotherapy alone.6 In a prospective trial addressing long-term survival in chemotherapy-refractory patients with AML who had undergone allogeneic HSCT, those with EM-AML had an inferior outcome after HSCT compared with those without EM-AML.7 In a large meta-analysis of eight prospective AML treatment trials, EM-AML was identified as an independent prognostic factor contributing to shorter overall survival in patients with chromosome 8 trisomy.8 There are no data on the prevalence of MS or EM-AML at initial diagnosis and the existing information about this condition is mostly based on retrospective and clinical analyses. 18Fluoro-deoxy-Glucose Positron Emission Tomography (18F-FDG-PET)/Computed Tomography (CT) is able to detect metabolically active tissue and has been shown to be effective in the detection and localization of various hematologic malignancies, particularly in Hodgkin’s lymphoma (HL) and non-Hodgkin’s lymphoma (NHL). It has been shown to be of prognostic importance and is, therefore, incorporated in the response criteria.9 Recent case reports have shown that 18F-FDG-PET/CT might be a useful tool in detecting extramedullary AML.10–13 In two retrospective studies of 5 and 6 patients with EM-AML, 18F-FDG-PET was more or at least equally effective in the detection of extramedullary disease.14 Furthermore, 18F-FDG-PET/CT was more accurate in detecting lesions than 18F-FDG-PET or CT alone.15 The aim of this study was to investigate the feasibility of using 18F-FDG-PET/CT scans to detect EM-AML in patients with newly diagnosed AML, as well as relapsed AML with histologically proven extramedullary disease.
Design and Methods
Patients
A total of 10 patients with AML and histologically proven extramedullary disease underwent total body 18F-FDG-PET/CT imaging at diagnosis to detect further manifestations. All patients had previously given their written informed consent for the procedure. Approval to review the medical records was obtained from the Ethics Committee of the Medical Faculty Carl Gustav Carus, Dresden (EK95032011). Blood, bone marrow, and MS (if available) tissue samples were obtained at diagnosis. Polymerase chain reaction (PCR) amplification of NPM1 exon 12 and FLT3-ITD mutation analyses were performed as previously described.16 Cytogenetic analyses were performed using standard techniques for chromosome banding and fluorescence in situ hybridization. MDS-derived AML (mdsAML) was defined by at least one documented bone marrow examination revealing MDS at least three months prior to the diagnosis of AML. Therapy-related AML (tAML) was defined as AML developing after cytotoxic chemotherapy and/or radiation therapy administered for a prior neoplastic or non-neoplastic disorder.
Imaging
Hybrid PET/CT scans were performed using a Biograph 16 W (Siemens Medical Solutions Inc., Knoxville, TN, USA) containing a 16-slice CT scanner. Unenhanced CT scans for attenuation correction were performed in a cranio-caudal direction from the skull base to lower thighs. Scanning parameters were as follows: 10 mAs, 120 kV, online tube current modulation, 1.5 mm slice collimation, 0.5–0.75 s rotation time, and reconstruction of 5 mm slices. The diagnostic CT scans of skull base, neck, thorax, abdomen and small pelvis were performed as primary contrast enhanced continuous scans. The scanning parameters were 1.5 mm slice collimation, 100 kV tube voltage, 180 mAs amperage with online current modulation using Siemens CareDose 4D® and a rotation time of 0.75 sec. As intravenous contrast media, we used 120 ml Ultravist® 370 (Bayer Schering Pharma, Leverkusen, Germany) followed by 50 ml of 0.9% NaCl solution to wash out remaining contrast media of the injection system. The injection was given automatically via a Medtron Accutron CT-D mobile system (Medtron AG, Saarbrücken, Germany) with a flow rate of 3 mL per sec. The CT scans were started automatically after a delay of 55 sec to the start of contrast media injection. Primary axial reconstructions in 3 mm slice thickness in soft tissue, lung and a bone window were calculated at post processing of the raw data.
All patients had a mean estimated glomerular filtration rate (eGFR) (according to the Modification of Diet in Renal Disease study equation)17 of 88 ml*min−1*1,73 m−2 prior to 18F-FDG-PET/CT (range 44–107) and a mean eGFR after 18F-FDG-PET/CT of 87 ml*min−1*1,73 m−2 (range 45–115) with no patient showing a continuous deterioration in eGFR thereafter (data not shown).
PET 3-dimensional (3D) emission scans with a median activity of 346 MBq (range 225–391 MBq) of FDG (GlucoRos® Helmholtz Center, Dresden-Rossendorf, Germany) were taken 60 min (±10 min) post injection.18 For each scan, the blood glucose level was measured immediately prior to FDG injection. Intraindividual variation was low (<20%) and the protocol was arranged to minimize differences in tumor uptake, resulting in intraindividual PET studies which could not be compared over time. The patients did not receive any sedative and/or diuretic medication in the FDG accumulation phase and they were instructed not to talk in order to avoid muscular FDG uptake, which could have hampered interpretation of the PET images.
The acquisition time per bed position, for the emission scan only, was 3 min. Uncorrected emission images and CT-based attenuation-corrected images were reconstructed using four iterations, eight subsets, and a 5 mm 3D Gaussian filter.
Results and Discussion
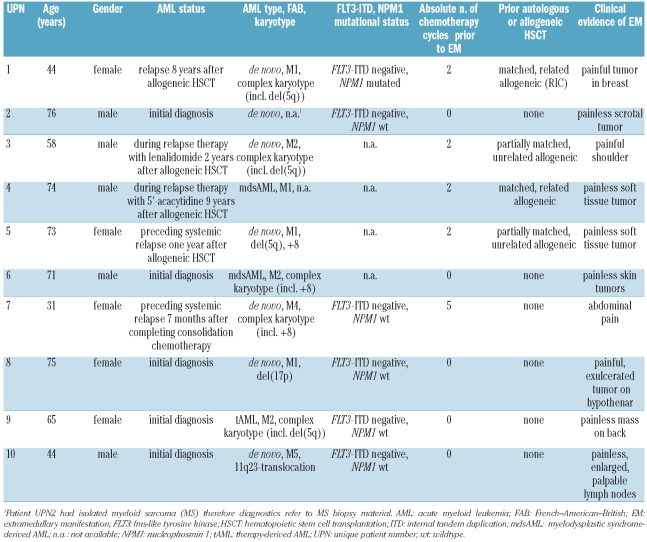
Patients’ characteristics at the time of 18F-FDG-PET/CT imaging are shown in Table 1. Notably, 5 of the 10 patients presented with EM-AML at relapse seven months to nine years after allogeneic HSCT. Cytogenetic analyses of bone marrow aspirates were available for 8 patients at initial diagnosis. Interestingly, 3 of the 5 patients who presented with EM-AML after allogeneic HSCT had a del(5q) karyotype. Three patients presented with chromosome 8 trisomy. Five patients had a complex karyotype. Only one of 6 patients had a mutated NPM1 gene at the time of diagnosis. None of the patients in whom the FLT3-mutational status was tested harbored a mutated FLT3 gene. Interestingly, only 4 patients complained of pain as a symptom of the EM mass while the remaining 6 patients had painless EM tumors.
Table 1.
Patients’ characteristics at the time of 18FDG-PET/CT.
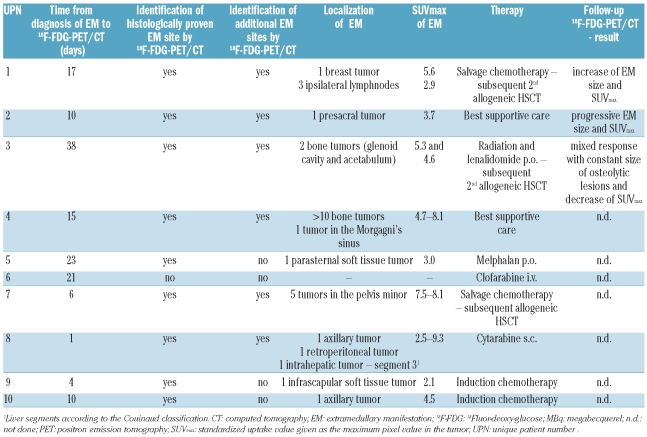
18F-FDG-PET/CT imaging time points, procedures, and findings are shown in Table 2. 18F-FDG-PET/CT imaging was performed in these 10 patients because they had his-tologically proven EM-AML; imaging was performed a median of 12 days (range 1–38 days) after histological diagnosis and prior to the initiation of systemic therapy. In 6 of the 10 AML patients (60%), 18F-FDG-PET/CT identified new EM manifestations that were not clinically detectable. This is in agreement with previous reports in smaller patient cohorts.14–15 In one patient (UPN 6), the histologically proven EM could not be detected by 18F-FDG-PET/CT. It is important to note that the SUVmax (standard uptake value given as the maximum pixel value in the tumor) ranged from 2.1 to 9.3 and showed intra- and interindividual variation; it was generally lower than that previously reported in patients with HL or NHL.13–15,19 Follow-up 18F-FDG-PET/CT was performed in 3 patients. Patient UPN 1 had follow-up imaging on day 25 after salvage chemotherapy and showed a decrease in breast tumor size (5.0 x 2.4 vs. 5.5 x 3.3 cm) and SUVmax (3.4 vs. 5.6) even though 18F-FDG-PET/CT was performed very soon after the initiation of treatment (Figure 1). Patient UPN 2, who had isolated MS, decided not to undergo any therapy but returned to the hospital 57 days later due to abdominal complaints. This patient then had follow-up 18F-FDG-PET/CT showing an increase in size of the presacral tumor (5.8×4.7 x 5.9 cm vs. 2.9 x 3.2 x 3.0 cm) and SUVmax (6.1 vs. 3.7). Patient UPN 3 received local irradiation of the glenoid cavity and acetabulum and systemic therapy with lenalidomide due to a del(5q) karyotype. This patient had a mixed response with no change in the dimensions of the osteolytic tumors and an increase in SUVmax in the glenoid (5.3 vs. 2.3) and the acetabulum (5.7 vs. 4.6) 27 days after first imaging and initiation of treatment.
Table 2.
18FDG-PET/CT imaging characteristics.
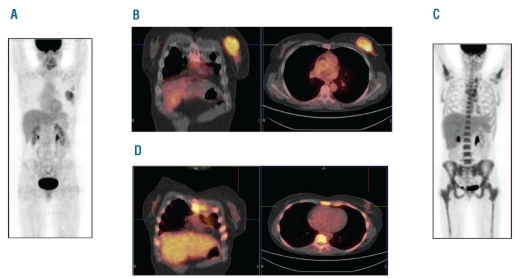
Figure 1.
(A) 18F-FDG-PET scan of patient UPN 1 at diagnosis of extramedullary AML. (B) Coronal and transversal 18F-FDG-PET/CT fusion images of the EM in the left mammary gland at diagnosis. (C) 18F-FDG-PET scan of the same patient after salvage chemotherapy (note: higher bone marrow 18F-FDG uptake due to higher bone marrow activity). (D) Coronal and transversal 18F-FDG-PET/CT fusion images of the EM in the left mammary gland after salvage chemotherapy (note: higher bone marrow 18F-FDG uptake due to higher bone marrow activity).
To the best of our knowledge, this is the largest study examining the suitability of 18F-FDG-PET/CT imaging for the detection of extramedullary disease in patients with AML. One study on the utility of 3′-deoxy-3′-18F-fluorothymidine (18F-FLT)-PET in a series of patients with AML, in which patients underwent either 18F-FLT-PET or -PET/CT before confirmation of EM-AML, has been reported.
Interestingly, 4 of the 10 patients were diagnosed as harboring EM-AML, suggesting a significant percentage of AML with occult extramedullary manifestations.20 This finding could be clinically relevant since EM-AML may respond differently to standard chemotherapy. By applying 18F-FDG-PET/CT in our study, we were able to detect EM-AML in patients with de novo AML, as well as AML relapsed after chemotherapy and allogeneic HSCT. EM appeared to occur more frequently in patients after allogeneic HSCT, supporting clinical observations and studies investigating the prevalence of extramedullary relapse in patients with AML after allogeneic HSCT.2,5 Cytogenetic studies of AML bone marrow aspirates in our patients showed a high proportion of del(5q) aberrations, which has not been observed at this frequency in EM-AML in the literature. However, the number of patients included in our study was too small to allow us to draw any definitive conclusions from this observation. Moreover, our result could reflect a bias since aggressive AML is associated with more frequent relapse and, therefore, occurs more often with extramedullary disease. Trisomy of chromosome 8 was also frequently observed in our patients as shown in previous reports.6,8 A high prevalence of NPM1 mutations has been reported in EM-AML.21–22 We could not confirm this observation nor the suggested inv(16) and t(8;21) alterations which might be associated with EM-AML1 due to the limitations imposed by the total number of only 10 patients. Lack of molecular information presented a further limitation. In most patients with EM-AML, 18F-FDG-PET/CT was able to detect additional extramedullary sites, whereas one patient with EM-AML had a false negative 18F-FDG-PET/CT result (UPN 6). We are aware that due to 18F-FDG distribution and metabolism, detection of EM in the heart, the central nervous system and urinary tract is difficult. However, these manifestations might be detected by routinely performed lumbar puncture in cases of neurological or psychiatric abnormalities and by echocardiography. Since the median SUVmax was lower than that observed in other hematologic diseases, a combination with CT seems reasonable in terms of anatomical land marking and collecting morphological aspects to determine the most exact diagnosis. Whether the lower SUVmax reflects lower growth kinetics in EM-AML as compared to other hematologic malignancies remains speculative. Therefore, 18F-FDG-PET may be less sensitive in the detection of extramedullary lesions with low FDG uptake, especially when located in areas with physiologically high background FDG uptake as previously suggested.14–15 In these cases, magnetic resonance imaging with diffusion weighted imaging (MRI-DWI) could be used as a complementary diagnostic tool, although this remains challenging since imaging findings lack specificity.15
It is generally accepted that local treatment of EM-AML is not sufficient for curative therapy and that allogeneic HSCT is superior compared to other therapies in this context.6,23 It remains a subject of debate whether quiescent AML cells serving as a reservoir for occult leukemia prefer infiltration and fusion with vascular endothelium, which has recently been observed, or whether they preferentially move toward and persist in bradytrophic sites (“sanctuary sites”).24 EM in AML is clinically difficult to diagnose, appears to have an inferior outcome without allogeneic HSCT, and its prevalence at diagnosis has still not been evaluated. These factors indicate the need for an increased level of alertness for this entity in patients with AML. Therefore, 18F-FDG-PET/CT should be applied in patients with EM-AML at diagnosis, before treatment initiation and thereafter as early assessment, and in complete remission (CR) in order to evaluate treatment response.
In conclusion, this single center study describes the detection of extramedullary disease by 18F-FDG-PET/CT in patients with histologically proven EM-AML. The ability to detect EM-AML, with its poor clinical trajectory, using a non-invasive diagnostic procedure would be a welcome adjunct to the management of this AML subtype. An observational trial with 18F-FDG-PET/CT for newly diagnosed and relapsed AML patients is currently underway to estimate the prevalence of EM in AML (ClinicalTrials.gov Identifier: NCT01278069).
Footnotes
Funding: this study was presented in part at the 52nd Annual Meeting of the American Society of Hematology, December 4–7, 2010, Orlando, Florida, USA.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 2008:140–1. [Google Scholar]
- 2.Chong G, Byrnes G, Szer J, Grigg A. Extramedullary relapse after allogeneic bone marrow transplantation for haematological malignancy. Bone Marrow Transplant. 2000;26(9):1011–5. doi: 10.1038/sj.bmt.1702659. [DOI] [PubMed] [Google Scholar]
- 3.Tsimberidou AM, Kantarjian HM, Wen S, Keating MJ, O’Brien S, Brandt M, et al. Myeloid sarcoma is associated with superior event-free survival and overall survival compared with acute myeloid leukemia. Cancer. 2008;113(6):1370–8. doi: 10.1002/cncr.23691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bekassy AN, Hermans J, Gorin NC, Gratwohl A. Granulocytic sarcoma after allogeneic bone marrow transplantation: a retrospective European multicenter survey. Acute and Chronic Leukemia Working Parties of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 1996;17(5):801–8. [PubMed] [Google Scholar]
- 5.Mortimer J, Blinder MA, Schulman S, Appelbaum FR, Buckner CD, Clift RA, et al. Relapse of acute leukemia after marrow transplantation: natural history and results of subsequent therapy. J Clin Oncol. 1989;7(1):50–7. doi: 10.1200/JCO.1989.7.1.50. [DOI] [PubMed] [Google Scholar]
- 6.Pileri SA, Ascani S, Cox MC, Campidelli C, Bacci F, Piccioli M, et al. Myeloid sarcoma: clinico-pathologic, phenotypic and cytogenetic analysis of 92 adult patients. Leukemia. 2007;21(2):340–50. doi: 10.1038/sj.leu.2404491. [DOI] [PubMed] [Google Scholar]
- 7.Schmid C, Schleuning M, Schwerdtfeger R, Hertenstein B, Mischak-Weissinger E, Bunjes D, et al. Long-term survival in refractory acute myeloid leukemia after sequential treatment with chemotherapy and reduced-intensity conditioning for allogeneic stem cell transplantation. Blood. 2006;108(3):1092–9. doi: 10.1182/blood-2005-10-4165. [DOI] [PubMed] [Google Scholar]
- 8.Schaich M, Schlenk RF, Al-Ali HK, Dohner H, Ganser A, Heil G, et al. Prognosis of acute myeloid leukemia patients up to 60 years of age exhibiting trisomy 8 within a non-complex karyotype: individual patient data-based meta-analysis of the German Acute Myeloid Leukemia Intergroup. Haematologica. 2007;92(6):763–70. doi: 10.3324/haematol.11100. [DOI] [PubMed] [Google Scholar]
- 9.Hutchings M, Specht L. PET/CT in the management of haematological malignancies. Eur J Haematol. 2008;80(5):369–80. doi: 10.1111/j.1600-0609.2008.01051.x. [DOI] [PubMed] [Google Scholar]
- 10.Karlin L, Itti E, Pautas C, Rachid M, Bories D, Cordonnier C, et al. PET-imaging as a useful tool for early detection of the relapse site in the management of primary myeloid sarcoma. Haematologica. 2006;91(12 Suppl):ECR54. [PubMed] [Google Scholar]
- 11.Kuenzle K, Taverna C, Steinert HC. Detection of extramedullary infiltrates in acute myelogenous leukemia with whole-body positron emission tomography and 2-deoxy-2-[18F]-fluoro-D-glucose. Mol Imaging Biol. 2002;4(2):179–83. doi: 10.1016/s1095-0397(01)00056-5. [DOI] [PubMed] [Google Scholar]
- 12.Mantzarides M, Bonardel G, Fagot T, Gontier E, Soret M, Revel TD, et al. Granulocytic sarcomas evaluated with F-18-fluorodeoxyglucose PET. Clin Nucl Med. 2008;33(2):115–7. doi: 10.1097/RLU.0b013e31815ef799. [DOI] [PubMed] [Google Scholar]
- 13.von Falck C, Laenger F, Knapp WH, Galanski M. F-18 FDG PET/CT showing bilateral breast involvement in acute myeloid leukemia relapse. Clin Nucl Med. 2009;34(10):713–5. doi: 10.1097/RLU.0b013e3181b53a6d. [DOI] [PubMed] [Google Scholar]
- 14.Ueda K, Ichikawa M, Takahashi M, Momose T, Ohtomo K, Kurokawa M. FDG-PET is effective in the detection of granulocytic sarcoma in patients with myeloid malignancy. Leuk Res. 2010;34(9):1239–41. doi: 10.1016/j.leukres.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 15.Aschoff P, Hantschel M, Oksuz M, Werner MK, Lichy M, Vogel W, et al. Integrated FDG-PET/CT for detection, therapy monitoring and follow-up of granulocytic sarcoma. Initial results. Nuklearmedizin. 2009;48(5):185–91. doi: 10.3413/nukmed-0236. [DOI] [PubMed] [Google Scholar]
- 16.Thiede C, Koch S, Creutzig E, Steudel C, Illmer T, Schaich M, et al. Prevalence and prognostic impact of NPM1 mutations in 1485 adult patients with acute myeloid leukemia (AML) Blood. 2006;107(10):4011–20. doi: 10.1182/blood-2005-08-3167. [DOI] [PubMed] [Google Scholar]
- 17.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 18.Boellaard R, O’Doherty MJ, Weber WA, Mottaghy FM, Lonsdale MN, Stroobants SG, et al. FDG PET and PET/CT: EANM procedure guidelines for tumour PET imaging: version 1.0. Eur J Nucl Med Mol Imaging. 2010;37(1):181–200. doi: 10.1007/s00259-009-1297-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Juweid ME. FDG-PET/CT in Lymphoma. Methods Mol Biol. 2011;727:1–19. doi: 10.1007/978-1-61779-062-1_1. [DOI] [PubMed] [Google Scholar]
- 20.Buck AK, Bommer M, Juweid ME, Glatting G, Stilgenbauer S, Mottaghy FM, et al. First demonstration of leukemia imaging with the proliferation marker 18F-fluorodeoxythymidine. J Nucl Med. 2008;49(11):1756–62. doi: 10.2967/jnumed.108.055335. [DOI] [PubMed] [Google Scholar]
- 21.Dohner K, Schlenk RF, Habdank M, Scholl C, Rucker FG, Corbacioglu A, et al. Mutant nucleophosmin (NPM1) predicts favorable prognosis in younger adults with acute myeloid leukemia and normal cytogenetics: interaction with other gene mutations. Blood. 2005;106(12):3740–6. doi: 10.1182/blood-2005-05-2164. [DOI] [PubMed] [Google Scholar]
- 22.Falini B, Lenze D, Hasserjian R, Coupland S, Jaehne D, Soupir C, et al. Cytoplasmic mutated nucleophosmin (NPM) defines the molecular status of a significant fraction of myeloid sarcomas. Leukemia. 2007;21(7):1566–70. doi: 10.1038/sj.leu.2404699. [DOI] [PubMed] [Google Scholar]
- 23.Cunningham I. Extramedullary sites of leukemia relapse after transplant. Leuk Lymphoma. 2006;47(9):1754–67. doi: 10.1080/10428190600632857. [DOI] [PubMed] [Google Scholar]
- 24.Masri A, Goldman DC, Asbaghi SA, Clark HA, Leon RP, Liu Q, Hickey RD, Grompe M, Kurre P, Fleming WH. Acute Myeloid Leukemia Cells Fuse with Vascular Endothelium. Blood (ASH Annual Meeting Abstracts) 2010;112:1490. [Google Scholar]