Acute promyelocytic leukemia (APL) is a distinct subtype of acute myeloid leukemia that is characterized by three distinct features: i) accumulation in the bone marrow (BM) of tumor cells with promyelocytic phenotype; ii) association with specific translocations which involve chromosome 17 at the retinoic acid receptor alpha (RARA) locus; iii) and the sensitivity of APL blasts to the differentiating action of retinoic acid (RA).1 The RARA locus was first demonstrated to be involved in the t(15;17)(q22;q21) that fuses the RARA and the PML genes. While the PML/RARA fusion transcript is present in over 95% of APL cases, variant rearrangements have been identified involving RARA, and at a lower frequency (>3%) the PLZF, or more rarely the NPM1, NUMA, STAT5b, PRKAR1A and FIP1L1 as partner genes.2,3 The nature of the fusion partner has an important role on the disease biology particularly regarding RA sensitivity, with PLZF-RARA patients characterized by RA resistance.4 Following the description published in the journal Haematologica in 2008,5 we describe here the second case of FIP1L1/RARA fusion gene in an APL patient.
A 77-year old female patient presented with a progressive history of asthenia which lasted for several weeks. Initial laboratory evaluation of peripheral blood revealed a white blood cell count of 59×109/L with 84% of abnormal promyelocyte cells, hemoglobin level of 9.2 g/dL, and a platelet count of 109×109/L. The coagulation function in this patient was normal and lactate dehydrogenase was 1,938 U/L. The BM aspirate showed a hypercellular marrow replaced by promyelocyte blasts with intense azurophilic granule and prominent nucleoli accounting for 93% of all nucleated cells, suggestive of APL (Figure 1A). Immunophenotype was: CD45+, CD45RA+, CD13+, CD15+low, CD33+, CD133+low, HLA-DR+, CD11c+low, CD65+low, CD71+low, CD117+low and CD38+low. The molecular analysis identified the FLT3-ITD mutation, being negative for the presence of FLT3-D835Y, CEBPA and NPM1 mutations, as well as for the presence of PML/RARA, AML1/ETO and CBFβ/MYH11 fusion genes. The patient was treated according to PETHEMA APL 2005 protocol. Unfortunately, she died after ten days of treatment, probably due to RA syndrome, and response to RA treatment could not be assessed in this case due to early death.
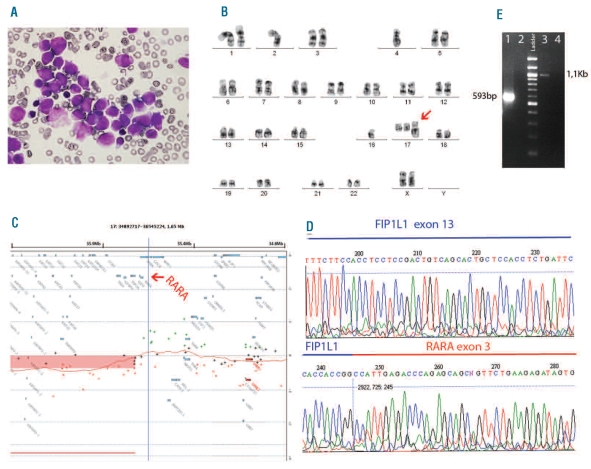
Figure 1.
Characterization of APL cells. (A) Morphology of the leukemia cells shows hypergranular promyelocytes with Auer roads in BM (100X). (B) A representative G-banded karyotype of the aberrant clone. The arrow indicates the derivative chromosome 17. (C) The panel shows the representative ideogram of the gain (40.8Mb) in chromosome 17q21.2q25.3, which results in the partial gain of the RARA locus and a possible rearrangement of this gene (arrow). (D) The sequence analysis of the identified fusion gene from the reverse sequence of RARA exon 3 identified FIP1L1 at exon 13 as the fusion partner gene. (E) Confirmation of the presence of the two reciprocal fusion transcripts by RT-PCR. Line 1: FIP1L1-RARA detection with a forward primer on FIP1L1 exon 10 (5′-ACAGCAGGGAAGAACTGGAA-3′), and a reverse primer on RARA exon 3 (5′-CCCCATAGTGGTAGCCTGAG). Line 3: RARA-FIP1L1 detection with a forward primer on RARA exon 1 (5′-ACACACCTGAGCAGCATCAC -3′), and a reverse primer on FIP1L1 exon 18 (5′-GTGTAGCTTCGGTGCTCTCC -3′). Lanes 2 and 4 are negative controls.
Cytogenetics revealed, in addition to normal metaphases, a complex karyotype with the presence of a der(17) in 50% of the metaphases with the following formula: 44,X,der(X)t(X;?)(p?;?),-2,-4,-16,+der(17)t(17;?) (q21;?) [cp10] (Figure 1B). FISH analysis with the PML-RARA dual-fusion translocation probe (Kreatech Diagnostics, Amsterdam, The Netherlands) identified no dual fusion signal but the presence of two copies of PML and three signals of RARA gene, indicating a possible variant rearrangement of this gene. The presence of DNA copy number changes was analyzed by array CGH with Agilent 44K platform (Agilent Technologies, Palo Alto, CA, USA). We found a mono-allelic gain of 40.8Mb in the long arm of chromosome 17 (Figure 1C). The duplicated region started within the locus of RARA gene, also indicating its possible rearrangement. To identify the 5′-fusion partner of RARA, we assayed the 5′-RACE method (SMARTer RACE cDNA, Clontech, Mountain View, CA, USA) designing a reverse primer complementary to exon 3 of the RARA gene. FIP1L1 was identified as the fusion partner of RARA. The rearrangement fused the RARA exon 3 with exon 13 of the FIP1L1 gene (Figure 1D). Direct sequencing of the reverse-transcriptase PCR products revealed that the FIP1L1/RARA and the RARA/FIP1L1 fusion transcripts were both in frame fusions (Figure 1E).
FIP1L1 is an integral subunit of cleavage and polyadenylation specificity factor and interacts with poly(A)polymerase to stimulate polyadenylation.6 This gene is recurrently fused to PDGFRA in patients with eosinophilia-associated myeloproliferative neoplasms (Eos-MPNs), with clinical response to imatinib.7,8 Although a FIP1L1/RARA fusion was described in a case of juvenile myelomonocytic leukemia,9 Kondo et al. described, for the first time, the FIP1L1/RARA fusion gene in APL.5 In both cases, the fusion gene was generated juxtaposing exons 15 and 3 of FIP1L1 and RARA, respectively. In the molecular pathogenesis of APL, fusion gene products must form homodimers to repress RA-responsive transcriptional activity.10,11 In fact, homodimerization, which seems to be dependent on the FIP1L1 portion, was demonstrated for the isoforms identified in the previous patient with the FIP1L1/RARA fusion that retained FIP1L1 exon 15 and responded to RA treatment.5 Conversely in the FIP1L1/PDGFRA fusion in Eos-MPNs, the breakpoint in FIP1L1 is variable and spreads from exon 10 to exon 13 (exons 7 to 9, according to Cools J et al.7), lacking the ability to form homodimers in a mouse pro-B cell line.12 It still remains to be clarified whether the RA syndrome observed in our case could be due to the abnormal homodimerization predicted by the breakpoint in FIP1L1, similar to that observed in Eos-MPNs.
In conclusion, we report the second occurrence of t(4;17)(q12;q21), with the reciprocal FIP1L1/RARA transcripts, in a very aggressive case of APL. Our results confirm FIP1L1 as a recurrent partner of RARA gene with breakpoint at intron 13 that was associated with the RA syndrome. We, therefore, propose the inclusion of the FIP1L1/RARA variant fusion gene in the screening in PML/RARA-negative APL patients in order to indicate alternatives therapies.
Acknowledgments
We thank Dr. Eva Yebra Fernandez from Severo Ochoa Hospital, who provided some clinical data to be evaluated in this study, and all the co-workers in our laboratory, for their excellent technical assistance. This work was supported by an INTRASALUD project PI 08-0440 to JCC and Obra Social-Fundación “La Caixa” to JM.
Footnotes
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.de The H, Chen Z. Acute promyelocytic leukaemia: novel insights into the mechanisms of cure. Nat Rev Cancer. 2010;10(11):775–83. doi: 10.1038/nrc2943. [DOI] [PubMed] [Google Scholar]
- 2.Zelent A, Guidez F, Melnick A, Waxman S, Licht JD. Translocations of the RARalpha gene in acute promyelocytic leukemia. Oncogene. 2001;20(49):7186–203. doi: 10.1038/sj.onc.1204766. [DOI] [PubMed] [Google Scholar]
- 3.Catalano A, Dawson MA, Somana K, Opat S, Schwarer A, Campbell LJ, et al. The PRKAR1A gene is fused to RARA in a new variant acute promyelocytic leukemia. Blood. 2007;110(12):4073–6. doi: 10.1182/blood-2007-06-095554. [DOI] [PubMed] [Google Scholar]
- 4.Lo Coco F, Ammatuna E, Noguera N. Treatment of acute promyelocytic leukemia with gemtuzumab ozogamicin. Clin Adv Hematol Oncol. 2006;4(1):57–62. [PubMed] [Google Scholar]
- 5.Kondo T, Mori A, Darmanin S, Hashino S, Tanaka J, Asaka M. The seventh pathogenic fusion gene FIP1L1-RARA was isolated from a t(4;17)-positive acute promyelocytic leukemia. Haematologica. 2008;93(9):1414–6. doi: 10.3324/haematol.12854. [DOI] [PubMed] [Google Scholar]
- 6.Kaufmann I, Martin G, Friedlein A, Langen H, Keller W. Human Fip1 is a subunit of CPSF that binds to U-rich RNA elements and stimulates poly(A) polymerase. EMBO J. 2004;23(3):616–26. doi: 10.1038/sj.emboj.7600070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cools J, DeAngelo DJ, Gotlib J, Stover EH, Legare RD, Cortes J, et al. A tyrosine kinase created by fusion of the PDGFRA and FIP1L1 genes as a therapeutic target of imatinib in idiopathic hypereosinophilic syndrome. N Engl J Med. 2003;348(13):1201–14. doi: 10.1056/NEJMoa025217. [DOI] [PubMed] [Google Scholar]
- 8.Walz C, Score J, Mix J, Cilloni D, Roche-Lestienne C, Yeh RF, et al. The molecular anatomy of the FIP1L1-PDGFRA fusion gene. Leukemia. 2009;23(2):271–8. doi: 10.1038/leu.2008.310. [DOI] [PubMed] [Google Scholar]
- 9.Buijs A, Bruin M. Fusion of FIP1L1 and RARA as a result of a novel t(4;17)(q12;q21) in a case of juvenile myelomonocytic leukemia. Leukemia. 2007;21(5):1104–8. doi: 10.1038/sj.leu.2404596. [DOI] [PubMed] [Google Scholar]
- 10.Kwok C, Zeisig BB, Dong S, So CW. Forced homo-oligomerization of RARalpha leads to transformation of primary hematopoietic cells. Cancer Cell. 2006;9(2):95–108. doi: 10.1016/j.ccr.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Sternsdorf T, Phan VT, Maunakea ML, Ocampo CB, Sohal J, Silletto A, et al. Forced retinoic acid receptor alpha homodimers prime mice for APL-like leukemia. Cancer Cell. 2006;9(2):81–94. doi: 10.1016/j.ccr.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 12.Stover EH, Chen J, Folens C, Lee BH, Mentens N, Marynen P, et al. Activation of FIP1L1-PDGFRalpha requires disruption of the juxtamembrane domain of PDGFRalpha and is FIP1L1-independent. Proc Natl Acad Sci USA. 2006;103(21):8078–83. doi: 10.1073/pnas.0601192103. [DOI] [PMC free article] [PubMed] [Google Scholar]