Abstract
Maintenance of a reducing redox balance is a critical physiologic function of red cells (RBC) that can be perturbed in variety of RBC pathologies. Here we describe a new approach to evaluate in vivo RBC redox status using a redox sensitive GFP (roGFP2) sensor under control of a β-globin mini-promoter, directing expression specifically to erythroid cells. RoGFP2 expressing RBCs demonstrate ratiometric and reversible shifts in fluorescence on exposure to oxidants and reductants. We demonstrate that roGFP2 expressing RBC can be used to monitor thiol redox status during in vitro phenylhydrazine treatment and over the course of in vivo RBC aging, where a shift to a more oxidized state is observed in older cells. Thus, roGFP2 transgenic mice are a new and versatile tool that can be used to probe how RBC redox status responds in the context of drug therapy, physiologic stressors and pathologic states.
Introduction
Many approaches for evaluation of RBC redox status have been established, including direct evaluation of the glutathione redox pair (GSH/GSSG),1 and indirect approaches such as measuring rates of reactive oxygen species (ROS) production using oxidation sensitive dyes2 and measurement of protein oxidation.3 While useful, these methods have specific limitations. Measurement of the GSH/GSSG ratio is notoriously difficult because of oxidation of GSH during cell disruption and sample preparation1,4; ROS sensitive dyes exhibit promiscuous reactivity with multiple radical species5; protein oxidation reflects accumulated damage, not current redox status.6 Importantly, none of these measurements is well suited for in vivo experimentation. Development of redox-sensitive green fluorescent protein variants (roGFPs) provides a new option for measuring cellular redox status.7–12 RoGFP2 has been engineered as a redox sensor by replacement of S147 and Q204 positions of EGFP with 2 cysteine residues. These cysteines are either reduced or form an intra-molecular disulfide bond in equilibrium with local redox status. Rising levels of oxidized roGFP indicate a shift in cellular redox status that is monitored by following the ratio of GFP fluorescence emission after excitation at 405 versus 488 nm.9–11,13 Here, we show how roGFP2-expressing RBC in transgenic mice can be used to follow, in vitro and in vivo, important changes in RBC redox status.
Methods
Generation of roGFP2 transgenic mice
roGFP2 was sub-cloned into a ß-globin minigene expression plasmid pμ'LCR-βpr-BglII-3′β(int2-enh),14 and micro-injected into pronuclei of fertilized C57BL/6J mouse oocytes. The best transgenic founder animal expressed GFP in ∼ 50% of peripheral RBC by FACS, and gave rise to progeny with the same expression pattern.
Flow cytometry
RBCs (1 × 106) were washed and resuspended in 1 mL FACS buffer (PBS/0.2%BSA/2mM EDTA). Cells were incubated with oxidant (H2O2 or t-butyl hydroperoxide), reductant (DTT) or Phenylhydrazine (PHZ) in the presence of 10mM glucose at 37°C for 60 minutes. Cells were analyzed on a LSRII flow cytometer (BD Biosciences). The emission wavelength/bandwidth for the 488nm channel is 530/30nm, and for the 405nm channel is 525/50nm. Data were analyzed with FlowJo Version 9.2 (TreeStar Inc). RoGFP2 ratio is the ratio of mean fluorescence intensity excited at 405 versus 488 nm.9,13
Calculation of fractional oxidation of roGFP2 and red cell redox potential
The redox potential (EroGFP2) was calculated according to the fractional oxidation of roGFP2:
and the Nernst equation:
10mM DTT or 100μM t-butyl hydroperoxide (t-BOOH) was used to generate fully reduced or oxidized roGFP2.
The redox potential of GSH (EGSH) was calculated according to the fractional oxidation of GSH
and the Nernst equation
Equation 1: I488red is fluorescence intensity for fully reduced and I488ox for fully oxidized roGFP2 at 488nm. Rred, Rox and R refer to fully reduced, fully oxidized or experimental measure of roGFP2 ratio.15
Equation 2: E'roGFP2 is the mid-point potential of roGFP2 (-280mV9,10), R is the gas constant (8.135JK−1mol−1), T is absolute temperature (298.15K), z is the number of electrons exchanged and F is the Faraday constant (96,485C mol−1).9,10,13
Measurement of redox changes during RBC aging
Mice were injected intravenously with 1 mg sulfo-NHS-Biotin (Pierce) in normal saline to label, in vivo, all RBC.17,18 Blood samples (5 μL) were taken and analyzed by flow cytometry after staining with streptavidin Cy-Chrom (BD Pharmingen) for determination of cell age and roGFP2 ratio. All animal protocols were approved by the TSRI IACUC.
Statistical analysis:
Data are presented as mean ± SEM, compared using unpaired Student t test. Calculation: PRISM Version 4 software (Graphpad Software).
Results and discussion
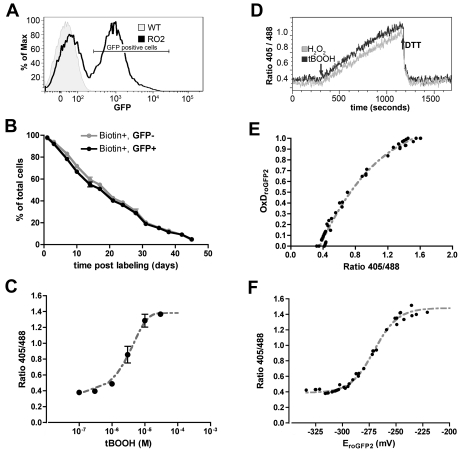
To create a model system for assessment of the redox status of erythrocytes, we have developed and characterized a line of transgenic mice that express a redox GFP transgene driven by a ß-globin mini-promoter.10,14 In transgenic animals ∼ 50% of peripheral RBCs express roGFP2 (Figure 1A). Expression of roGFP2 had no effect on RBC survival as the survival curves are identical for both the GFP positive and GFP negative RBC (Figure 1B) and agree with studies performed by our group using nontransgenic mice on the same strain (B6/J) background (data not shown).17–19 We compared complete blood counts of transgenic and nontransgenic mice and found no significant differences in RBC indices, WBC parameters or platelet count (supplemental Table 1, available on the Blood Web site; see Supplemental Materials link at the top of the online article), indicating that expression of roGFP2 does not impact RBC survival or development.
Figure 1.
Characterization of RBC of roGFP2 transgenic mice. (A) FACS analysis of RBC from a roGFP2 transgenic mouse and a nontransgenic littermate (WT) demonstrates that ∼ 55% of peripheral RBC express roGFP2. Histogram is representative of 4 experiments using a total of 20 mice. (B) Survival curves of GFP(+) and GFP(-) RBC are identical. Peripheral RBCs of transgenic mice were biotinylated and the percentage of Biotin(+) GFP(+) and Biotin(+) GFP(-) cells was tracked by FACS (mean ± SEM; N = 12). (C) RoGFP2 ratio in RBC treated with the indicated concentration of tBOOH (mean ± SEM from 5 independent experiments). (D) Real-time recording of dynamic and reversible changes in the roGFP2 sensor. RoGFP2 expressing RBC were treated with 1μM H2O2 or 10μM tBOOH for 15 minutes, followed by administration of 10mM DTT, all at room temperature. The ratio 405/488 was recorded by continuous flow cytometry. (E) The relationship between roGFP2 ratio and the degree of roGFP2 oxidation (OxDroGFP2). For maximal reduction, RBCs were treated with 10mM DTT; oxidation was measured over a range of tBOOH concentrations (0.1μM to 100μM). Fluorescence intensity was measured by FACS to define fully reduced (I488red), fully oxidized (I488ox) and intermediate states of roGFP2. The OxDroGFP2 was calculated according to Equation 1, and plotted versus the 405/488 ratio. (F) The relationship between R405/488nm and the roGFP2 redox potentials. The roGFP2 redox potentials were calculated according to the known OxDroGFP2 in panel E and Equation 2, and then plotted against ratio 405/488nm. GFP emission was recorded for both excitation wavelengths (405 and 488 nm) and is shown as the ratio of 405/488.
We performed in vitro flow cytometry experiments using roGFP2-expressing RBCs to characterize the response and dynamic fluorescence range of the redox sensor. When transgenic RBCs are incubated with t-butyl hydroperoxide (tBOOH), fluorescence intensity excited at 405 nm (emitted at 520 nm) increases while fluorescence intensity excited at 488nm (emitted at 520 nm) decreases (supplemental Figure 1). This emission intensity ratio (R405/488) increases in a dose-dependent manner, reaching a plateau value at 1.43 ± 0.03 (Figure 1C). R405/488 decreases subtly but significantly from 0.36 ± 0.03 to 0.34 ± 0.03 when RBCs are incubated with 10mM DTT (data not shown). Kinetics and reversibility of redox changes in transgenic RBC were monitored by continuous flow cytometry (Figure 1D). After a 5-minute lead-in period to demonstrate the stability of R405/488, 1μM H2O2 or 10μM tBOOH was added. R405/488 increased steadily for 15 minutes, and was reversed promptly and totally by addition of 10mM DTT. These data show that roGFP2 can function as an effective, real-time sensor of RBC redox status.
Based on titration experiments, we used treatment with 10mM DTT or 100μM tBOOH to represent maximally reduced and maximally oxidized states of the roGFP2 sensor. The degree of oxidation of roGFP2 (OxDroGFP2) was calculated according to the measured ratio R405/488, and the fully reduced and oxidized 488nm fluorescence intensity values (Figure 1E).9,15,16 Since the redox sensor is thermodynamically reversible in RBCs, the actual redox potential of roGFP2 in RBCs can be estimated according to the degree of oxidization of roGFP2 and the known midpoint potential E'roGFP (-280 mV) using the Nernst equation.9,13 The correlation between R405/488 and EroGFP2 is shown in Figure 1F. The effective redox sensitivity range in RBCs for roGFP2 is from −333.75 to −236.94 mV, corresponding to the measured range of R405/488 values (0.39 to 1.43). In unperturbed transgenic RBCs, we calculate the OxDroGFP2 to be 5.42 ± 2.92%, and the corresponding RBC redox potential to be −318.5 ± 8.07 mV. Many insults causing hemolysis such as sickle cell anemia, thalassemia, certain drugs or toxins are associated with increased redox stress, for which the roGFP2 sensor is well suited.
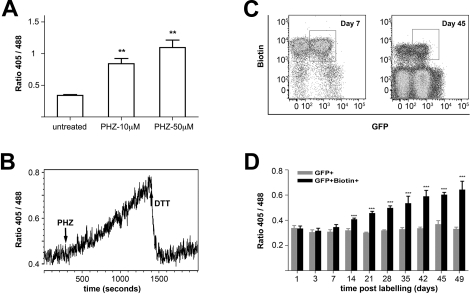
We used roGFP2 transgenic cells and mice to successfully monitor redox changes in vitro on exposure to the pro-hemolytic compound phenylhydrazine (PHZ) and in vivo to monitor redox changes during the normal physiologic process of RBC aging. PHZ causes ROS production and oxidative damage in RBC.20 In vitro incubation with PHZ resulted in a dose-dependent increase of R405/488 that was reversed by administration of 10mM DTT (Figure 2A-B). We next probed for redox changes accompanying normal RBC aging using an in vivo biotin labeling method17,21 (Figure 2C). R405/488 of the labeled cells (increasing in age) was followed over time and compared with that of unlabeled, GFP positive RBC in control mice. At early time points, R405/488 remains stable. By 14 days postlabeling, the ratio shifts indicating a more oxidized state, and with increasing age the ratio continues to rise (Figure 2D). The increase in R405/488 can be partially reversed by DTT (not shown). This provides direct evidence that changes in redox balance occur as a normal part of RBC aging, implying that oxidative damage over time contributes to RBC senescence.22,23
Figure 2.
Using roGFP2 to monitor redox dynamics on in vitro PHZ treatment or during in vivo normal RBC aging. (A) RoGFP2 responses to the RBCs oxidative stress caused phenylhydrazine (PHZ). Transgenic RBCs were treated with 10μM and 50μM PHZ at room temperature for 60 minutes. The ratio 405/488 was determined by FACS. Data were from 3 independent experiments, shown as mean ± SEM. (B) DTT reversed the increase in roGFP2 ratio caused by PHZ treatment. Continuous flow cytometry was used to follow dynamic redox changes in RBCs on exposure to 10μM PHZ for 15 minutes followed by 10mM DTT. (C) RBCs of roGFP2 transgenic mice were biotin labeled and analyzed over time by flow cytometry for GFP and biotin. Shown are representative plots from day 7 and day 45 after labeling. (D) RoGFP2 ratio was monitored by flow cytometry using RBC harvested at intervals up to 49 days after labeling. From day +14 after labeling, there is a progressive increase in oxidation of the roGFP2 sensor. Data are mean ratio ± SEM (n = 23); representative of 4 experiments. *** P < .001.
Based on previous reports, roGFP2 predominantly equilibrates with GSH/GSSG rather than other cellular redox pairs, through an interaction that can be facilitated by glutaredoxins.9,15,16,24 The relationship of roGFP2 fluorescence and the degree of oxidation of glutathione (OxDGSH) in RBCs was examined (supplemental Figure 2A). If completely equilibrated, the EGSH should be equal to EroGFP2. In fact, the calculated EroGFP2 is always much more negative than the EGSH (supplemental Figure 2B). A possible reason for this discrepancy includes spuriously high measured GSSG concentration because of oxidation during cell disruption.1
To our knowledge, this is the first biosensor described for measuring real time redox status in RBC. We show that roGFP2 expressing RBCs, both in vitro and in vivo, can be used to monitor RBC redox status avoiding artifacts associated with cell disruption. There are limitations of the sensor, including lack of complete reversibility on exposure to high concentration of oxidants, and after prolonged oxidant stress in vivo—indicating modifications to the sensor outside of the labile cysteine residues may occur. Future studies will attempt to identify the nature of modifications that leave the sensor stuck in the “on” position. Despite these limits, roGFP2 transgenic animals make accessible a novel approach for probing and monitoring RBC redox changes in response to exogenous insults, genetic defects or physiologic challenges, and will improve our understanding of the role of redox changes across many RBC pathologies.
Supplementary Material
Acknowledgments
The authors appreciate the excellent technical services provided by the TSRI Mouse Genetics Core Facility in production of roGFP2 transgenic mice. RoGFP2 cDNA was a gift of Dr James Remington, University of Oregon. The ß-globin minigene expression construct was a gift of Dr Ken Peterson, University of Kansas.
This work was funded by grants R21DK075762 from the NIDDK and UM/W81XWH-07-02-0095 from the US Army.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: J.S.F. designed the research; J.S.F., X.X.L., D.N., K.S., and A.V. performed the experiments; and J.S.F., X.X.L., and K.v.L. analyzed the data and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jeffrey S. Friedman, MD, PhD, Department of Molecular and Experimental Medicine, MEM151, 10550 N Torrey Pines Rd, La Jolla, CA 92037; e-mail: friedman@scripps.edu.
References
- 1.Lee R, Britz-McKibbin P. Differential rates of glutathione oxidation for assessment of cellular redox status and antioxidant capacity by capillary electrophoresis-mass spectrometry: an elusive biomarker of oxidative stress. Anal Chem. 2009;81(16):7047–7056. doi: 10.1021/ac901174g. [DOI] [PubMed] [Google Scholar]
- 2.Chen XP, Zhong ZF, Xu ZT, Chen LD, Wang YT. 2′,7′-Dichlorodihydrofluorescein as a fluorescent probe for reactive oxygen species measurement: forty years of application and controversy. Free Radic Res. 2010;44(6):587–604. doi: 10.3109/10715761003709802. [DOI] [PubMed] [Google Scholar]
- 3.England K, Driscoll CO, Cotter TG. ROS and protein oxidation in early stages of cytotoxic drug induced apoptosis. Free Radic Res. 2006;40(11):1124–1137. doi: 10.1080/10715760600838209. [DOI] [PubMed] [Google Scholar]
- 4.Hafer K, Iwamoto KS, Schiestl RH. Refinement of the dichlorofluorescein assay for flow cytometric measurement of reactive oxygen species in irradiated and bystander cell Populations. Radiat Res. 2008;169(4):460–468. doi: 10.1667/RR1212.1. [DOI] [PubMed] [Google Scholar]
- 5.Bonini MG, Rota C, Tomasi A, Mason RP. The oxidation of 2′,7′-dichlorofluorescin to reactive oxygen species: a self-fulfilling prophesy? Free Radic Biol Med. 2006;40(6):968–975. doi: 10.1016/j.freeradbiomed.2005.10.042. [DOI] [PubMed] [Google Scholar]
- 6.Stadtman ER. Protein oxidation in aging and age-related diseases. Ann N Y Acad Sci. 2001;928:22–38. doi: 10.1111/j.1749-6632.2001.tb05632.x. [DOI] [PubMed] [Google Scholar]
- 7.Guzman JN, Sanchez-Padilla J, Wokosin D, et al. Oxidant stress evoked by pacemaking in dopaminergic neurons is attenuated by DJ-1. Nature. 2010;468(7324):696–U119. doi: 10.1038/nature09536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang K, Schwarzer C, Lally E, et al. Expression and characterization of a redox-sensing green fluorescent protein (reduction-oxidation-sensitive green fluorescent protein) in Arabidopsis. Plant physiol. 2006;141(2):397–403. doi: 10.1104/pp.106.078246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meyer AJ, Dick TP. Fluorescent protein-based redox probes. Antioxid Redox Signal. 2010;13(5):621–650. doi: 10.1089/ars.2009.2948. [DOI] [PubMed] [Google Scholar]
- 10.Hanson GT, Aggeler R, Oglesbee D, et al. Investigating mitochondrial redox potential with redox-sensitive green fluorescent protein indicators. J Biol Chem. 2004;279(13):13044–13053. doi: 10.1074/jbc.M312846200. [DOI] [PubMed] [Google Scholar]
- 11.Merksamer PI, Trusina A, Papa FR. Real-time redox measurements during endoplasmic reticulum stress reveal interlinked protein folding functions. Cell. 2008;135(5):933–947. doi: 10.1016/j.cell.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Remington SJ. Green fluorescent protein: A perspective. Protein Sci. 2011;20(9):1509–1519. doi: 10.1002/pro.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dooley CT, Dore TM, Hanson GT, Jackson WC, Remington SJ, Tsien RY. Imaging dynamic redox changes in mammalian cells with green fluorescent protein indicators. J Biol Chem. 2004;279(21):22284–22293. doi: 10.1074/jbc.M312847200. [DOI] [PubMed] [Google Scholar]
- 14.Peterson KR, Fedosyuk H, Zelenchuk L, et al. Transgenic Cre expression mice for generation of erythroid-specific gene alterations. Genesis. 2004;39(1):1–9. doi: 10.1002/gene.20020. [DOI] [PubMed] [Google Scholar]
- 15.Meyer AJ, Brach T, Marty L, et al. Redox-sensitive GFP in Arabidopsis thaliana is a quantitative biosensor for the redox potential of the cellular glutathione redox buffer. Plant J. 2007;52(5):973–986. doi: 10.1111/j.1365-313X.2007.03280.x. [DOI] [PubMed] [Google Scholar]
- 16.Gutscher M, Pauleau AL, Marty L, et al. Real-time imaging of the intracellular glutathione redox potential. Nat Methods. 2008;5(6):553–559. doi: 10.1038/nmeth.1212. [DOI] [PubMed] [Google Scholar]
- 17.Hoffmann-Fezer G, Maschke H, Zeitler HJ, et al. Direct in vivo biotinylation of erythrocytes as an assay for red cell survival studies. Ann Hematol. 1991;63(4):214–217. doi: 10.1007/BF01703446. [DOI] [PubMed] [Google Scholar]
- 18.Xu X, Martin F, Friedman JS. The familial Parkinson's disease gene DJ-1 (PARK7) is expressed in red cells and plays a role in protection against oxidative damage. Blood Cells Mol Dis. 2010;45(3):227–232. doi: 10.1016/j.bcmd.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dale GL, Daniels RB, Beckman J, Norenberg SL. Characterization of senescent red cells from the rabbit. Adv Exp Med Biol. 1991;307:93–103. doi: 10.1007/978-1-4684-5985-2_9. [DOI] [PubMed] [Google Scholar]
- 20.Amer J, Goldfarb A, Fibach E. Flow cytometric analysis of the oxidative status of normal and thalassemic red blood cells. Cytometry A. 2004;60(1):73–80. doi: 10.1002/cyto.a.20017. [DOI] [PubMed] [Google Scholar]
- 21.Friedman JS, Lopez MF, Fleming MD, et al. SOD2-deficiency anemia: protein oxidation and altered protein expression reveal targets of damage, stress response, and antioxidant responsiveness. Blood. 2004;104(8):2565–2573. doi: 10.1182/blood-2003-11-3858. [DOI] [PubMed] [Google Scholar]
- 22.Sambrano GR, Parthasarathy S, Steinberg D. Recognition of oxidatively damaged erythrocytes by a macrophage receptor with specificity for oxidized low density lipoprotein. Proc Natl Acad Sci U S A. 1994;91(8):3265–3269. doi: 10.1073/pnas.91.8.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang TK. Free radical theory of erythrocyte aging. J Formos Med Assoc. 1997;96(10):779–783. [PubMed] [Google Scholar]
- 24.Meyer AJ. The integration of glutathione homeostasis and redox signaling. J Plant Physiol. 2008;165(13):1390–1403. doi: 10.1016/j.jplph.2007.10.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.