Abstract
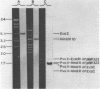
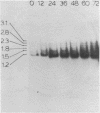
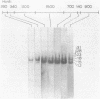
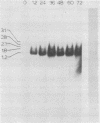
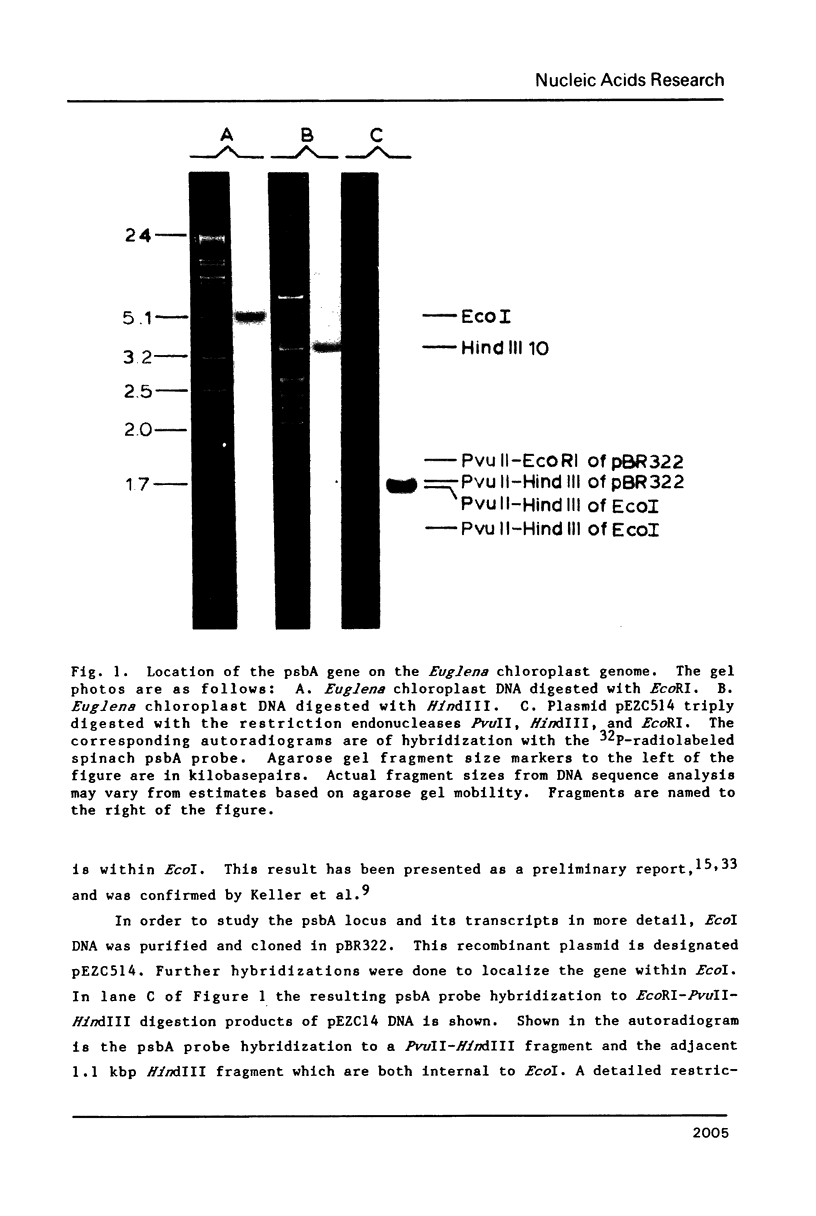
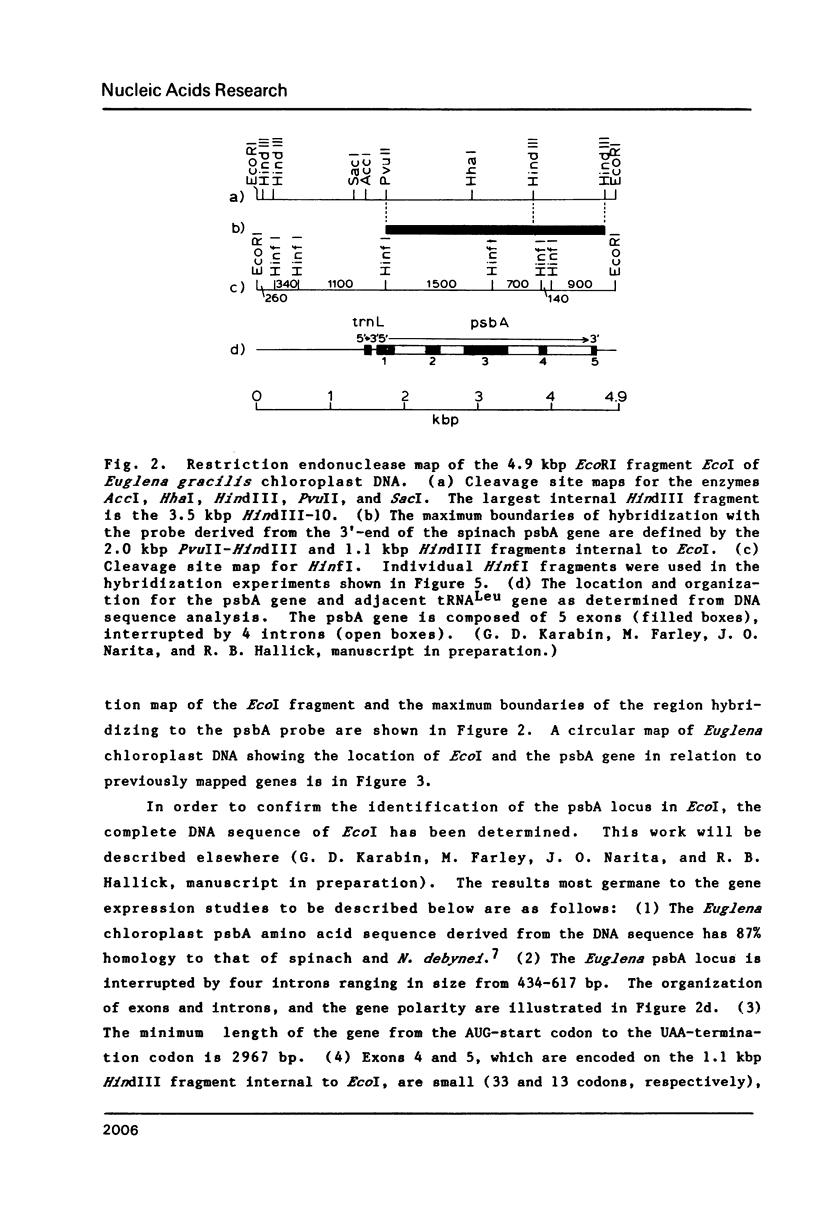
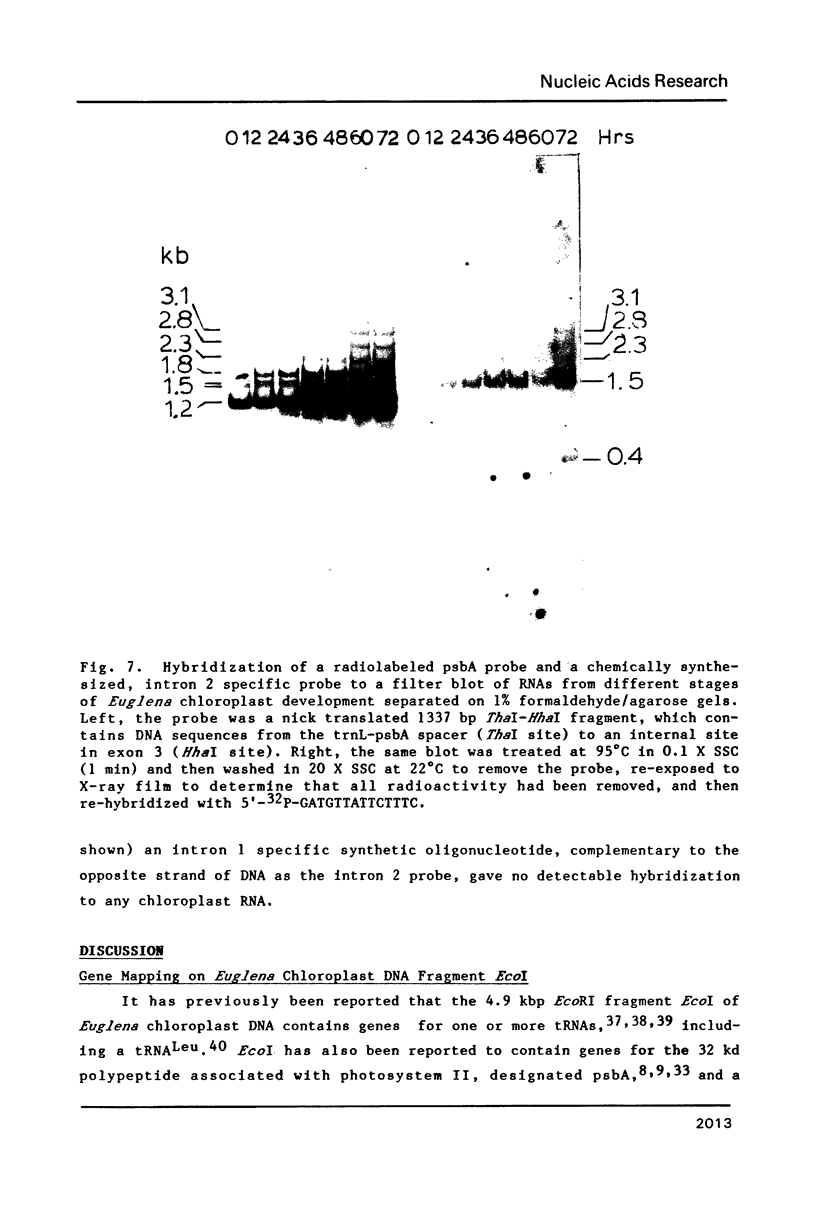
The psbA gene is the coding locus for a polypeptide of 32 kilodaltons that is involved in electron transport through photosystem II. The 4.9 kilobasepair (kbp) EcoRI restriction endonuclease fragment EcoI from the 145 kbp Euglena gracilis chloroplast DNA was shown to encode psbA. Five transcripts of size 3.1, 2.8, 2.3, 1.8, and 1.2 kilobases were detected by hybridization of psbA probes to nitrocellulose filter blots of electrophoretically separated RNAs. This same pattern was observed when the hybridization probe consisted of only exon sequences from this split gene. A synthetic, intron specific probe hybridized to all RNA precursors except the 1.2 kb mature RNA. These results and psbA DNA sequence data lead to the conclusion that the four higher molecular weight transcripts are unprocessed precursors of the 1.2 kilobase RNA, some of which contain unspliced intervening sequences. There is an increase in psbA transcripts during light induced maturation of the chloroplasts.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bahl C. P., Marians K. J., Wu R. A general method for inserting specific DNA sequences into cloning vehicles. Gene. 1976;1(1):81–92. doi: 10.1016/0378-1119(76)90008-1. [DOI] [PubMed] [Google Scholar]
- Ben-Shaul Y., Schiff J. A., Epstein H. T. Studies of Chloroplast Development in Euglena. VII. Fine Structure of the Developing Plastid. Plant Physiol. 1964 Mar;39(2):231–240. doi: 10.1104/pp.39.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham S., Schiff J. A. Events surrounding the early development of Euglena chloroplasts. 16. Plastid thylakoid polypeptides during greening. Biochim Biophys Acta. 1979 Sep 11;547(3):531–543. doi: 10.1016/0005-2728(79)90032-x. [DOI] [PubMed] [Google Scholar]
- Blair G. E., Ellis R. J. Protein synthesis in chloroplasts. I. Light-driven synthesis of the large subunit of fraction I protein by isolated pea chloroplasts. Biochim Biophys Acta. 1973 Aug 24;319(2):223–234. doi: 10.1016/0005-2787(73)90013-0. [DOI] [PubMed] [Google Scholar]
- Chelm B. K., Hallick R. B., Gray P. W. Transcription program of the chloroplast genome of Euglena gracilis during chloroplast development. Proc Natl Acad Sci U S A. 1979 May;76(5):2258–2262. doi: 10.1073/pnas.76.5.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelm B. K., Hoben P. J., Hallick R. B. Expression of the chloroplast ribosomal RNA genes of Euglena gracilis during chloroplast development. Biochemistry. 1977 Feb 22;16(4):776–781. doi: 10.1021/bi00623a032. [DOI] [PubMed] [Google Scholar]
- Chua N. H., Gillham N. W. The sites of synthesis of the principal thylakoid membrane polypeptides in Chlamydomonas reinhardtii. J Cell Biol. 1977 Aug;74(2):441–452. doi: 10.1083/jcb.74.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Driesel A. J., Speirs J., Bohnert H. J. Spinach chloroplast mRNA for a 32 000 dalton polypeptide: size and localization on the physical map of the chloroplast DNA. Biochim Biophys Acta. 1980 Dec 11;610(2):297–310. doi: 10.1016/0005-2787(80)90011-8. [DOI] [PubMed] [Google Scholar]
- Gilbert C. W., Buetow D. E. Two-dimensional gel analysis of polypeptide appearance in forming thylakoid membranes. Biochem Biophys Res Commun. 1982 Jul 30;107(2):649–655. doi: 10.1016/0006-291x(82)91540-6. [DOI] [PubMed] [Google Scholar]
- Gray P. W., Hallick R. B. Isolation of Euglena gracilis chloroplast 5S ribosomal RNA and mapping the 5S rRNA gene on chloroplast DNA. Biochemistry. 1979 May 1;18(9):1820–1825. doi: 10.1021/bi00576a029. [DOI] [PubMed] [Google Scholar]
- Grebanier A. E., Coen D. M., Rich A., Bogorad L. Membrane proteins synthesized but not processed by isolated maize chloroplasts. J Cell Biol. 1978 Sep;78(3):734–746. doi: 10.1083/jcb.78.3.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallick R. B., Chelm B. K., Gray P. W., Orozco E. M., Jr Use of aurintricarboxylic acid as an inhibitor of nucleases during nucleic acid isolation. Nucleic Acids Res. 1977 Sep;4(9):3055–3064. doi: 10.1093/nar/4.9.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschberg J., McIntosh L. Molecular Basis of Herbicide Resistance in Amaranthus hybridus. Science. 1983 Dec 23;222(4630):1346–1349. doi: 10.1126/science.222.4630.1346. [DOI] [PubMed] [Google Scholar]
- Hoffman-Falk H., Mattoo A. K., Marder J. B., Edelman M., Ellis R. J. General occurrence and structural similarity of the rapidly synthesized, 32,000-dalton protein of the chloroplast membrane. J Biol Chem. 1982 Apr 25;257(8):4583–4587. [PubMed] [Google Scholar]
- Langridge J., Langridge P., Bergquist P. L. Extraction of nucleic acids from agarose gels. Anal Biochem. 1980 Apr;103(2):264–271. doi: 10.1016/0003-2697(80)90266-3. [DOI] [PubMed] [Google Scholar]
- Link G., Coen D. M., Bogorad L. Differential expression of the gene for the large subunit of ribulose bisphosphate carboxylase in maize leaf cell types. Cell. 1978 Nov;15(3):725–731. doi: 10.1016/0092-8674(78)90258-1. [DOI] [PubMed] [Google Scholar]
- Mattoo A. K., Pick U., Hoffman-Falk H., Edelman M. The rapidly metabolized 32,000-dalton polypeptide of the chloroplast is the "proteinaceous shield" regulating photosystem II electron transport and mediating diuron herbicide sensitivity. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1572–1576. doi: 10.1073/pnas.78.3.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco E. M., Jr, Hallick R. B. Euglena gracilis chloroplast transfer RNA transcription units. I. Physical map of the transfer RNA gene loci. J Biol Chem. 1982 Mar 25;257(6):3258–3264. [PubMed] [Google Scholar]
- Palmer J. D., Edwards H., Jorgensen R. A., Thompson W. F. Novel evolutionary variation in transcription and location of two chloroplast genes. Nucleic Acids Res. 1982 Nov 11;10(21):6819–6832. doi: 10.1093/nar/10.21.6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passavant C. W., Stiegler G. L., Hallick R. B. Location of the single gene for elongation factor Tu on the Euglena gracilis chloroplast chromosome. J Biol Chem. 1983 Jan 25;258(2):693–695. [PubMed] [Google Scholar]
- Pfister K., Steinback K. E., Gardner G., Arntzen C. J. Photoaffinity labeling of an herbicide receptor protein in chloroplast membranes. Proc Natl Acad Sci U S A. 1981 Feb;78(2):981–985. doi: 10.1073/pnas.78.2.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rave N., Crkvenjakov R., Boedtker H. Identification of procollagen mRNAs transferred to diazobenzyloxymethyl paper from formaldehyde agarose gels. Nucleic Acids Res. 1979 Aug 10;6(11):3559–3567. doi: 10.1093/nar/6.11.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawson J. R., Boerma C. L., Andrews W. H., Wilkerson C. G. Complexity and abundance of ribonucleic acid transcribed from restriction endonuclease fragments of Euglena chloroplast deoxyribonucleic acid during chloroplast development. Biochemistry. 1981 Apr 28;20(9):2639–2644. doi: 10.1021/bi00512a043. [DOI] [PubMed] [Google Scholar]
- Renger G. Studies on the structural and functional organization of system II of photosynthesis. The use of trypsin as a structurally selective inhibitor at the outer surface of the thylakoid membrane. Biochim Biophys Acta. 1976 Aug 13;440(2):287–300. doi: 10.1016/0005-2728(76)90063-3. [DOI] [PubMed] [Google Scholar]
- Schiff J. A., Zeldin M. H., Rubman J. Chlorophyll Formation and Photosynthetic Competence in Euglena During Light-Induced Chloroplast Development in the Presence of 3, (3,4-dichlorophenyl) 1,1-Dimethyl Urea (DCMU). Plant Physiol. 1967 Dec;42(12):1716–1725. doi: 10.1104/pp.42.12.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinback K. E., McIntosh L., Bogorad L., Arntzen C. J. Identification of the triazine receptor protein as a chloroplast gene product. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7463–7467. doi: 10.1073/pnas.78.12.7463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiegler G. L., Matthews H. M., Bingham S. E., Hallick R. B. The gene for the large subunit of ribulose-1,5-bisphosphate carboxylase in Euglena gracilis chloroplast DNA: location, polarity, cloning, and evidence for an intervening sequence. Nucleic Acids Res. 1982 Jun 11;10(11):3427–3444. doi: 10.1093/nar/10.11.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock R., Sweet R., Weiss M., Cedar H., Axel R. Intragenic DNA spacers interrupt the ovalbumin gene. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1299–1303. doi: 10.1073/pnas.75.3.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski G., Bohnert H. J., Whitfeld P. R., Bottomley W. Nucleotide sequence of the gene for the M(r) 32,000 thylakoid membrane protein from Spinacia oleracea and Nicotiana debneyi predicts a totally conserved primary translation product of M(r) 38,950. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7699–7703. doi: 10.1073/pnas.79.24.7699. [DOI] [PMC free article] [PubMed] [Google Scholar]