Abstract
Osteonecrosis of the jaw is an uncommon but potentially serious complication of bisphosphonate therapy in multiple myeloma. Previous studies showed that the presence of one or two minor alleles of the cytochrome P450, subfamily 2C polypeptide 8 gene (CYP2C8) polymorphism rs1934951 was an independent prognostic marker associated with development of osteonecrosis of the jaw in multiple myeloma patients treated with bisphosphonates. The aim of this study was to validate the frequency of SNP rs193451 in 79 patients with multiple myeloma. In 9 (22%) patients developing osteonecrosis of the jaw, a heterozygous genotype was found, in contrast with those who did not develop osteonecrosis of the jaw (n=4, 11%) or healthy individuals (n=6, 13%). We found no differences in the cumulative risk of developing osteonecrosis of the jaw between patients homozygous and heterozygous for the major allele. We were unable to confirm a significant association between this polymorphism and the risk of developing osteonecrosis of the jaw.
Keywords: CYP2C8, ONJ, bisphosphonates, multiple myeloma
Introduction
Multiple myeloma (MM) is characterized by anemia, increased susceptibility to infections, and severe pain as a result of osteolytic lesions. The destruction of bone is the result of multiple factors and causes several skeletal complications such as bone pain, pathological fractures, spinal cord compression, and hypercalcemia.1,2 Many of these complications are associated with significant morbidity and can have a negative impact on survival.2 To reduce and delay the skeletal morbidity caused by MM, treatment with bisphosphonates (BP) has become the standard of care for patients with MM and bone disease.3
Osteonecrosis of the jaw (ONJ) is an uncommon but potentially serious complication of intravenous BPs, which is characterized by the presence of exposed bone in the mouth.4,5 Currently, the cause of ONJ is not certain and is likely multi-factorial. The risk for ONJ increases the longer BP treatment is continued and has been shown to be 5–15% at four years.2 Although ONJ has been described during therapy with any BP, the possibility of developing ONJ may increase with the use of the more potent BPs, with a higher incidence for zoledronic acid (ZOL).6–8
Recently, several guidelines for the prevention, diagnosis, and management of ONJ have been released. These mainly focus on the relevant clinical aspects of prevention.1 Conversely, there is little data concerning biological markers that could help to predict which of these patients receiving BP therapy are at risk of developing this complication.
With the availability of the human genome sequence, the impact of single nucleotide polymorphism (SNP) variations on disease became a primary focus in many research areas. Effects on RNA stability, splicing, or mRNA folding have been suggested as potential mechanisms to explain how SNPs can affect drug sensitivity or biological functions. In MM, Sarasquete et al. published a study using the Affymetrix GeneChip Mapping 500K showing that the presence of one or two minor alleles of the cytochrome P450, subfamily 2C polypeptide 8 gene (CYP2C8) SNP rs1934951 was an independent prognostic marker associated with development of ONJ in MM patients treated with BPs.9 CYP2C8 gene, mapped in chromosome 10q23, codify for a significant enzyme in metabolism of numerous therapeutic drugs.10,11 The aim of this study was to validate the incidence of SNP rs1934951 polymorphism in an independent series of patients with MM treated with BP therapy.
Design and Methods
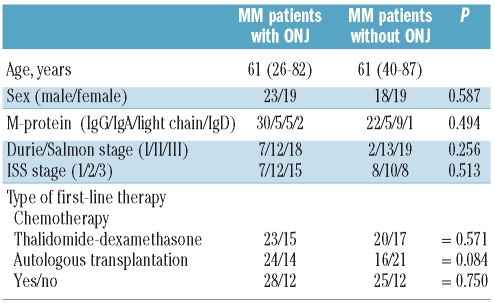
Two series of patients from the University Hospital La Fe (Valencia, Spain, n=59) and the Alexandra General Hospital (Athens, Greece, n=20) diagnosed with MM with and without ONJ were pooled and studied for the incidence of the rs1934951 polymorphism. Clinical and biological characteristics of the patients at diagnosis and their response to therapy were homogeneous between both series (Table 1). All together, a total of 79 patients with symptomatic MM diagnosed according to the International Myeloma Working Group criteria12,13 between 1993 and 2008, and receiving treatment with BPs constitute the basis of the present report. Those patients included in the study and diagnosed before 2003 started BP treatment from this date on. Forty-two out of the 79 patients had ONJ and the remaining 37 did not develop ONJ. For all patients, diagnostic procedures for ONJ were carried out by stomatologists with extensive experience in the diagnosis and management of this complication. ONJ was defined as a non-specific necrotic lesion of bone tissue that occurred spontaneously or after a dental procedure without any evidence of healing of the mucosal covering for at least eight weeks. In keeping with the guidelines of the Declaration of Helsinki, this retrospective non-interventional study was conducted with the approval of the internal review board of the Bioethics and Medical Research at the University Hospital La Fe. All patients received BP therapy with ZOL alone (median duration of treatment 18 months, range 8–84 months).
Table 1.
Main clinical and biological characteristics of the patients.
Using dbSNP, the International HapMap Project, Applied Biosystems’ SNP Browser and the UCSC Genome Browser, all functional information and frequency data in the polymorphic range were compiled, i.e. the frequency of the less common allele in a Caucasian population from the SNP selected for this study. Additionally, samples from 45 healthy volunteers provided by the Biobanco Hospital La Fe were analyzed for the presence of CYP2C8 polymorphisms to determine the polymorphic range in the general Spanish population and to confirm that there were no differences from the reported Caucasian population series. DNA samples were extracted from whole peripheral blood or serum blood by standard procedures. The SNP rs1934951 was analyzed by a TaqMan® assay method (TaqMan® SNP genotyping Assay, Applied Biosystems. Assay Reference C____361409_1_). The manufacturer’s protocols were followed, and samples were run in 96-well plates and read on LightCycler 480 Endpoint Genotyping Software.
Fisher’s exact test was used to test for the allelic association between SNP rs1934951 and ONJ. Unadjusted time-to-event analyses were performed using the Kaplan-Meier14 estimate and log rank tests for comparisons15. The probability of ONJ was also estimated by the cumulative incidence method, and univariate comparison between the curves was performed using Gray’s test16. All descriptive statistics and tests were performed using the statistical SPSS package, version 17.0 (SPSS Inc., Chicago, IL, USA) and R 2.7.2 software package.
P<0.05 was considered significant.
Results and discussion
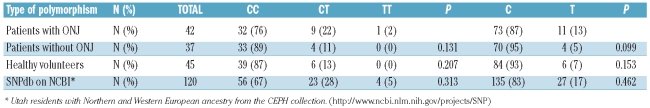
The series included 45 (54%) males and 38 (46%) females with a median age of 62 years (range 26–87 years). The median follow up was 84 months (range 10–256 months). Homozygous genotype for the major allele (CYP2C8CC) was the most frequent genotype in the three populations studied and was observed in 32 (76%) patients with ONJ, in 33 (89%) patients without ONJ, and in 39 (86%) healthy volunteers. These proportions were somewhat higher than those previously reported in SNP databases (Table 2). Conversely, heterozygous genotype (CYP2C8CT) was present in 9 patients with ONJ (22%), in 4 patients without ONJ (11%), and in 6 healthy volunteers (13%). Finally, only one of the individuals analyzed was found to be homozygous for the T allele (Table 1). Thus, although roughly twice the number of the MM patients developing ONJ showed heterozygous genotype CYP2C8CT as compared with MM patients who did not develop ONJ or healthy individuals, these differences did not reach statistical significance (P=0.13). These results contrast with those reported by Sarasquete et al. who found a 66% of heterozygous genotype CYP2C8CT in MM cases with ONJ versus 25% of controls.9 Additionally, they also found 14% homozygous for the CYP2C8TT allele within the ONJ group versus 0% within the controls.9 Perhaps the limited number of cases in both groups could have influenced the results; however, our P values are very different from those related to the comparisons made in the previously published report by Sarasquete et al.9
Table 2.
Allelic and genotypic distribution of the CYP2C8 SNP rs1934951.
These borderline results meant no concrete conclusions could be drawn. We, therefore, estimated the cumulative incidence of developing ONJ in patients with CYP2C8CT genotype and in patients with CYP2C8CC genotype. At three years, actuarial risk for patients with CYP2C8CT genotype was 35% and for those with CYP2C8CC 53%, with no statistically significant differences observed.
The two studies used slightly different methodologies. Besides these, several factors could explain, at least in part, the differences observed in the CYP2C8 allele proportion between our series and in the previous report by Sarasquete et al.9 First, there may have been a bias in patient selection between both series due to the small numbers of patients. Second, patients in the previous series were uniformly treated with polychemotherapy followed by autologous transplant whereas in our series patients received different induction regimens, including new drugs (thalidomide). Additionally, all patients in our study received ZOL as BP therapy whereas in the series by Sarasquete et al., the main BP administered was pamidronate.9 Finally, in our series we could not evaluate the potential role of oral risk factors (oral surgery, previous dental assessment, control of pre-existing implants and prosthesis) or the application of strategies to prevent ONJ in this group of patients.17 This could help to explain the different results observed in the two studies. Overall, although our results showed a trend towards a higher proportion of the SNP rs1934951 polymorphism on CYP2C8 gene in those patients developing ONJ, there was no significant difference in the cumulative risk compared with patients with the homozygous major allele. In conclusion, we were unable to confirm in an independent series a significant association between polymorphisms in the CYP2C8 gene and the risk of developing osteonecrosis of the jaw in patients with MM receiving treatment with BP.
Acknowledgments
The authors would like to thank Beatriz Costan for her daily support in performing molecular studies.
Footnotes
Funding: this study was supported in part by research funding from grants “Red Tematica de Investigación Cooperativa en Cancer” RD06/0020/0031 and “Red de Biobancos Hospitalarios” RD09/0076/00021, research project PI09/01882 from the “Instituto de Salud Carlos III”, research grant CA08/00141, CM10/00321 and CM09/00038 from the “Instituto de Salud Carlos III”, and “Ministerio de Ciencia e Innovación” grant BES2008-008053.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Pearse RN, Sordillo EM, Yaccoby S, Wong BR, Liau DF, Colman N, et al. Multiple myeloma disrupts the TRANCE/osteoprotegerin cytokine axis to trigger bone destruction and promote tumor progression. Proc Natl Acad Sci USA. 2001;98(20):11581–6. doi: 10.1073/pnas.201394498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kyle RA, Rajkumar SV. Multiple myeloma. N Engl J Med. 2004;351(18):1860–73. doi: 10.1056/NEJMra041875. [DOI] [PubMed] [Google Scholar]
- 3.Terpos E, Sezer O, Croucher PI, García-Sanz R, Boccadoro M, San Miguel J, et al. The use of bisphosphonates in multiple myeloma: recommendations of an expert panel on behalf of the European Myeloma Network. Ann Oncol. 2009;20(8):1303–17. doi: 10.1093/annonc/mdn796. [DOI] [PubMed] [Google Scholar]
- 4.Badros A, Terpos E, Katodritou E, Goloubeva O, Kastritis E, Verrou E, et al. Natural history of osteonecrosis of the jaw in patients with multiple myeloma. J Clin Oncol. 2008;26(36):5904–9. doi: 10.1200/JCO.2008.16.9300. [DOI] [PubMed] [Google Scholar]
- 5.Bagan JV, Murillo J, Jimenez Y, Poveda R, Milian MA, Sanchis JM, et al. Avascular jaw osteonecrosis in association with cancer chemotherapy: series of 10 cases. J Oral Pathol Med. 2005;34(2):120–3. doi: 10.1111/j.1600-0714.2004.00269.x. [DOI] [PubMed] [Google Scholar]
- 6.Dimopoulos MA, Kastritis E, Anagnostopoulos A, Melakopoulos I, Gika D, Moulopoulos LA, et al. Osteonecrosis of the jaw in patients with multiple myeloma treated with bisphosphonates: evidence of increased risk after treatment with zoledronic acid. Haematologica. 2006;91(7):968–71. [PubMed] [Google Scholar]
- 7.Mhaskar R, Redzepovic J, Wheatley K, Clark OA, Miladinovic B, Glasmacher A, et al. Bisphosphonates in multiple myeloma. Cochrane Database Syst Rev. 2010;(3):CD003188. doi: 10.1002/14651858.CD003188.pub2. [DOI] [PubMed] [Google Scholar]
- 8.Cafro AM, Barbarano L, Nosari AM, D'Avanzo G, Nichelatti M, Bibas M, et al. Osteonecrosis of the jaw in patients with multiple myeloma treated with bisphosphonates: definition and management of the risk related to zoledronic acid. Clin Lymphoma Myeloma. 2008;8(2):111–6. doi: 10.3816/clm.2008.n.013. [DOI] [PubMed] [Google Scholar]
- 9.Sarasquete ME, García-Sanz R, Marín L, Alcoceba M, Chillón MC, Balanzategui A, et al. Bisphosphonate-related osteonecrosis of the jaw is associated with polymorphisms of the cytochrome P450 CYP2C8 in multiple myeloma: a genome-wide single nucleotide polymorphism analysis. Blood. 2008;112(7):2709–12. doi: 10.1182/blood-2008-04-147884. [DOI] [PubMed] [Google Scholar]
- 10.Rodríguez-Antona C, Niemi M, Backman JT, Kajosaari LI, Neuvonen PJ, Robledo M, et al. Characterization of novel CYP2C8 haplotypes and their contribution to paclitaxel and repaglinide metabolism. Pharmacogenomics J. 2008;8(4):268–77. doi: 10.1038/sj.tpj.6500482. [DOI] [PubMed] [Google Scholar]
- 11.Speed WC, Kang SP, Tuck DP, Harris LN, Kidd KK. Global variation in CYP2C8-CYP2C9 functional haplotypes. Pharmacogenomics J. 2009;9(4):283–90. doi: 10.1038/tpj.2009.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.International Myeloma Working Group. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol. 2003;121(5):749–57. [PubMed] [Google Scholar]
- 13.Dimopoulos M, Terpos E, Comenzo RL, Tosi P, Beksac M, Sezer O, et al. International myeloma working group consensus statement and guidelines regarding the current role of imaging techniques in the diagnosis and monitoring of multiple Myeloma. Leukemia. 2009;23(9):1545–56. doi: 10.1038/leu.2009.89. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan EL, Meier P. Nonparametric estimations from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 15.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50(3):163–70. [PubMed] [Google Scholar]
- 16.Gray RJ. A class of K-sample test for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–54. [Google Scholar]
- 17.Vahtsevanos K, Kyrgidis A, Verrou E, Katodritou E, Triaridis S, Andreadis CG, et al. Longitudinal Cohort Study of Risk Factors in Cancer. Patients of Bisphosphonate-Related Osteonecrosis of the Jaw. J Clin Oncol. 27(32):5356–62. doi: 10.1200/JCO.2009.21.9584. [DOI] [PubMed] [Google Scholar]