Abstract
Mast cell maturation is poorly understood. We show that enhanced PI3K activation results in accelerated maturation of mast cells by inducing the expression of microphthalmia transcription factor (Mitf). Conversely, loss of PI3K activation reduces the maturation of mast cells by inhibiting the activation of AKT, leading to reduced Mitf but enhanced Gata-2 expression and accumulation of Gr1+Mac1+ myeloid cells as opposed to mast cells. Consistently, overexpression of Mitf accelerates the maturation of mast cells, whereas Gata-2 overexpression mimics the loss of the PI3K phenotype. Expressing the full-length or the src homology 3– or BCR homology domain–deleted or shorter splice variant of the p85α regulatory subunit of PI3K or activated AKT or Mitf in p85α-deficient cells restores the maturation but not growth. Although deficiency of both SHIP and p85α rescues the maturation of SHIP−/− and p85α−/− mast cells and expression of Mitf; in vivo, mast cells are rescued in some, but not all tissues, due in part to defective KIT signaling, which is dependent on an intact src homology 3 and BCR homology domain of p85α. Thus, p85α-induced maturation, and growth and survival signals, in mast cells can be uncoupled.
Introduction
Mast cells are considered to be the primary cell type responsible for allergic disease and play an important role in modulating both innate and adaptive immune responses in humans.1–4 More recently, these cells have been implicated in autoimmune and inflammatory diseases. Mast cells are fairly long lived and have the capacity to proliferate on stimulation in vivo. Furthermore, enhanced recruitment of these cells in response to allergens or other stimuli results in their expansion in vivo.1–4 Although it has been known for some time that the expansion, survival, and maturation of mast cells in the bone marrow (BM) and in various tissues are largely controlled by signals generated in response to KIT receptor stimulation by its ligand, stem cell factor (SCF; KIT ligand), and by IL-3 receptor stimulation by IL-3, how precisely this occurs is still not fully understood.1–4
Class IA phosphatidylinositol-3-kinases (PI3Ks) are heterodimeric lipid kinases composed of p110 catalytic subunits and p50, p55, or p85 regulatory subunits. The regulatory subunits, via their src homology (SH) 2 domains, bring the enzyme complex to activated cytokine or tyrosine kinase-containing receptors, enabling the catalytic subunits to convert plasma membrane associated phosphatidylinositol-4,5-bisphosphate to the important second messenger phosphatidylinositol-3,4,5-trisphosphate (PIP3).5,6 In the hematopoietic system, there are 2 genes, PIK3R1 and PIK3R2, that encode p85α and its splice variants p55α, p50α, and p85β. In addition to the regulatory subunits, 3 separate genes, PIK3CA, PIK3CB, and PIK3CD, encode p110α, p110β, and p110δ catalytic subunits, respectively. It is generally believed that each p85 isoform is equally capable of binding all p110 isoforms and vice versa.6
PIP3 acts as a lipid second messenger to amplify cytokine or growth factor-induced signals by binding to pleckstrin homology domains of several proteins, including the serine/threonine kinase AKT. Importantly, the hematopoietic-restricted SH2-containing inositol 5′-phosphatase SHIP inhibits the PI3K pathway primarily by hydrolyzing PIP3 to phosphatidylinositol-(3,4)-bisphosphate.7 Whereas PIP3 levels are regulated by its synthesis by PI3K and its degradation by phosphoinositide phosphatases such as SHIP, the relative contribution of PIP3, PI3K, and SHIP to maturation of bone marrow cells into mast cells is not known.
Recent studies have shown that both p85α and SHIP contribute to many mast cell functions8–10; however, how these 2 proteins with opposing biochemical functions contribute to mast cell maturation and survival is unclear. In the current study, we define a novel pathway involving p85α/AKT/Mitf in regulating the maturation of mast cells and demonstrate that p85α-induced maturation and growth signals can be uncoupled.
Methods
Mice
p85α−/−, SHIP−/−, and Wsh mice (all on C57/BL6 backgrounds) have been described previously.8–9,11 All mice were used between 6-10 weeks of age. Mice doubly deficient in p85α and SHIP were generated by crossing p85α+/− and SHIP+/− mice. To better understand the role of p85α, p55α, and p50α in mast cell development, we also used the Cre/lox approach to generate mice in which all 3 p85α isoforms were deleted in a tissue-specific manner as described previously.12 To generate mice deficient in the expression of p85α, p55α, and p50α (p85αfl/fl) in hematopoietic cells, we crossed p85αfl/fl mice with Mx-Cre mice to generate p85αfl/fl/Cre+ mice in which induction of Cre recombinase by poly I:C injection deletes the gene that encodes p85α, p55α, and p50α subunits. Progeny from these mice were genotyped by polymerase chain reaction (PCR). All mice were maintained under specific pathogen-free conditions at the Indiana University Laboratory Animal Research Center (Indianapolis, IN). All studies were approved by the Indiana University Laboratory Animal Resource Center.
Transplantation of p85α−/−:SHIP−/− bone marrow cells into Wsh mice and identification of mast cells in tissues
Low-density BM cells (1 × 106) from wild-type (WT), SHIP−/−, p85α−/−, or SHIP−/−:p85α−/− mice and 0.1 × 106 supporting splenocytes from Wsh mice were transplanted intravenously by tail vein injection into lethally irradiated (1100 cGy-split dose) Wsh mice. Mice were killed after 4 months, and low-density BM cells and tissues were collected to analyze mast cell growth, maturation, and tissue distribution. Ear and small gastrointestinal tract (stomach, duodenum, jejunum, ileum, and colon) harvested from transplanted Wsh mice were fixed in 10% buffered formalin, sectioned, and stained with toluidine blue (Sigma-Aldrich). For each sample, mast cells stained in purple were counted in 5-12 fields under ×200 magnification using a microscope (Leica Microsystems). Average numbers of mast cells in a given field are represented. For identification of mast cells in the peritoneal cavity, 5 mL of sterile PBS free of Ca2+/Mg+ was injected intraperitoneally into mice, and fluid was allowed to equilibrate in the peritoneum for 5 minutes. Three milliliters of lavage fluid was retrieved, and total cells were counted after red cell lysis.
Transduction and expression of various constructs in BMMCs
Retroviral supernatants for transduction of mast cell progenitors (MCps) were generated using the Phoenix ecotropic packaging cell line transfected with retroviral vector plasmids (cDNAs encoding Mitf, Gata-2, and various p85 mutants) using a calcium phosphate transfection kit (Invitrogen). Supernatants were collected 48 hours after transfection and filtered through 0.45-μm membranes. BM-derived mast cells (BMMCs) were suspended in IMDM containing 20% FBS and 2% penicillin/streptomycin, and prestimulated in nontissue culture plates supplemented with SCF (50 ng/mL) and IL-3 (10 ng/mL) for 48 hours before retroviral infection on fibronectin fragments. After infection, cells were sorted to homogeneity based on enhanced green fluorescent protein (EGFP) expression and used to perform all experiments. BMMCs infected with MSCV-p110CAAX were cultured in BMMC media for 2 days followed by selection with puromycin.13 Hemagglutinin (HA)–tagged ActAKT is a constitutively active form of AKT that has a myristoylation sequence at the amino terminal, which causes AKT to associate with the membrane, leading to its constitutive phosphorylation and activation.14 Transduced cells were grown in BMMC media for an additional 2-3 weeks and analyzed by flow cytometry for the expression of KIT and IgE receptor.
Results
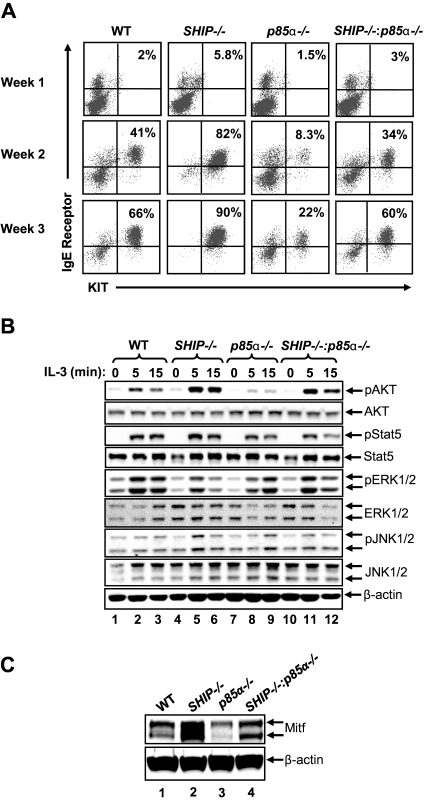
Because SHIP has been shown to play a central role in repressing the activation of the PI3K pathway after cytokine and growth factor stimulation in BMMCs by hydrolyzing PIP3 to phosphatidylinositol-(3,4)-biphosphate,7,15 we assessed the effect of the presence or absence of SHIP on the maturation of BMMCs from its precursors (see supplemental Methods, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Specifically, BM from WT and SHIP−/− mice were cultured for 4 weeks with IL-3, and then their maturation into mast cells was assessed by carrying out cytospins over time. We observed significant differences in the morphology of cells derived from WT and SHIP−/− mice. In particular, we observed that deficiency of SHIP alone in cultures induced the development of a higher percentage of cells that resembled more mature mast cells compared with WT controls. The differences in morphology were apparent at the end of second, third, and fourth week of culture (data not shown). To further explore the possibility of accelerated mast cell maturation because of elevated PI3K activity, we systematically monitored the acquisition and emergence of KIT and IgE receptor double-positive cells at the end of every week of culture in vitro in the 2 genotypes. Although the percentage of KIT and IgE receptor double-positive cells on day 0 in the BM from both genotypes was similar (data not shown); soon after, and at the end of weeks 1, 2, and 3, the maturation of SHIP-deficient BMMCs was significantly enhanced relative to controls (Figure 1A).
Figure 1.
Deficiency of p85α in SHIP−/− bone marrow cells rescues BMMC maturation in vitro. (A) Flow cytometric analysis demonstrating the expression of KIT and IgE receptor double-positive cells at the indicated times in BMMCs derived from the indicated genotypes. BM cells were harvested from WT, SHIP−/−, p85α−/−, and SHIP−/−:p85α−/− mice, and BMMCs were derived in vitro. Cells were harvested and stained with antibodies that recognize KIT and IgE receptor after the indicated weeks followed by flow cytometry. Numbers in the top right quadrant of each dot blot indicate the percentage of BMMCs that are double positive for KIT and IgE receptor expression during different times of culture. n = 5. (B) Hyperactivation of AKT in SHIP−/− BMMCs is reduced in the setting of p85α deficiency. BMMCs derived from the indicated genotypes were starved in the absence of growth factors and stimulated for the indicated times. Cell lysates were subjected to Western blot analysis using antibodies that recognize the activated version of the indicated signaling proteins. Similar findings were observed in additional 1 to 3 independent experiments. (C) Loss of SHIP expression in BMMCs results in enhanced expression of Mitf. BMMCs derived from WT, SHIP−/−, p85α−/−, and SHIP−/−:p85α−/− mice were lysed and subjected to Western blot analysis using an anti-Mitf antibody. Arrows indicate the level of expression of Mitf and β-actin (loading control) in each lane. n = 3.
To assess the signaling molecules that might contribute to the enhanced maturation of SHIP-deficient BMMCs, we examined the activation of AKT, Stat5, JNK, and ERK in the 2 genotypes. Although a subtle change in the activation of most of the above-mentioned signaling molecules was observed in the 2 genotypes; activation of AKT was significantly enhanced in SHIP−/− BMMCs relative to WT controls (Figure 1B lane 2 and compared with lanes 5 and 6). To assess whether the enhanced maturation observed in SHIP-deficient BMMCs was mediated by elevated PI3K activity in response to the binding of p85α–p110 complex to the activated receptor, we crossed SHIP-deficient mice with p85α-deficient mice and generated SHIP and p85α double knockout mice (SHIP−/−:p85α−/− or DKO). Cytospins performed on BMMCs from the 4 genotypes showed significant differences in cell morphology during maturation. Specifically, we observed that deficiency of p85α alone in MCps induced the development of fewer mature BMMCs compared with WT or SHIP−/− cells (data not shown). Furthermore, although the frequency of KIT and IgE receptor double-positive cells on day 0 in the BM from all 4 genotypes was similar (data not shown), MCps lacking p85α showed a profound reduction in the expression of KIT and IgE receptor double-positive cells, whereas MCps deficient in the expression of both SHIP and p85α behaved in a manner similar to WT BMMCs (Figure 1A). Importantly, the reduced AKT activation associated with reduced maturation of p85α−/− BMMCs was rescued in DKO BMMCs (Figure 1B lanes 8 and 9 compared with lanes 11 and 12). Thus, PI3K-induced AKT activation probably contributes to the maturation of BMMCs.
Maturation of mast cells, including the expression of KIT, is regulated to a large extent by the transcription factor Mitf.16–19 Two alternatively spliced forms of Mitf have been reported previously in mast cells.20 Loss of Mitf expression or mutations in the Mitf gene has been shown to impact the expression of multiple mast cell-specific genes, including KIT.21 To determine whether the increased rate of BMMC maturation, including the expression of KIT, because of SHIP deficiency is a result of increased Mitf expression, we performed Western blot analysis on BMMCs derived from WT, SHIP−/−, p85α−/−, and DKO mice. Figure 1C demonstrates a significant increase in the expression of Mitf in BMMCs derived from SHIP−/− mice compared with WT controls. Importantly, and consistent with the reduced expression of KIT in BMMCs lacking p85α, expression of Mitf was significantly reduced in cells deficient in the expression of p85α relative to controls (Figure 1C lane 1 compared with lane 3). Furthermore, DKO BMMCs showed significant correction in the expression of Mitf expression relative to SHIP−/− or p85α−/− BMMCs (Figure 1C).
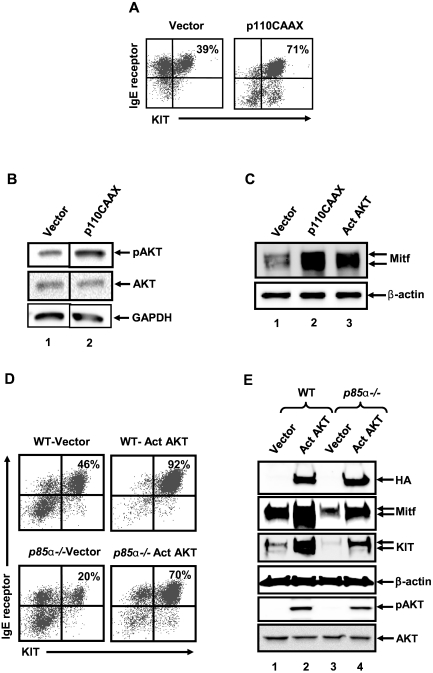
To directly assess the consequence of hyperactivation of the PI3K/AKT pathway on the maturation of BMMCs, we compared the maturation of WT MCps expressing a constitutively activated version of PI3K (p110CAAX) versus an empty vector control. Transduced cells were selected in the presence of puromycin. As seen in Figure 2A, whereas introduction of the empty vector encoding the puromycin resistance gene led to the acquisition of ∼ 40% KIT and IgE receptor double-positive BMMCs; expression of p110CAAX resulted in ∼ 70% KIT and IgE receptor double-positive BMMCs during the same time period under identical culture conditions. The increase in the maturation of p110CAAX-expressing MCps was associated with enhanced constitutive activation of AKT (Figure 2B lane 2). To further determine whether the increased rate of BMMC maturation in cells bearing activated version of PI3K is a result of increased Mitf expression, we performed Western blot analysis on WT BMMCs expressing an empty vector or p110CAAX. As seen in Figure 2C, a significant increase in the expression of Mitf was observed in BMMCs expressing p110CAAX compared with empty vector-transduced cells. Thus, similar to the results obtained using SHIP−/− MCps, expression of a constitutively active version of PI3K in WT MCps also results in increased activation of AKT, increased expression of Mitf and accelerated BMMC maturation.
Figure 2.
Expression of activated PI3K (p110CAAX) accelerates the rate of WT BMMC maturation by inducing the expression of Mitf. WT MCps were transduced with either an empty vector control or a vector expressing an activated version of PI3K (p110CAAX). Transduced cells were selected in puromycin for 2-3 weeks and analyzed for maturation. (A) Cells were subjected to flow cytometric analysis to assess the coexpression of KIT and IgE receptor double-positive cells after culture. Numbers in the top right quadrant indicate the percentage of cells that are double positive for KIT and IgE receptor expression. Shown are representative dot blots from 1 of 4 independent experiments. (B) Lysates derived from cells in panel A were subjected to Western blot analysis using antibodies against phospho-AKT and total AKT. The level of activated AKT is indicated by an arrow. (C) Lysates derived from cells in panels A and D were subjected to Western blot analysis using an anti-Mitf antibody. Arrows indicate the level of expression of Mitf and β-actin (loading control) in each lane. (D) Expression of activated AKT in p85α−/− BMMCs rescues mast cell differentiation. MCps from WT or p85α−/− mice were transduced with retrovirus expressing either an empty vector or an activated version of AKT with an HA tag. Transduced cells were cultured for another 2 to 3 weeks, and EGFP-positive cells were sorted and analyzed for the expression of KIT and IgE receptor by flow cytometry. Percentage of KIT and IgE receptor double-positive cells is indicated in the top right quadrant, n = 3. (E) Cells generated in panel D were subjected to Western blot analysis using an anti-HA, anti-Mitf, anti-KIT, and anti–β-actin antibody. The level of expression of KIT, Mitf, HA-tagged AKT, and β-actin is indicated. Cells generated in panel D also were analyzed for AKT activation (bottom 2 panels).
Because loss of SHIP and expression of p110CAAX in BMMCs demonstrated hyperactivation of AKT, which was significantly reduced in p85α BMMCs, we directly assessed the role of activated AKT in the maturation of WT BMMCs. Specifically, WT MCps were infected with an activated version of AKT (Act AKT) or empty vector and assessed for maturation. As seen in Figure 2D, hyperactivation of AKT not only accelerated the rate of WT BMMC maturation but also led to increased expression of Mitf (Figure 2C lane 1 compared with lane 3). Importantly, and consistent with reduced AKT activation observed in p85α−/− BMMCs (Figure 1B), expression of an activated version of AKT in p85α−/− MCps not only restored BMMC maturation, including the expression of KIT but also the expression of Mitf (Figure 2D-E). These results suggest that both p85α-induced AKT activation and Mitf expression are essential for normal BMMC maturation.
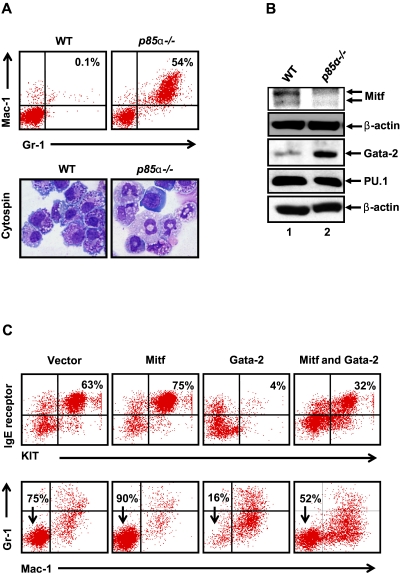
Because MCps derived from p85α−/− mice showed impaired BMMC maturation, we asked whether deficiency of p85α in MCps accumulates other lineages including myeloid cells. We cultured BM cells from WT and p85α−/− mice for 3 weeks under conditions that induce mast cell maturation and analyzed the expression of myeloid cell markers including Gr-1 and Mac-1 by flow cytometry. Interestingly, cultures deficient in the expression of p85α showed a dramatic increase in the frequency of Gr-1 and Mac-1–positive cells compared with controls, suggesting that deficiency of p85α in BM cells tends to promote the generation of Gr-1/Mac-1–positive myeloid cells as opposed to BMMCs (Figures 1B and 3A). Consistent with flow cytometric analysis, cytospins performed on p85α−/− BMMCs showed more number of cells with morphology similar to Gr-1/Mac-1–positive cells compared with WT BMMCs (Figure 3A). To identify the mechanism behind this alteration, we analyzed the expression of transcription factors that are important for the maturation and generation of BMMCs and Gr-1/Mac-1–positive myeloid cells, including Mitf, Gata-2, and PU.1.22 As seen in Figure 3B, p85α-deficient cells showed reduced expression of Mitf relative to WT cells. In contrast, increased expression of Gata-2 was observed in p85α-deficient cells compared with controls (Figure 3B). No difference in the expression of PU.1 was observed between the 2 genotypes (Figure 3B). To assess the physiologic relevance of the differential expression of Mitf and Gata-2 in p85α−/− progenitors, we infected WT MCps with Mitf or Gata-2 alone or in combination and analyzed BMMC maturation. Although overexpression of Mitf in WT BMMCs led to accelerated BMMC maturation; Gata-2 overexpression reduced WT BMMC maturation compared with vector-transduced cells (Figure 3C). Interestingly, coexpression of Mitf and Gata-2 showed significantly enhanced BMMC maturation relative to Gata-2–overexpressing cells (Figure 3C). Importantly, Gata-1 overexpression showed no significant effect on the maturation of BMMCs (data not shown). Consistent with the association between enhanced Gata-2 expression and increased frequency of Gr-1+/Mac-1+ cells, overexpression of Gata-2 in WT mast cell progenitors also led to increased frequency of Gr-1+/Mac-1+ cells, which was modulated as a result of Mitf coexpression (Figure 3C).
Figure 3.
Loss of p85α in MCps results in altered expression of key transcription factors associated with mast cell maturation. (A) BM cells from WT and p85α−/− mice were cultured for 3 weeks, after which cells were stained with Giemsa (bottom) or antibodies that recognize Gr-1 and Mac-1 followed by flow cytometry (top). Shown is a dot blot profile from 1 of 4 independent experiments. Percentage of Gr-1 and Mac-1 double-positive cells are indicated in the top right quadrant. (B) Differential expression of Mitf and Gata-2 in WT and p85α-deficient cells. Cells described in panel A were lysed, and equal amounts of protein lysate were subjected to Western blot analysis using an anti-Mitf, anti-Gata-2, anti-PU.1, and anti–β-actin antibody as indicated. Shown is a representative Western blot from 2-3 independent experiments. (C) Overexpression of Gata-2 in WT BMMCs mimics the p85α−/− differentiation phenotype. WT BMMCs transduced with vector, Mitf, and Gata-2 alone or in combination were sorted to homogeneity and cultured for 3 to 4 weeks. Maturation of BMMCs and Gr-1/Mac-1 myeloid cells was analyzed by staining the cells with antibodies that recognize KIT and IgE receptor as well as the presence of Gr-1 and Mac-1 by flow cytometry. Shown are dot blots from 1 representative experiment performed 2-3 independent times.
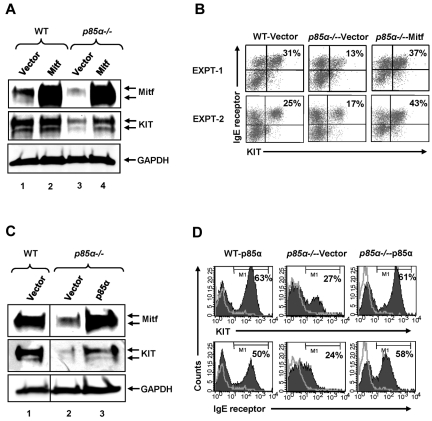
To further assess the importance of Mitf in regulating PI3K-mediated BMMC maturation, we expressed Mitf in p85α−/− BMMCs. Figure 4A demonstrates rescue in the expression of KIT by Western blotting in p85α−/− BMMCs engineered to express the Mitf cDNA relative to the vector-infected control p85α−/− cells. Figure 4B shows flow cytometry profiles of BMMCs infected with various constructs. As seen in 2 independent experiments, expression of MITF in p85α−/− cells rescued the maturation of BMMCs, including the expression of KIT and IgE receptor to near WT levels. Furthermore, restoring the expression of full-length p85α in p85α−/− BMMCs restored the expression of KIT and Mitf as well as the maturation of these cells, as determined by the expression of KIT and IgE receptor (Figure 4C-D). Together, these results demonstrate that an essential balance in the expression of Mitf and Gata-2 in adult BM-derived MCps is essential for normal BMMC maturation, which is tightly controlled by signals emanating from the p85α regulatory subunit of the class IA PI3K/AKT pathway.
Figure 4.
Mitf expression rescues BMMC maturation defect because of p85α deficiency. (A) WT and p85α−/− MCp cells were transduced with a retroviral vector expressing the Mitf cDNA. EGFP-positive cells were sorted to homogeneity and grown for additional weeks. Cells were harvested and subjected to Western blot analysis using an anti-Mitf or KIT antibody. The arrow in the top panel indicates the level of Mitf expression in various genotypes. The middle panel indicates the level of KIT expression in the various genotypes, and the lower bottom panel shows the level of GAPDH expression in each lane. n = 2. (B) Cells generated in panel A were harvested and subjected to flow cytometric analysis using antibodies against KIT and IgE receptor. The percentage of KIT and IgE receptor positive cells are indicated. Two representative experiments are shown. n = 3. (C-D) Reconstituting the expression of p85α in p85α-deficient MCps restored Mitf and KIT expression. MCps from WT or p85α-deficient mice were transduced with either the full-length form of p85α or the empty vector. Transduced cells were sorted on the basis of EGFP expression and cultured for 2 weeks. After 2 weeks, cells were harvested and subjected to Western blot analysis using an anti-KIT, anti-Mitf, or anti-GAPDH antibodies (C) or stained with antibodies against KIT or the IgE receptor and analyzed by flow cytometry (D). Percentages in each panel correspond to the level of KIT and IgE receptor levels in indicated genotypes. One of several independent experiments is shown.
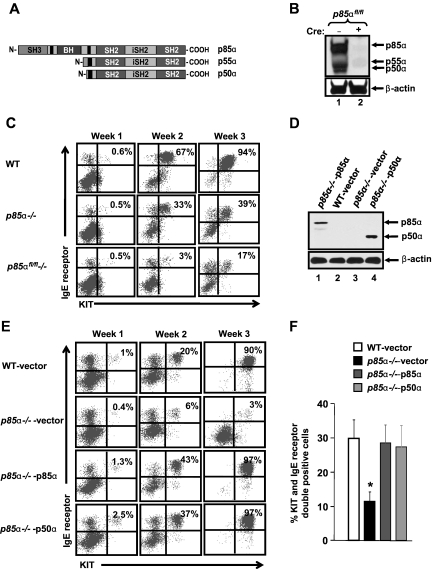
p85α, p50α, and p55α are splice variants encoded by PIK3R1. Previous studies have shown that mice lacking full-length p85α are viable but that lack of all subunits of p85α, ie, p85α, p50α, and p55α, result in embryonic lethality, suggesting an essential role for the shorter isoforms of p85α for normal development. Because abnormal maturation of BMMCs was observed in cells deficient in the full-length version of p85α in spite of the presence of p50α and p55α, we assessed the role of these shorter subunits in BMMC maturation. We conditionally deleted p55α and p50α along with the full-length version of p85α using the Cre-Lox system, and we compared maturation with cells deficient in only the full-length version of p85α. Figure 5A shows a schematic of the 3 p85α isoforms. Figure 5B shows complete deletion of all 3 p85α isoforms after poly I:C injection in p85αfl/fl−/− mice. As seen in Figure 5C, further reduction in the maturation of BMMCs was observed in p85αfl/fl−/− BMMCs (which lack p85α, p55α, and p50α) compared with p85α−/− cells (which only lack p85α). These results suggest that reduction in the expression of the smaller isoforms of p85α, namely, p50α and p55α further impairs the maturation of BMMCs.
Figure 5.
p50α and p55α isoforms of p85 are important for mast cell maturation. (A) Schematic of regulatory subunits p85α, p55α, and p50α. (B) Deletion of all regulatory subunits of p85α. To delete all regulatory subunits of p85α (p85α, p55α, and p50α) in BM cells, p85αfl/fl/ Mx-Cre− mice (WT), and p85αfl/fl/Mx-Cre+ mice (p85αfl/fl−/−) were injected intraperitoneally with poly I:C (300 μg) 3 times on alternate days, and BM was harvested 3 weeks after the final injection. BM from WT and p85αfl/fl−/− mice was cultured, and equal amounts of protein lysate were subjected to Western blot analysis using a pan–anti-p85 antibody (this antibody recognizes all regulatory subunits of class IA PI3K) to confirm the deletion of PI3K subunits. (C) Deficiency of p50α and p55α further impairs the maturation of p85α−/− BMMCs. BM cells from WT, p85α−/−, and p85αfl/fl−/− mice were cultured, and at indicated time points mast cell maturation was analyzed by staining the cells with antibodies that recognize KIT and IgE receptor by flow cytometric analysis. Shown is a dot blot profile of 1 of 3 independent experiments. (D) Expression of p50α in p85α−/− BMMCs. MCps from WT and p85α−/− mice were transduced with vector, HA-tagged full-length p85α, or p50α and sorted to homogeneity. Expression of p85α and p50α in sorted cells was analyzed by Western blotting using anti-HA and β-actin antibodies. (E-F) Restoration of p50α corrects the defective maturation of p85α-deficient BMMCs. BMMCs in panel D were collected at indicated time points. Mast cell differentiation was analyzed by staining the cells with antibodies that recognize KIT and IgE receptor followed by flow cytometric analysis. Shown is a representative dot blot profile (E) and quantitative data (F) from 5 independent experiments. *P < .05, WT versus p85α−/−vector.
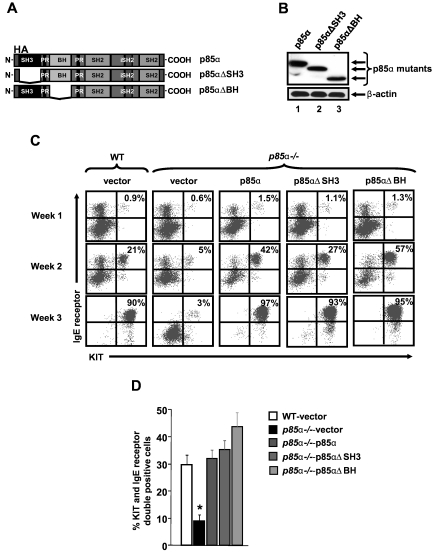
To determine whether the impact of the full-length p85α and its shorter isoforms (p50α and p55α) on BMMC maturation was qualitative or quantitative, we cloned p50α into a bicistronic retroviral vector (MIEG3) and overexpressed it in MCps lacking only the full-length form of p85α. Figure 5D shows the expression of p85α and p50α in transduced p85α-deficient MCps. As seen in Figure 5E, overexpression of p50α in p85α-deficient MCps completely restored the defective BMMC maturation to levels comparable with that of cells in which the full-length form of p85α was restored. Quantitative data from 5 independent experiments is shown in Figure 5F. These results suggest that the amino terminal sequences of the full-length form of p85α may not be necessary for regulating mast cell maturation. To further explore this notion, we created p85α mutant constructs that lack either the SH3 (p85αΔSH3) or the BCR homology (BH) domains (p85αΔBH) of the full-length p85α. Cells lacking only the full-length form of p85α were infected with these constructs and subjected to differentiation. Figure 6A shows a schematic of the full-length p85α and p85α mutant's ΔSH3 and ΔBH. Figure 6B shows the expression of the full-length p85α and the mutants in p85α-deficient cells. As seen in Figure 6C and D, expression of the mutant versions of p85α in p85α-deficient cells completely corrected the defective BMMC maturation as well as Mitf expression (data not shown) to near WT levels, further confirming that the amino terminal domains of p85α are not essential for the maturation of mast cells from its precursors.
Figure 6.
Cooperation between the SH3 and the BH domain of p85α is not required for BMMC maturation. (A) Schematic of full-length p85α and p85α mutants lacking either the SH3 or the BH domain. Full-length p85α or p85α mutants lacking either the amino terminal SH3 domain (1-80 aa) or the BH domain (102-288 aa) were cloned into a bicistronic retroviral vector MIEG3. The constructs were HA tagged at the amino terminus to distinguish exogenous expression from endogenous p85 protein. (B) Expression of full-length p85α or p85α mutants. MCps from p85α−/− mice were transduced with vector, full-length p85α, or p85α mutants lacking either the SH3 domain (p85αΔSH3) or the BH domain (p85αΔBH) and sorted to homogeneity. Cells were harvested and subjected to Western blot analysis using an anti-HA antibody or β-actin antibody as indicated. Expression of various p85α mutants is indicated in the top panel. (C-D) Expression of p85α mutants into p85α−/− MCps corrects defective mast cell differentiation. Cells in panel B were sorted to homogeneity and grown. At indicated times, maturation was evaluated by staining the cells with antibodies that recognize KIT and IgE receptor by flow cytometry. Shown is a representative dot blot (C) and quantitative data (D) from 5 independent experiments. *P < .05, WT versus p85α−/−vector.
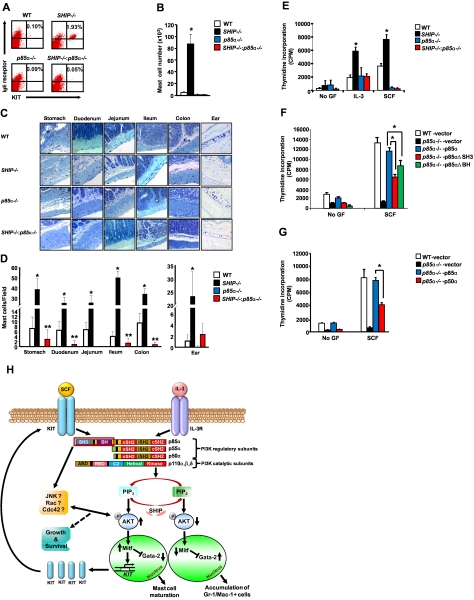
Next, we assessed whether the in vitro observations with respect to SHIP-p85α interactions also occur in vivo. We intravenously injected low-density BM cells from WT, SHIP−/−, p85α−/−, and DKO mice into Wsh mice, which lack endogenous mast cells. Four months after transplantation, mice were killed, and peritoneal lavage fluid and tissues were harvested to enumerate mast cells in vivo. We first harvested mast cells from the peritoneal cavity of recipient Wsh mice, and then we counted and stained cells with anti-KIT and anti-IgE receptor antibody followed by flow cytometric analysis. As seen in Figure 7A and B, loss of SHIP alone resulted in a significantly enhanced percentage and number of mast cells in the peritoneal cavity of Wsh-transplanted mice. In contrast, deficiency of both SHIP and p85α reduced the increased number of mast cells associated with SHIP deficiency to levels observed with p85α−/− BM cells (Figure 7A-B). These results suggest that the enhanced number of mature mast cells in the peritoneal cavity of SHIP−/− BM-transplanted mice is because of enhanced PI3K activity mediated via the p85α regulatory subunit of class IA PI3K.
Figure 7.
Deficiency of p85α in SHIP−/− bone marrow cells rescues the increase in mast cell numbers in vivo in various tissues. Wsh mice lacking mast cells were lethally irradiated and transplanted with 1 million bone marrow cells derived from WT, SHIP−/−, p85α−/−, and SHIP−/−:p85α−/− mice. Four months later, cells from the peritoneal cavity or the indicated tissues of recipient Wsh mice were harvested and stained with antibodies against KIT and IgE receptor or embedded tissue sections were stained with toluidine blue. (A) Representative dot blots indicate peritoneal cavity-derived mast cells stained with an anti-KIT and anti-IgE receptor antibody from the indicated genotypes. Numbers in the top right quadrant of dot blot indicate the percentage of peritoneal cells that were double positive for KIT and IgE receptor expression. (B) Bar graph represents quantitative assessment of the total number of mast cells in 5 mL of peritoneal lavage of Wsh mice transplanted with BM cells from the indicated genotypes. n = 5 (mean ± SEM), *P < .05, WT versus SHIP−/−. (C) Representative photomicrographs of toludine blue-stained tissue sections derived from Wsh mice transplanted with BM cells from the indicated genotypes. (D) Bar graph indicating quantitative assessment of toludine blue-positive mast cells in the indicated tissues. n = 3 ∼ 5 (mean ± SEM). *P < .05, WT versus SHIP−/−. **P < .05, WT versus SHIP−/−:p85α−/−. (E) BMMCs from the indicated genotypes were starved for 6 hours and cultured in the presence or absence of SCF or IL-3. After 48 hours, proliferation was evaluated by [3H]thymidine incorporation. Bar represents the mean [3H]thymidine incorporation in BMMCs (cpm ± SD) from 1 representative experiment performed in quadruplicate. Similar results were obtained in 3 independent experiments. *P < .05, WT versus SHIP−/−. (F-G) Overexpression of p50α or N-terminal deletion mutants of p85α partially correct the defective growth of p85α−/− BMMCs. p85α-deficient BMMCs expressing the indicated versions of p85α were starved of growth factors and stimulated with SCF. After 48 hours, proliferation was evaluated by [3H]thymidine incorporation. Bars represent the mean [3H]thymidine incorporation (cpm ± SD). Shown are data from one of many independent experiments. *P < .05, p85α−/− -p85α versus p85α−/− -p50α or p85α−/− -p85αΔSH3 or p85α−/− -p85αΔBH. (H) A model describing the mechanisms involved in regulating growth and maturation of mast cells via the PI3K pathway (see “Discussion” for details).
To further assess the distribution of mast cells in various tissues of Wsh-transplanted mice, we harvested, embedded, sectioned, and stained various tissues of mice transplanted with BM cells from the 4 genotypes. As seen in Figure 7C and D, we found an increase in the number of mast cells in Wsh mice lacking SHIP in various tissues, including stomach, duodenum, ileum, colon, and ear. Interestingly, however, although the number of mast cells in the ear was reduced to WT levels in mice deficient in both SHIP and p85α, mast cells in tissues such as the stomach, duodenum, ileum, and colon were significantly lower than that obtained with WT BM (Figure 7C-D). These results further demonstrate that PI3K activity plays a major role in regulating mast cell numbers in vitro and in vivo. Furthermore, although deficiency of p85α in SHIP−/− BM cells shows similar BMMC maturation in vitro compared with WT cells, in vivo it normalizes mast cell numbers to WT levels in some tissues but not all, suggesting subtle but differential in vivo localized tissue dependence of mast cells on this pathway.
The lack of equivalent number of tissue mast cells in vivo in spite of similar maturation of BMMCs in vitro in cells lacking both SHIP and p85α led us to examine the growth potential of DKO BMMCs. In vivo, KIT–SCF interactions play an essential role in maintaining the growth of tissue mast cells. To assess whether defective KIT signaling may explain the lack of complete rescue in the number of mast cells in some tissues of DKO mice, we examined SCF-induced growth in the 4 genotypes. As seen in Figure 7E, although IL-3–induced growth in DKO BMMCs is normalized to WT levels, SCF-induced growth in DKO BMMCs was similar to that of p85α-deficient BMMCs. Because loss of p85α in the setting of SHIP deficiency completely corrects the maturation of BMMCs and the amino terminal sequences of p85α are not essential for this process, we examined whether amino terminal sequences of p85α are necessary for KIT-induced growth and mast cell homeostasis in vivo. As seen in Figure 7F and G, p85α-deficient BMMCs expressing either the SH3 or the BH domain deleted version of p85α or the p50α subunit of p85 resulted in only 50% correction in KIT-induced proliferation in vitro and significant reduction in the repopulating ability of mast cells in vivo relative to controls (data not shown). Thus, although the amino terminal sequences of p85α are nonessential for driving the maturation of BMMCs, they play an essential role in regulating the SCF-induced growth and survival of mast cells once the maturation of these cells has been accomplished.
Discussion
Although the predominant role of SHIP seems to involve the hydrolysis of PIP3 and it is generally believed that most phenotypes because of SHIP deficiency are a result of elevated PIP3; SHIP also performs other functions, including functioning as an adaptor protein. In this regard, in some scenarios, SHIP is likely to compete with Grb2 for Shc to modulate Ras activation.23 SHIP also has been shown to recruit p62Dok and to modulate Ras activity and also may induce the activation of SHP2.24,25 Our in vitro and in vivo findings suggest that the increased rate of BMMC maturation and tissue mast cell numbers because of SHIP deficiency are predominantly a result of enhanced PI3K activity via the recruitment and activation of the p85α–p110 PI3K complex, because deficiency of p85α alone in BMMCs lacking SHIP corrects the enhanced maturation defect in vitro and partially restores normal mast cell numbers in vivo. These findings are further supported by conducting experiments using WT and p85α−/− MCps expressing activated versions of PI3K and AKT, respectively. Thus, even though SHIP has been implicated in regulating many functions, including levels of Ras, our results suggest that its predominant role is to break down PIP3 generated via the p85α–p110 complex in mast cells. Our results also demonstrate that downstream from PI3K/AKT, the transcription factor Mitf plays an essential role in driving the maturation of mast cells and that the expression of Mitf in MCps is regulated in part via the PI3K/AKT pathway. Mitf is a basic helix-loop-helix leucine zipper transcription factor. Mice with mutations in Mitf demonstrate severe defects in the maturation of mast cells. Mitf functions by regulating several mast cell genes, including mast cell proteases, adhesion and cell surface signaling molecules, and transporters.
Our results show that too much Gata-2 expression as reflected by its overexpression in WT MCps or because of p85α deficiency results in accumulation of Gr-1/Mac-1 double-positive myeloid cells as opposed to mast cells. Previous studies have shown that overexpression of Gata-2 in purified BM cells can result in very different outcomes.26,27 Our data suggest that some down-regulation of Gata-2 is essential for normal BMMC maturation to occur. Likewise, up-regulation of Mitf is essential during early phases of BMMC maturation. Our results demonstrate that this process is tightly controlled by PI3K. When PI3K activity is elevated in MCps, Mitf is induced, which drives BMMC maturation in an AKT-dependent manner. In the absence of or reduced PI3K activity, AKT levels are reduced, which impairs the development of BMMCs and modulates the maturation of BM cells toward Gr-1/Mac-1 double-positive myeloid cells, which is associated with the accumulation of Gata-2 and repression of Mitf (Figure 7H).
Although maturation of BM cells deficient in the expression of both SHIP and p85α is similar to WT levels; the number of tissue mast cells in vivo in mice lacking SHIP and p85α was not equivalent to WT levels. This is probably because of KIT-induced defects in growth and survival in cells lacking both SHIP and p85α. Thus, although maturation in DKO BMMCs is normalized to WT levels, once mature, these cells may require additional signals in the form of KIT to maintain survival and growth of fully mature cells, and perhaps it is the survival and growth signals in DKO cells that remain defective in some tissues in vivo. To this end, we found IL-3–induced proliferation and survival to be normalized to WT levels in DKO BMMCs, whereas SCF-induced survival signals were only partially rescued. After 24 hours of culture, a 50% difference in the survival was observed between BMMCs derived from p85α−/− mice versus DKO mice (data not shown). Therefore, it is likely that lack of complete in vivo correction of tissue mast cells in DKO mice is a result of impaired SCF-induced survival signals. Consistent with this observation, only a partial rescue in SCF induced Bcl-xL and Bcl-2 expression was observed in DKO BMMCs stimulated with SCF compared with WT controls, whereas IL-3–induced Bcl-xL and Bcl-2 expression was completely normalized (data not shown). Previous studies by us and others have shown that Bcl-xL/Bcl-2 play an essential role in regulating tissue mast cell numbers downstream from Stat5 and Rac2.28,29 Together, our results provide evidence to suggest that PI3K's mast cell maturation function, including the regulation of Mitf, can be uncoupled from its proliferation and survival function.
To further assess the role of the p85α–p110 PI3K complex in regulating maturation versus proliferation and survival of BMMCs, we examined the role of shorter isoforms of p85α. Our results demonstrate that although shorter isoforms of p85α are sufficient to restore BMMC maturation, KIT induced growth and survival functions require the N-terminal SH3 and BH domains of p85α. Several lines of evidence indicate that the unique N terminus of p85α is required for the activation of the Rho/cdc42/JNK pathway.30,31 For example, restoring the expression of full-length p85α in p85α-deficient livers restores JNK activation, whereas re-expression of the shorter isoforms, that is, p55α and p50α, does not.30 Interestingly, deletion of either the SH3 or the BH domain from the full-length form of p85α impairs its ability to activate cdc42 without altering its ability to activate PI3K, suggesting that reduced cdc42 activation is probably not a result of impaired p85 function but a result of not having the SH3 and the BH domains.30 Thus, the defect in BMMC maturation is probably because of quantitative reduction in p85 subunits rather than because of unique loss of the full-length form of p85α. In contrast, SCF-induced growth clearly requires the presence of an intact SH3 and BH domain of p85α because mutants of p85 lacking these domains only partially restore BMMC proliferation. Together, our results provide new evidence to suggest a dual role for p85α subunits in BMMC maturation and growth, which can be uncoupled.
Supplementary Material
Acknowledgments
The authors thank Marilyn Wales for administrative support.
This work was supported in part by National Institute of Health grants R01HL075816 and R01HL077177 (R.K).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: P.M., R.S.M., V.M., S.K., B.R., E.M., H.M., J.G., and S.L. performed research and analyzed data; R.J.C. provided reagents; G.K., A.W.C., and C.T. provided reagents, interpreted research, and edited the manuscript; and R.K. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Reuben Kapur, Herman B Wells Center for Pediatric Research, Indiana University School of Medicine, 1044 W Walnut St, Rm W168, Indianapolis, IN 46202; e-mail: rkapur@iupui.edu.
References
- 1.Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat Immunol. 2005;6(2):135–142. doi: 10.1038/ni1158. [DOI] [PubMed] [Google Scholar]
- 2.Kitamura Y. Heterogeneity of mast cells and phenotypic change between subpopulations. Annu Rev Immunol. 1989;7:59–76. doi: 10.1146/annurev.iy.07.040189.000423. [DOI] [PubMed] [Google Scholar]
- 3.Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997;77(4):1033–1079. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- 4.Galli SJ, Zsebo KM, Geissler EN. The kit ligand, stem cell factor. Adv Immunol. 1994;55:1–96. doi: 10.1016/s0065-2776(08)60508-8. [DOI] [PubMed] [Google Scholar]
- 5.Yu J, Zhang Y, McIlroy J, Rordorf-Nikolic T, Orr GA, Backer JM. Regulation of the p85/p110 phosphatidylinositol 3′-kinase: stabilization and inhibition of the p110alpha catalytic subunit by the p85 regulatory subunit. Mol Cell Biol. 1998;18(3):1379–1387. doi: 10.1128/mcb.18.3.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fruman DA, Meyers RE, Cantley LC. Phosphoinositide kinases. Annu Rev Biochem. 1998;67:481–507. doi: 10.1146/annurev.biochem.67.1.481. [DOI] [PubMed] [Google Scholar]
- 7.Rauh MJ, Kalesnikoff J, Hughes M, Sly L, Lam V, Krystal G. Role of Src homology 2-containing-inositol 5′-phosphatase (SHIP) in mast cells and macrophages. Biochem Soc Trans. 2003;31(Pt 1):286–291. doi: 10.1042/bst0310286. [DOI] [PubMed] [Google Scholar]
- 8.Huber M, Helgason CD, Damen JE, Liu L, Humphries RK, Krystal G. The src homology 2-containing inositol phosphatase (SHIP) is the gatekeeper of mast cell degranulation. Proc Natl Acad Sci U S A. 1998;95(19):11330–1135. doi: 10.1073/pnas.95.19.11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huber M, Helgason CD, Scheid MP, Duronio V, Humphries RK, Krystal G. Targeted disruption of SHIP leads to Steel factor-induced degranulation of mast cells. EMBO J. 1998;17(24):7311–7319. doi: 10.1093/emboj/17.24.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fukao T, Yamada T, Tanabe M, et al. Selective loss of gastrointestinal mast cells and impaired immunity in PI3K-deficient mice. Nat Immunol. 2002;3(3):295–304. doi: 10.1038/ni768. [DOI] [PubMed] [Google Scholar]
- 11.Munugalavadla V, Vemula S, Sims EC, et al. The p85alpha subunit of class IA phosphatidylinositol 3-kinase regulates the expression of multiple genes involved in osteoclast maturation and migration. Mol Cell Biol. 2008;28(23):7182–7198. doi: 10.1128/MCB.00920-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo J, McMullen JR, Sobkiw CL, et al. Class IA phosphoinositide 3-kinase regulates heart size and physiological cardiac hypertrophy. Mol Cell Biol. 2005;25(21):9491–9502. doi: 10.1128/MCB.25.21.9491-9502.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samayawardhena LA, Kapur R, Craig AW. Involvement of Fyn kinase in Kit and integrin-mediated Rac activation, cytoskeletal reorganization, and chemotaxis of mast cells. Blood. 2007;109(9):3679–3686. doi: 10.1182/blood-2006-11-057315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kohn AD, Takeuchi F, Roth RA. Akt, a pleckstrin homology domain containing kinase, is activated primarily by phosphorylation. J Biol Chem. 1996;271(36):21920–62192. doi: 10.1074/jbc.271.36.21920. [DOI] [PubMed] [Google Scholar]
- 15.Liu Q, Sasaki T, Kozieradzki I, et al. SHIP is a negative regulator of growth factor receptor-mediated PKB/Akt activation and myeloid cell survival. Genes Dev. 1999;13(7):786–791. doi: 10.1101/gad.13.7.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stechschulte DJ, Sharma R, Dileepan KN, et al. Effect of the mi allele on mast cells, basophils, natural killer cells, and osteoclasts in C57Bl/6J mice. J Cell Physiol. 1987;132(3):565–570. doi: 10.1002/jcp.1041320321. [DOI] [PubMed] [Google Scholar]
- 17.Stevens J, Loutit JF. Mast cells in spotted mutant mice (W Ph mi). Proc R Soc Lond B Biol Sci. 1982;215(1200):405–409. doi: 10.1098/rspb.1982.0050. [DOI] [PubMed] [Google Scholar]
- 18.Isozaki K, Tsujimura T, Nomura S, et al. Cell type-specific deficiency of c-kit gene expression in mutant mice of mi/mi genotype. Am J Pathol. 1994;145(4):827–836. [PMC free article] [PubMed] [Google Scholar]
- 19.Tsujimura T, Morii E, Nozaki M, et al. Involvement of transcription factor encoded by the mi locus in the expression of c-kit receptor tyrosine kinase in cultured mast cells of mice. Blood. 1996;88(4):1225–33. [PubMed] [Google Scholar]
- 20.Takemoto CM, Yoon YJ, Fisher DE. The identification and functional characterization of a novel mast cell isoform of the microphthalmia-associated transcription factor. J Biol Chem. 2002;277(33):30244–30252. doi: 10.1074/jbc.M201441200. [DOI] [PubMed] [Google Scholar]
- 21.Shahlaee AH, Brandal S, Lee YN, Jie C, Takemoto CM. Distinct and shared transcriptomes are regulated by microphthalmia-associated transcription factor isoforms in mast cells. J Immunol. 2007;178(1):378–388. doi: 10.4049/jimmunol.178.1.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takemoto CM, Lee YN, Jegga AG, et al. Mast cell transcriptional networks. Blood Cells Mol Dis. 2008;41(1):82–90. doi: 10.1016/j.bcmd.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coggeshall KM. Inhibitory signaling by B cell Fc gamma RIIb. Curr Opin Immunol. 1998;10(3):306–312. doi: 10.1016/s0952-7915(98)80169-6. [DOI] [PubMed] [Google Scholar]
- 24.Tamir I, Stolpa JC, Helgason CD, et al. The RasGAP-binding protein p62dok is a mediator of inhibitory FcgammaRIIB signals in B cells. Immunity. 2000;12(3):347–358. doi: 10.1016/s1074-7613(00)80187-9. [DOI] [PubMed] [Google Scholar]
- 25.Koncz G, Toth GK, Bokonyi G, et al. Co-clustering of Fcgamma and B cell receptors induces dephosphorylation of the Grb2-associated binder 1 docking protein. Eur J Biochem. 2001;268(14):3898–3906. doi: 10.1046/j.1432-1327.2001.02295.x. [DOI] [PubMed] [Google Scholar]
- 26.Iwasaki H, Mizuno S, Arinobu Y, et al. The order of expression of transcription factors directs hierarchical specification of hematopoietic lineages. Genes Dev. 2006;20(21):3010–21. doi: 10.1101/gad.1493506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Persons DA, Allay JA, Allay ER, et al. Enforced expression of the GATA-2 transcription factor blocks normal hematopoiesis. Blood. 1999;93(2):488–499. [PubMed] [Google Scholar]
- 28.Yang FC, Kapur R, King AJ, et al. Rac2 stimulates Akt activation affecting BAD/Bcl-XL expression while mediating survival and actin function in primary mast cells. Immunity. 2000;12(5):557–568. doi: 10.1016/s1074-7613(00)80207-1. [DOI] [PubMed] [Google Scholar]
- 29.Shelburne CP, McCoy ME, Piekorz R, et al. Stat5 expression is critical for mast cell development and survival. Blood. 2003;102(4):1290–1297. doi: 10.1182/blood-2002-11-3490. [DOI] [PubMed] [Google Scholar]
- 30.Taniguchi CM, Aleman JO, Ueki K, et al. The p85alpha regulatory subunit of phosphoinositide 3-kinase potentiates c-Jun N-terminal kinase-mediated insulin resistance. Mol Cell Biol. 2007;27(8):2830–2840. doi: 10.1128/MCB.00079-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hill KM, Huang Y, Yip SC, Yu J, Segall JE, Backer JM. N-terminal domains of the class ia phosphoinositide 3-kinase regulatory subunit play a role in cytoskeletal but not mitogenic signaling. J Biol Chem. 2001;276(19):16374–16378. doi: 10.1074/jbc.M006985200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.