Abstract
Advanced glycation end products (AGEs) accumulate in diabetic patients due to high blood glucose levels and cause multiple deleterious effects. In this study, we provide evidence that the AGE increased cell death, one such deleterious effect. Methyl glyoxal-coupled human serum albumin (AGE-HSA) induced transcription factors such as NF-κB, NF-AT, and AP-1. AGE acts through its cell surface receptor, RAGE, and degranulates vesicular contents including interleukin-8 (IL-8). The number of RAGEs, as well as the amount of NF-κB activation, is low, but the cell death is higher in neuronal cells upon AGE treatment. Degranulated IL-8 acts through its receptors, IL-8Rs, and induces sequential events in cells: increase in intracellular Ca2+, activation of calcineurin, dephosphorylation of cytoplasmic NF-AT, nuclear translocation of NF-AT, and expression of FasL. Expressed FasL increases activity of caspases and induces cell death. Although AGE increases the amount of reactive oxygen intermediate, accompanying cell death is not dependent upon reactive oxygen intermediate. AGE induces autophagy, which partially protects cells from cell death. A novel mechanism of AGE-mediated cell death in different cell types, especially in neuronal cells where it is an early event, is provided here. Thus, this study may be important in several age-related neuronal diseases where AGE-induced apoptosis is observed because of high amounts of AGE.
Keywords: Aging, Apoptosis, Autophagy, Calcineurin, Chemokines, Degranulation, FasL, Glycated End Products, IL-8, NF-AT
Introduction
The formation of advanced glycation end products (AGEs)2 by non-enzymatic reactions is implicated in aging. The AGE products are involved in age-related pathologies such as atherosclerosis and neurodegenerative disorders and in the development of long-term complications of diabetes. The neurodegenerative diseases often occur due to loss of function or death of neuronal or neuroglial cells. Chronic inflammation, triggered by intrinsic factors and nutrients, affects tissue environment, especially in age-related diseases such as diabetes, atherosclerosis, cancer, etc. (1). Glycation is a process in which reducing sugars or their reactive carbonyl metabolites such as methyl glyoxal (MGO), glycolaldehyde, d-glucose, etc. react with the free amino groups of predominantly long-lived proteins to produce cross-links non-enzymatically that accumulate in the aging tissue. In diabetes mellitus (hyperglycemic conditions), the production of several carbonyl metabolites such as MGO increases. AGE interacts with its specific receptors, RAGEs, and results in cell signaling that induces various cellular consequences leading to several ailments such as retinopathy, neuropathy, nephropathy, and atherosclerosis, especially in patients with diabetes (2). Several studies have shown that diabetics have increased MGO in their plasma when compared with non-diabetics (3–5). These carbonyl metabolites of sugar result in an increase in the glycation of proteins, leading to tissue damage (6). AGE interacts with its receptors, generates reactive oxygen species, and induces inflammatory responses (7). Hyperglycemia generates mitochondrial reactive oxygen species and activates stress-sensitive pathways such as NF-κB, p38 MAPK, and JAK/STAT pathways; polyol (sorbitol) and hexosamine pathways; and PKC and AGEs pathways.
Several studies suggest that AGE generates oxidative stress that leads to DNA damage and culminates in cell death through mitotic catastrophe (8–10). Aberrantly active NF-κB complexes can contribute in cell proliferation by regulating genes that promote the growth and survival of cells (11–13). NF-κB, a heterodimer of two subunits, p50 (NF-κB 1) and p65 (RelA), is normally present in the cytoplasm in an inactive state in complex with an inhibitory subunit of κB (IκBα). Upon phosphorylation and subsequent degradation of IκBα, a nuclear localization signal on the p50-p65 heterodimer is exposed, leading to nuclear translocation of NF-κB. The p50-p65 heterodimer binds with a specific sequence in DNA, resulting in gene transcription. Phosphorylation and acetylation of p65 subunit potentiate NF-κB-dependent gene transcription for rapid cell proliferation, the hallmark for tumorigenic response (14, 15). Expression of adhesion molecules, cyclins, Bax, Bcl2, and several cytokines including IL-8, etc. is NF-κB-dependent. IL-8 induces signal through its cell surface receptors (IL-8Rs) via recruitment of TNF receptor-associated factor (TRAF) 6 (16).
Several stimuli induce cells by recruiting specific receptors to release several proteolytic enzymes as an early event through degranulation of azurophilic and specific granules and secretory vesicles as a first line of defense to combat foreign microbes and particulates. Several cellular proteins are recruited in the degranulation, especially endocytosis and exocytosis processes. Transglutaminase (TG), involved in endocytosis, is targeted in several neurodegenerative disorders (17, 18). As a late response, these stimuli induce many transcription factors such as NF-κB and activator protein (AP)-1 and thereby are involved in inducing biological responses including inflammation, apoptosis, etc. Some inducers also increase intracellular Ca2+, which acts as a secondary messenger molecule to induce cellular activities.
Upon stimulation of calcineurin, a downstream target of intracellular Ca2+ signaling (19), several phosphate residues at the regulatory domain of NF-AT, the target substrate for calcineurin (20–22), are dephosphorylated, and this leads to nuclear translocation of NF-AT and activation of target genes of AP-1 (23), MEF2 (24), FasL (25, 26), and GATA (27) proteins. FasL acts through binding its cell surface receptors, CD95 or Fas, and induces cell death via activation of cysteine proteases, the caspases that execute apoptosis. The nuclear translocation of NF-AT and activity of calcineurin are blocked by cyclosporine A (CsA), thereby affecting downstream signals and cell death (25). Many physiological processes such as neuronal death, T-cell activation, and cardiac and skeletal myocyte differentiation were found to be regulated by calcineurin (20). Calcineurin activity was found to decrease significantly in tissues of cervical carcinoma patients (28).
Dysfunctional organelles and misfolded proteins are utilized for survival in the cells (29) during autophagy. Some studies have suggested that autophagy induces cell death (30, 31). AGE is known to induce autophagy (32), which is also supported by our data. In addition, this increase partially protects cells from death. In this study, we provide evidences for the first time that AGE-mediated cell death proceeds through the signaling cascade: early increases of IL-8 by degranulation by AGE-RAGE interaction increased intracellular Ca2+ activation of calcineurin nuclear translocation of NF-AT expression of NF-AT-dependent FasL FasL-mediated caspase activation induction of cell death. Thus, the suggested novel pathway for AGE-mediated cell death in neurodegenerative diseases in aged persons due to high amounts of accumulated AGE might help in formulating molecules for combination therapy.
EXPERIMENTAL PROCEDURES
Materials
MGO, human serum albumin (HSA), dihydrorhodamine, propidium iodide, MTT, monodansyl cadaverine (MDC), glycine, BAPTA-AM, diltiazem, cystamine, brefeldin A, CsA, RII [(pSer95, Ala97)-cAMP-dependent protein kinase regulatory subunit type II (81–99)] phosphopeptide, para-nitrophenyl phosphate, ortho-phenylenediamine, β-d-glucuronide, dimethyl sulfoxide (DMSO), and anti-tubulin antibody were obtained from Sigma-Aldrich. Penicillin, streptomycin, neomycin, RPMI 1640, Iscove's modified Dulbecco, DMEM medium, and fetal bovine serum (FBS) were obtained from Invitrogen. Caspase substrates (Ac-DVED-pNA, Ac-ITED-pNA, and Ac-LEHD-pNA) were obtained from Calbiochem (Darmstadt, Germany). DAPI, Fura-2AM, and anti-rabbit IgG conjugated with HRP were obtained from Molecular Probes (Eugene, OR). Antibodies against cytochrome c, CRM1, IκBα, p65, IL-8, RAGE, FasL, cleaved caspase 3, and alkaline phosphatase and gel shift oligonucleotides for NF-κB, AP-1, and NF-AT were obtained from Santa Cruz Biotechnology (Santa Cruz, CA).
Cell Lines
The cell lines used in this study were as follows: MCF-7 (human breast carcinoma), U-937 (human histiocytic lymphoma), and U373 (human glioblastoma). These cells were obtained from the American Type Culture Collection (Manassas, VA). Cells were cultured in DMEM medium containing 10% FBS, penicillin (100 units/ml), and streptomycin (100 μg/ml). All cells were found to be free from mycoplasma by the Gen-Probe mycoplasma detection kit (Fisher Scientific).
Coupling of AGE and Assay for the Concentration of AGE-coupled Protein
HSA was incubated with MGO for 7 days aseptically at 37 °C. Proteins were dialyzed, and concentrations were measured and kept the same as the concentrations of AGE. The amount of protein glycation was measured by the periodate method. Briefly, 100 μl of MGO solution or unknown MGO-coupled HSA was treated with periodic acid. These reaction mixtures or a known amount of HCHO solution were incubated with 200 μl of HCHO assay solution (46 μl of acetyl acetone in 10 ml of 3.3 m ammonium acetate). Absorbance was measured at 405 nm to measure diacetyl dihydrolutidine, which was formed due to the reaction of HCHO with acetyl acetone with ammonia. Absorbance of diacetyl dihydrolutidine at 405 nm is 7778/mol. After calculation, we have found that incorporated MGO is 4 nmol/mg of HSA (equivalent to 0.31 mol/mol of HSA). Dialyzed AGE-HSA is free from MGO, although 100 nm MGO-treated cells did not show any cell death at 72 h of incubation (data not shown).
NF-κB, AP-1, and NF-AT DNA Binding Assay
DNA binding activity of NF-κB, AP-1, and NF-AT was determined by EMSA (33). Briefly, after different treatments, cells were used to prepare cytoplasmic and nuclear extracts. Nuclear extract proteins (8 μg) were incubated with 32P end-labeled double-stranded NF-κB oligonucleotide of HIV-LTR, 5′-TTGTTACAAGGGACTTTCCGCTGGGGACTTTCCAGGGAGGCGTGG-3′, for 30 min at 37 °C, and the DNA-protein complex was separated from free oligonucleotide on 6.6% native PAGE. Similarly, NF-AT was assayed using specific double-stranded labeled oligonucleotide of 5′-CGCCCAAAGAGGAAAATTTGTTTCATA-3′, and AP-1 was assayed using double-stranded oligonucleotide of 5′-CGCTTGATGACTCAGCCGGAA-3′. Visualization of radioactive bands was done in a Fluorescent Image Analyzer FLA-3000 (Fuji, Japan).
FasL and NF-AT-dependent Luciferase Gene Transcription Assay
The expression of FasL- and NF-AT-dependent luciferase reporter gene was carried out as described previously (16). Cells were transiently transfected with SuperFect transfection reagent containing 0.5 μg of each reporter plasmid containing FasL or NF-AT binding site cloned upstream of luciferase (designated as FasL-luciferase or NF-AT-luciferase) and GFP constructs. After 3 h of transfection, cells were washed and cultured for 12 h followed by different treatments. GFP-positive cells were counted. The cell pellets were extracted with lysis buffer (part of the luciferase assay kit from Promega), and the extracts were incubated with the firefly luciferin (substrate from Promega). Light emission was monitored with a luminometer, and values were calculated as -fold of activation over vector-transfected value.
Assay of Enzymes Released
Cells (1 × 107 cells/ml), suspended in RPMI 1640 medium (phenol red-free) with 10% FBS, were treated with AGE-HSA for varying times at 37 °C. The supernatant was collected and used for assay of different enzymes (34). Myeloperoxidase, alkaline phosphatase, and elastase activities were measured using chromogenic substrates ortho-phenylenediamine, p-nitrophenyl phosphate, and N-methoxysuccinyl-Ala-Ala-Pro-Val p-nitroaniline as substrates, respectively.
Calcineurin Activity Assay
After different treatments, cells (2 × 106) were extracted in 200 μl of 2× assay buffer (200 mm NaCl, 100 mm Tris (pH 7.5), 12 mm MgCl2, 1 mm CaCl2) and 0.05% Nonidet P-40. The extracts were passed through a Sephadex G-25 column to eliminate the free phosphate, and the pooled proteins fractions were used for the calcineurin assay (26). The extract (10 μg of protein, almost 5 μl) was mixed with 25 μl of 2× assay buffer, and the mixture was incubated without or with RII [(pSer95, Ala97)-cAMP-dependent protein kinase regulatory subunit type II (81–99)] phosphopeptide (5 μm) for 10 min at 30 °C. The reaction was terminated by the addition of 100 μl of Malachite green mix (3 volumes of 0.045% Malachite green and 1 volume of 4.2% ammonium molybdate in 4 n HCl). After a 30-min incubation at 30 °C, the absorbance was read at 660 nm. The calcineurin activity (-fold) was calculated in terms of inorganic phosphate released under these conditions, taking the value of unstimulated cells as 1-fold.
Intracellular Ca2+ Release Assay
Intracellular Ca2+ mobilization was measured using the Fura-2AM method as described (35). Briefly, cells were incubated in Low Calcium Buffer A (136 mm NaCl, 2.7 mm KCl, 2 mm MgCI2, 5 mm HEPES, 5.6 mm glucose, 0.42 mm NaH2PO4, and 0.5 mm EGTA buffer, pH 6.6). Cells were washed and incubated in Low Calcium Buffer B (same composition as Buffer A, except 0.16 mm CaCl2 and without EGTA, pH 7.4) and incubated with 2.5 μm Fura-2AM at 37 °C for 30 min. Then the cells were washed, scraped, and suspended in Buffer B, and fluorescence intensity was monitored at an excitation wavelength at 330 nm and an emission wavelength at 510 nm in a PerkinElmer Life Sciences spectrofluorometer.
Cytotoxicity Assay
The cytotoxicity was measured by MTT assay (36). Briefly, cells (1 × 104 cells/well of 96-well plate) were treated with different agents at the indicated concentrations and times, and thereafter 25 μl of MTT solution (5 mg/ml in PBS) was added. After a 2-h incubation, 100 μl of Extraction Buffer (20% SDS in 50% dimethylformamide) was added. After an overnight incubation at 37 °C, absorbance was read at 570 nm with the Extraction Buffer as blank.
Measurement of Reactive Oxygen Intermediate (ROI)
The production of ROI in cells treated with AGE was determined by flow cytometer as described (12). Briefly, cells (5 × 105 cells/ml), after different treatments, were incubated with dihydrorhodamine 123, and fluorescence intensity was measured by FACSCalibur (BD Biosciences) with excitation at 488 nm and emission between 515 and 550 nm. Data were analyzed using the CellQuest software (BD Biosciences).
Caspase Assay
To evaluate caspase 3, 8, and 9 activities, cell lysates were prepared after the treatments. Cell lysate (50 μg of protein) was incubated with 200 μm caspase 3 substrate (Ac-DVED-pNA), caspase 8 substrate (Ac-ITED-pNA), or caspase 9 substrate (Ac-LEHD-pNA) in 100 μl of Reaction Buffer (1% Nonidet P-40, 20 μm Tris-HCl, pH 7.5, 137 mm NaCl, and 10% glycerol) and incubated for 2 h at 37 °C. The release of chromophore pNA (para-nitroaniline) was monitored spectrophotometrically at 405 nm (26).
MDC Staining
After different treatments, cells were washed and incubated with MDC for 10 min at 37 °C and then washed three times with PBS, pH 7.4. Intracellular MDC was measured by fluorescence photometry (excitation at 380 nm and emission at 525 nm) (37). Autophagy was measured by the increase in fluorescence intensity of MDC-stained autophagosomes. The amount of fluorescence was expressed as relative fluorescent units.
Reverse Transcriptase (RT)-PCR
Total RNA was isolated using the standard TRIzol method (Invitrogen). 1 μg of total RNA (quantified by absorbance at 260 nm) was used to reverse-transcribe into cDNA by the One-step Access RT-PCR kit (Promega, Madison, WI) followed by the amplification of the gene of interest using gene-specific primers for IL-8, ICAM1, FasL, TNF, and actin. PCR was performed after avain myeloblastosis virus RT inactivation and RNA/cDNA/primer denaturation for 4 min at 94 °C and by repeating the cycles 94, 55, and 72 °C. Amplified products were separated by agarose gel electrophoresis (2%) and visualized by ethidium bromide staining. The primer sequence and product size are as follows: IL-8, 190 bp, forward, 5′-CAGACAGAGCTCTCTTCCAT-3′, reverse, 5′-GCAGCTCTGTGTGAAGGTGCA-3′; TNF, 324 bp, forward, 5′-CGAGTGACAAGCCTGTAGCC-3′, reverse, 5′-CATACCAGGGCTTGGCCTCA-3′; ICAM1, 450 bp, forward, 5′-AGGGAGGCTCCGTGCTGGTGA-3′, reverse, 5′-TCAGTGCGGCACGAGAAATTG-3′; FasL, 344 bp, forward, 5′-GGATTGGGCCTGGGGATGTTTCA-3′, reverse, 5′-TTGTGGCTCAGGGGCAGGTTGTTG-3′; actin, 616 bp, forward, 5′-CCAACCGTGAAAAGATGACC-3′, reverse, 5′-GCAGTAATCTCCTTCTGCATCC-3′.
RESULTS
HSA was dissolved in saline (4 mg/ml) and incubated with 10 μm MGO for 7 days at 37 °C. The mixture was dialyzed, and its protein concentration was determined. The MGO-HSA complex contained 4 nmol MGO/mg of HSA, equivalent to 0.31 mol/mol of HSA used in this study as AGE-HSA to examine its effects.
No Necrosis on AGE Treatment
There is no necrosis/cytolysis of cells upon treatment with AGE until 72 h as judged by the absence of lactate dehydrogenase (LDH) activity in the culture supernatant, indicated by the unused NADH in the calorimetric assay (OD340 were 1.012 ± 0.082, 0.992 ± .067, and 1.008 ± .077 in the culture supernatant, on the addition of AGE of 0, 100, and 200 μg/ml, respectively). The amount of incorporated MGO is much lower, and even 100 nm concentration of MGO alone did not show any cell death.
AGE Increases Cell Death
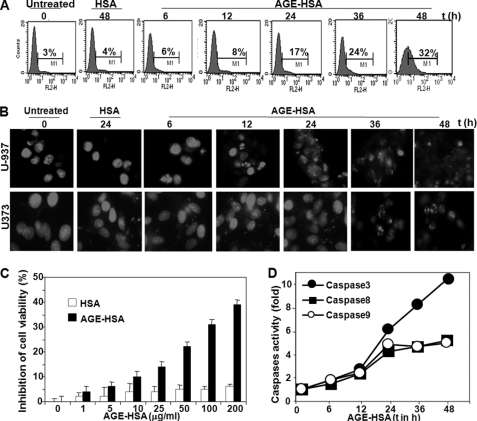
The amount of AGE present is always correlated with age, and aged persons are always prone to neurodegenerative diseases. It is therefore of interest to study the effects of AGE on aging processes and on cell death. U-937 and U373 cells were incubated with 100 μg/ml MGO-coupled HSA (known as AGE-HSA in this study) for different time intervals. The data on annexin V-phycoerythrin binding, as assayed by FACS, showed that AGE-HSA treatment increased cell death with increasing time (Fig. 1A). After 48 h of AGE-HSA treatment, 32% (p < 0.005) cell death was observed. Nuclear fragmentation data, as shown by propidium iodide-stained cells, confirmed similar cell death (Fig. 1B). Cell viability was shown almost 32% (p < 0.001) at 100 μg/ml AGE-HSA at 48 h of incubation, as shown by MTT assay (Fig. 1C). AGE-HSA increased caspase 3, 8, and 9 activities after 12 h of incubation (Fig. 1D). All these data support that AGE induces cell death.
FIGURE 1.
Effect of AGE-HSA on cell death. A, U-937 cells were treated with AGE-HSA (100 μg/ml) for different times. After these treatments, cell death was detected by annexin V-phycoerythrin and analyzed in FACS. U-937 cells were treated with AGE-HSA (100 μg/ml) for different times. Then cells were washed, fixed with methanol, and stained with propidium iodide. B, cells were then taken in slides and visualized in fluorescence microscopes. U-937 cells were treated with different concentrations of AGE-HSA in triplicate for 48 h. Cell viability was assayed using MTT dye. C, results are represented as inhibition of cell viability in percentage, which is calculated from mean absorbance ± S.D. of triplicate samples. D, U-937 cells were treated with AGE-HSA (100 μg/ml) for different times, and caspase 3, 8, and 9 activities were measured and indicated as -fold of activation considering untreated value as 1-fold.
AGE-mediated Cell Death Is Independent of Cell Types
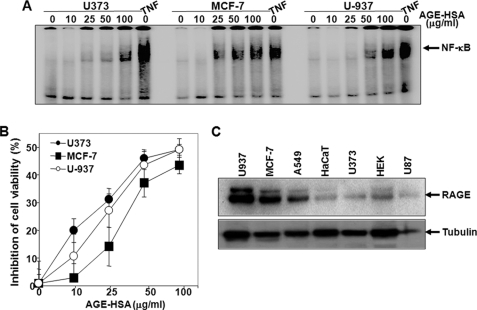
Neuronal and breast carcinoma cells were incubated with different concentrations of AGE-HSA for 24 h, and NF-κB was assayed to test whether the effect of AGE is specific to cell types. Binding of NF-κB to DNA increased in all these cells, more effectively in MCF-7 and U-937 cells than in U373 cells (Fig. 2A). The cell death activity, measured by MTT assay, was more prominent and faster in U373 cells when compared with U-937 and MCF-7 cells (Fig. 2B). On the other hand, the amount of expressed RAGEs was considerably more in U-937 and MCF-7 cells (Fig. 2C). These data indicate that neuronal cells have low expression of RAGE and high AGE-mediated cell death when compared with lymphoid or breast carcinoma cells.
FIGURE 2.
Effect of AGE-HSA on cell death in different cell types. A, U373, MCF-7, and U-937 cells were treated with different concentrations of AGE-HSA or 100 nm TNF for 24 h. Nuclear extracts were prepared and assayed for the NF-κB gel shift assay. These cells were treated with different concentrations of AGE-HSA for 72 h, and cell viability was assayed by MTT dye. B, results are represented as inhibition of cell viability in percentage, which is calculated from mean absorbance ± S.D. of triplicate samples. C, the amount of RAGE was measured from whole cell extracts (100 μg of proteins) by Western blot.
AGE Increases NF-κB, AP-1, and NF-AT DNA Binding, IκBα Degradation, p65 Nuclear Translocation, and NF-κB-dependent Gene Expression
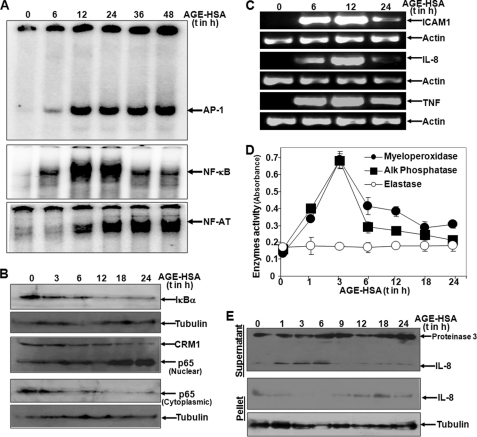
The role of AGE in induction of different transcription factors was examined by treating U-937 cells with AGE-HSA (100 μg/ml) for different time intervals and assaying for NF-κB, AP-1, and NF-AT by gel shift assay in their nuclear extracts. As shown in Fig. 3A, NF-κB DNA binding increased progressively until 24 h and decreased by 36 h of AGE-HSA treatment of the cells. Also, AP-1 and NF-AT DNA binding increased upon AGE-HSA treatment. In the cytoplasmic extracts, the amounts of IκBα decreased and the amounts of p65 increased with the time of AGE-HSA treatment (Fig. 3B). The amounts of ICAM1, IL-8, and TNF increased with increasing time of AGE-HSA treatment, as shown by RT-PCR followed by PCR data (Fig. 3C). These data indicate that AGE-HSA-mediated activation of NF-κB involves degradation of IκBα, nuclear translocation of p65, and NF-κB-dependent gene transcription.
FIGURE 3.
Effect of AGE-HSA on activation of transcription factors, NF-κB-dependent gene activation, and degranulation. A, U-937 cells were incubated with 100 μg/ml AGE-HSA for different times. Nuclear extracts were assayed for NF-κB, AP-1, and NF-AT DNA binding by gel shift assay. B, amounts of IκBα and p65 were measured from cytoplasmic extracts and p65 was measured from nuclear extracts by Western blot. C, the amounts of IL-8, ICAM1, and TNF were measured by RT-PCR from total RNA isolated from AGE-HSA-stimulated cells for different times. D, the cell culture supernatant was taken from AGE-HSA-stimulated cells for different times, and the amount of myeloperoxidase, alkaline phosphatase (Alk Phosphatase), and elastase was measured by measuring the activities of these enzymes as detected by specific chromogenic substrate. Error bars indicate ± S.D. of triplicate samples. E, upper panel, the culture supernatants were concentrated 10 times with a 3-kDa cut filter, and the amounts of IL-8 and proteinase 3 were measured by Western blot. E, lower panel, the amount of IL-8 was measured from whole cell extracts (200 μg of proteins) of the cell pellet upon similar treatment by Western blot.
The activity of myeloperoxidase and alkaline phosphatase, but not elastase activities, increased between 1 and 6 h of incubation of cells with AGE-HSA (Fig. 3D). The amount of IL-8 released into the culture supernatant increased during 1–6 h of incubation of cells with AGE-HSA with a corresponding decrease in the cell pellet in those time points (Fig. 3E). The amount of proteinase 3 releases in the supernatant was not altered at any time of incubation. These data suggest that AGE treatment triggers cells to degranulate granular contents that contain myeloperoxidase, alkaline phosphatase, and IL-8.
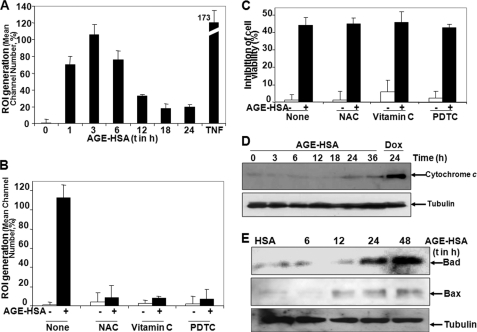
AGE Induces ROI, and Antioxidants Are Unable to Block AGE-mediated Cell Death
AGE-HSA treatment increased the amount of ROI as measured by rhodamine in FACS between 1 and 6 h of incubation (Fig. 4A), and antioxidants such as N-acetylcysteine, vitamin C, or pyrrolidine dithiocarbamate inhibited AGE-HSA-induced ROI generation completely (Fig. 4B). AGE induced almost 40% cell death, and antioxidants did not alter AGE-mediated cell death (Fig. 4C). The amount of cytoplasmic cytochrome c increased at 24 and 36 h of AGE-HSA treatment (Fig. 4D). AGE-HSA treatments increased the amounts of Bad and Bax at 24 and 48 h of incubation (Fig. 4E). These data suggest that AGE induces cell death, that antioxidants cannot protect the AGE-mediated cell death, and that cytochrome c release is a late response of AGE treatment.
FIGURE 4.
Effect of antioxidants on AGE-HSA-induced ROI generation and cell death. A, U-937 cells were treated with 100 μg/ml AGE-HSA for different times or 100 pm TNF for 2 h. ROI generation was measured using dihydrorhodamine by FACS and indicated as the percentage detected from mean channel number. U-937 cells were pretreated with N-acetylcysteine (NAC, 10 mm), vitamin C (2 mm), or pyrrolidine dithiocarbamate (PDTC, 100 μm) for 2 h and then stimulated with 100 μg/ml AGE-HSA for 4 h. B, ROI generation was measured using dihydrorhodamine by FACS. Cells were pretreated with N-acetylcysteine (10 mm), vitamin C (2 mm), or pyrrolidine dithiocarbamate (100 μm) for 2 h and then stimulated with 100 μg/ml AGE-HSA for 48 h. C, cell death was determined by MTT assay and indicated in percentage. Error bars in A–C indicate ± S.D. of triplicate samples. D, the amount of cytochrome c was measured from cytoplasmic fraction of AGE-HSA (100 μg/ml)-treated cells for different times, keeping doxorubicin (Dox) (1 μm for 24 h) as positive control for different times by Western blot. E, the amounts of Bad and Bax were determined in the whole cell extracts obtained from AGE-HSA-treated cells for different times by Western blot.
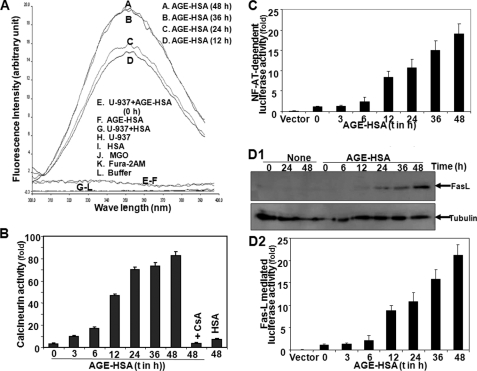
AGE Increases Intracellular Ca2+, Calcineurin Activity, Nuclear Translocation of NF-AT, and FasL Expression
Intracellular Ca2+, measured by the fluorescence of Fura-2AM dye, increased in cells with increasing time of incubation of 100 μg/ml AGE-HSA (100 μg/ml) (Fig. 5A). AGE-HSA treatment also increased calcineurin activity in a time-dependent manner, and the addition of CsA completely inhibited this effect (Fig. 5B). AGE-HSA treatment increased nuclear NF-AT activity in a time-dependent manner, as shown by NF-AT-dependent reporter gene and luciferase activity (Fig. 5C). AGE-HSA treatment increased the amount of FasL (Fig. 5D1) and FasL-dependent luciferase activity (Fig. 5D2). These data suggest that AGE-HSA increases intracellular Ca2+ that leads to activation of calcineurin, nuclear translocation of NF-AT, and NF-AT-dependent FasL expression.
FIGURE 5.
Effect of AGE-HSA on intracellular Ca2+ release, calcineurin activation, NF-AT-dependent luciferase activity, and FasL expression. A, U-937 cells were treated with AGE-HSA (100 μg/ml) for different times. Intracellular free Ca2+ was measured using Fura-2AM as fluorescent probe in a fluorometer. Cells were treated with 100 μg/ml AGE-HSA for different times or CsA (2.5 μm) for 2 h and then treated with AGE-HSA for 48 h. B, calcineurin activity was assayed from whole cell extracts. U-937 cells were transfected with Qiagen SuperFect reagent for 3 h with plasmids for NF-AT or FasL promoter DNA that had been linked to luciferase (NF-AT-luciferase or FasL-luciferase) and GFP. After washing, cells were cultured for 12 h. The GFP-positive cells were, counted and transfection efficiency was calculated. C and D2, cells, treated with AGE-HSA (100 μg/ml) for different times, were extracted, and the luciferase activity was measured as per Promega protocol and indicated as -fold of activation. D1, the amount of FasL was measured from whole cell extracts upon similar treatment by Western blot. Error bars in B, C, and D2 indicate ± S.D. of triplicate samples.
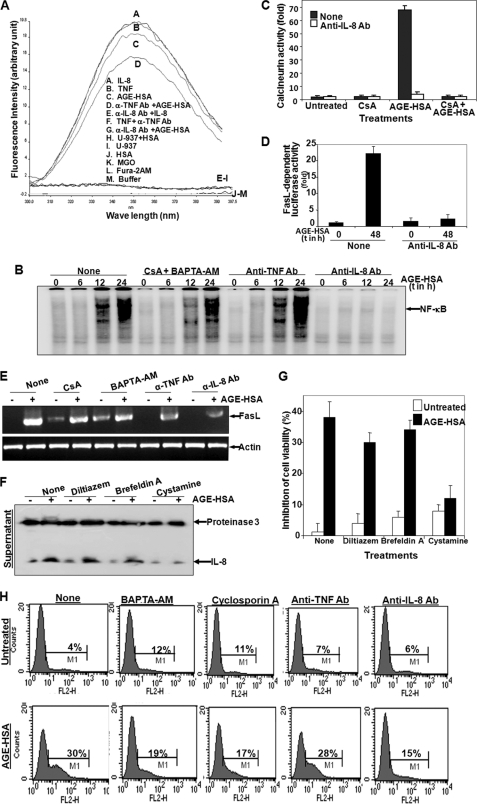
Depletion of Ca2+, Inhibition of Calcineurin, or Incubation of Anti-IL-8 or -FasL Ab Protects AGE-mediated Cell Death
The fact that AGE-HSA, IL-8, or TNF increases intracellular Ca2+ and causes apoptosis to the cells was substantiated in the following experiments. U-937 cells were preincubated with anti-IL-8 Ab or anti-TNF Ab for 2 h and then incubated with AGE-HSA for 24 h. Anti-IL-8 Ab, but not anti-TNF Ab, inhibited AGE-HSA-induced increase in Ca2+ (Fig. 6A). Removal of Ca2+ by incubating cells with cyclosporine A and BAPTA-AM did not block AGE-HSA-mediated increase in NF-κB DNA binding (Fig. 6B). Anti-IL-8 Ab, but not anti-TNF Ab, completely inhibited AGE-HSA-mediated activation of NF-κB, suggesting that AGE-mediated signaling is going through IL-8. Cells preincubated with cyclosporine A or anti-IL-8 Ab showed complete inhibition of calcineurin activity (Fig. 6C). These data suggest that AGE induces calcineurin and that calcineurin activity depends upon IL-8-mediated action. Anti-IL-8 Ab preincubated cells completely inhibited AGE-HSA-induced expression of luciferase mediated by FasL (Fig. 6D). These data suggest that IL-8 is involved in AGE-mediated expression of FasL via increase in Ca2+ and calcineurin activity, which then leads to cell death. Pretreatment with cystamine completely inhibited IL-8 release from cells upon stimulation of AGE-HSA, but brefeldin A partially inhibited IL-8 release from cells upon stimulation of AGE-HSA. Diltiazem (inhibitor of intracellular Ca2+) was unable to increase the amount of IL-8 in the cell supernatant upon incubation with AGE-HSA (Fig. 6F). These data suggest that inhibition of endocytosis inhibits AGE-mediated release of IL-8. AGE-HSA-induced cell death was almost completely inhibited by cystamine-pretreated cells, but not by brefeldin A- or diltiazem-pretreated cells (Fig. 6G). Cystamine alone induced 8% cell death, which suggests that interference of cell growth by the growth factors in the cell medium through endocytosis of these ligands. Inhibition of calcineurin or chelation of intracellular Ca2+ led to the partial inhibition of AGE-HSA-mediated cell death. Anti-IL-8 Ab, but not anti-TNF Ab, protected almost complete inhibition of AGE-mediated cell death (Fig. 6H). These data suggest that AGE increases release of IL-8 and that released IL-8 increases cell death.
FIGURE 6.
Effect of Ca2+ chelator and anti-IL-8, -TNF, or -FasL Ab on AGE-mediated cell death. A, U-937 cells were preincubated with 1 μg/ml anti-IL-8 or -TNF Ab for 2 h and then stimulated with AGE-HSA (100 μg/ml) for 24 h or with IL-8 (100 ng/ml) or TNF (100 pm) for 2 h. Cells were washed, and intracellular Ca2+ was measured using Fura-2AM as fluorescence probe in a fluorometer fixing emission at 510 nm and excitation from 300 to 400 nm. B, U-937 cells were preincubated with CsA (2.5 μm) and BAPTA-AM (5 μm) or with anti-TNF or -IL-8 Ab for 2 h and then stimulated with AGE-HSA for different times. Nuclear extracts were used to assay NF-κB DNA binding by gel shift assay. Cells were preincubated with CsA and anti-IL-8 Ab for 2 h and then stimulated with AGE-HSA for 24 h. C, calcineurin activity was measured from whole cell extracts and indicated as -fold of activation considering value of unstimulated cells as 1-fold. U-937 cells were transfected with Qiagen SuperFect reagent for 3 h with plasmids for FasL promoter DNA that had been linked to luciferase (FasL-luciferase) and GFP. After washing, cells were cultured for 12 h. Cells, incubated with anti-IL-8 Ab for 2 h, were stimulated with AGE-HSA (100 μg/ml) for 48 h. D, the luciferase activity was measured from whole cell extracts and indicated as -fold of activation. E, cells were preincubated with CsA, BAPTA-AM, anti-TNF Ab, or anti-IL-8 Ab for 2 h and then stimulated with AGE-HSA for 48 h. FasL was detected by RT-PCR from total RNA isolated from cells. F, U-937 cells, treated with cystamine (500 μm), brefeldin A (5 μg/ml), or diltiazem (100 μm) for 2 h, were stimulated with AGE-HSA for 24 h. Culture supernatant was concentrated 10 times and used to detect IL-8 and proteinase 3 by Western blot. Cells, treated with diltiazem, brefeldin A, or cystamine for 2 h in triplicate, were stimulated with AGE-HSA for 48 h. G, the MTT assay was done and indicated as inhibition of cell death in percentage. U-937 cells, incubated with anti-IL-8 Ab, anti-TNF Ab, CsA, or BAPTA-AM for 2 h, were stimulated with AGE-HSA for 48 h. Error bars in C, D, and G indicate ± S.D. of triplicate samples. H, after these treatments, cell death was detected by annexin V-phycoerythrin and analyzed in FACS.
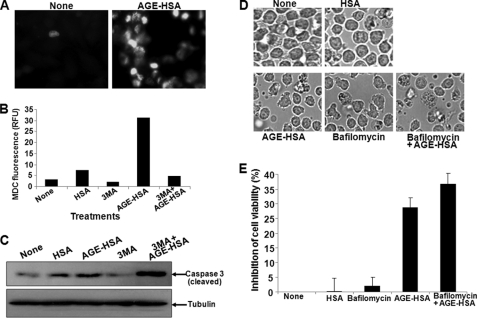
AGE Induces Autophagy, Which Partially Protects Cell Death
U-937 cells were treated with AGE (100 μg/ml) for 48 h, and autophagy was assayed by MDC staining. AGE increased the number of MDC-stained cells when compared with untreated cells (Fig. 7A). When cells were pretreated with 5 mm 3-methyl adenine, an autophagy inhibitor (38), AGE-induced increase in fluorescence of MDC was decreased (Fig. 7B) and the AGE-mediated cleavage of caspase 3 was potentiated (Fig. 7C). Pretreatment with bafilomycin, a known inhibitor of autophagosomal degradation (39), for 3 h before the completion of AGE stimulation inhibits degradation of autophagosome-potentiated AGE-mediated cell death as detected by morphology (Fig. 7D) and as measured by MTT assay (Fig. 7E). These data suggest that AGE induces autophagosome formation and that this leads to partial protection from AGE-mediated cell death.
FIGURE 7.
Effect of AGE in inducing autophagy. A, U-937 cells were incubated with 100 μg/ml AGE-HSA for 48 h. Cells were washed and incubated with MDC (0.05 mm) for 10 min. The fluorescent cells were visualized under a fluorescence microscope. U-937 cells were pretreated with 3-methyl adenine (3MA, 5 mm) and then incubated with AGE-HSA (100 μg/ml) for 48 h. After incubation, cells were washed and incubated with MDC. B, intracellular MDC was measured by fluorescence photometry (excitation 380 nm and emission 525 nm). RFU, relative fluorescence units. C, the MDC incorporated was expressed as specific activity (arbitrary units). U-937 cells were pretreated with 3-methyl adenine (5 mm) and then incubated with AGE-HSA for 48 h. D, the cells were observed under bright field microscopy. U-937 cells, incubated with AGE-HSA (100 μg/ml) for 45 h, were co-incubated with bafilomycin (100 nm) for 3 h. E, cell viability was measured by MTT assay and indicated as inhibition of cell viability in percentage. Error bars in B and E indicate ± S.D. of triplicate samples.
DISCUSSION
Advanced glycation end products are formed due to a high amount of carbohydrates in the blood typically in diabetic patients and to some extent in aged persons. Dysfunction of several physiological functions is observed under these conditions. The question often arises about how biological functions are suppressed in aged or diabetic persons. This study provides clues that AGE-mediated cell signaling is involved. AGE treatment often causes DNA damage and oxidative stress leading to death of cells, almost 30% at 24–36 h of incubation. ROI-mediated mitochondrial membrane potential change releases cytochrome c, which activates downstream caspases such as caspase 3 and 9. Caspase 8 is also activated, a little bit earlier than caspase 3 and 9. Caspase 8 activation requires recruitment of death domain and death effector domain-containing proteins such as Fas-associated death domain (FADD), TNF receptor-associated death domain (TRADD), etc. AGE induced NF-κB activation in different cell types at variable potencies upon the amount of cell surface RAGEs. NF-κB activation was decreased in neuronal cells upon AGE treatment with more pronounced cell death, but being anti-apoptotic, less activation of NF-κB may induce more cell death. The amount of receptor proteins sometimes may not reflect biological activities mediated by a specific ligand. The affinity of receptors toward their ligands is very important for receptor-mediated biological activities. Although the amount of RAGE in neuronal cells was lower, the affinity toward AGE may be different in these cells than in other cell types, and this needs to be studied further. AGE induced IκBα degradation via activation of IκB kinase complex as IκB kinase activation is redox-sensitive. AGE is a well known inducer of ROI (30), and it also activates NF-κB and other transcription factors such as AP-1 and NF-AT, as expected. Different antioxidants inhibit AGE-induced NF-κB activation, but these have no effect on AGE-mediated cell death. This suggests that AGE-mediated ROI may be causing the activation of NF-κB and that AGE-mediated cell death is independent of ROI. AGE-induced ROI is an early event, but activation of caspases must await cytochrome c release from mitochondria, which occurs very late. The increased amount of NF-κB DNA binding was again reflected by its dependent genes, such as expression of Bax and Bad. Bax and Bad might be altering the change in mitochondrial potential that leads to cell death, which needs to be studied further.
AGE potently induces autophagosome formation, confirming previous observations (32). Autophagy leads to survival by remaining cells by removing the non-functional proteins in the cytoplasm as well as turnover of the cellular proteins. It may also cause cell death, as shown by rapid degradation of cellular proteins. However, we have shown that AGE-mediated autophagy might lead to cell survival as AGE-mediated cell death is enhanced by inhibiting autophagy upon treatment with 3-methyl adenine or bafilomycin. AGE-induced cell death is 30–40%; it may be more if autophagy is impaired. In neurodegenerative disorders, autophagy is often reported to be impaired. Although AGE-induced autophagy is protective in nature, the more pronounced cell death mechanism may lead to neurodegeneration in aged person. How AGE-mediated cell death is dominated over autophagy needs to be studied further.
Receptor-ligand interaction leads to activation of several signaling cascades and releases several preformed granular contents as a first line of defense. AGE-RAGE interaction leads to release of several proteolytic enzymes such as myeloperoxidase and alkaline phosphates but not elastase. It also releases specific granular contents such as IL-8 at an early hour of treatment. Secretory IL-8 again acts through its specific receptors, IL-8Rs. Surprisingly, IL-8-mediated cell signaling proved to be the important determinant for AGE-mediated cell death. IL-8 is a potent inducer of intracellular Ca2+ (40). AGE treatment increased intracellular Ca2+, calcineurin activity, nuclear NF-AT DNA binding activity, and FasL expression. Inhibition of Ca2+ substantially inhibited AGE-mediated cell death. Late activation of caspases further suggests the role of FasL in AGE-mediated cell signaling. Substantial abrogation of AGE-mediated cell death and NF-κB activation was observed by preincubation with anti-IL-8 Ab, affirming the role of IL-8 as an intermediate factor in AGE-mediated cell signaling. Expression of IL-8 and TNF is NF-κB-dependent, and TNF is known to induce cell death via recruitment of TNF receptor-associated death domain and procaspase 8, although TNF had no role in AGE-mediated cell death. Inhibition of calcineurin and intracellular Ca2+ level did not alter AGE-induced NF-κB activation but inhibited AGE-mediated cell death significantly.
AGE interacts with its receptors and influences the cellular structural proteins that might alter cellular behaviors such as apoptosis. TG is one of the important molecules involved in endocytosis. Cystamine inactivates TG activity by forming a mixed disulfide (17, 41). Brefeldin A, an endocytosis blocker, disrupts membrane polarity and thereby inhibits polar sorting of common endosomes (42). AGE-mediated degranulation of IL-8 has been blocked by cystamine completely, but partially blocked by brefeldin A and diltiazem. AGE-mediated degranulation of IL-8 is not inhibited by diltiazem, an inhibitor of intracellular Ca2+. Transglutaminase might be involved more in AGE-mediated signaling. TG is a therapeutic target for several neurodegenerative disorders. Cystamine, which inactivates TG, inhibits other thiol-dependent enzymes such as caspases (43) and thus plays an important role in AGE-mediated IL-8 release and cell death, which means that it might be an important therapeutic molecule in neuroprotection. Secretion of several proteolytic enzymes might also trigger AGE-mediated inflammatory responses. The events that occurred in AGE-treated cells were: calcineurin activation, NF-AT nuclear translocation, FasL expression, and cell death. We were unable to inhibit complete inhibition of FasL expression induced by AGE upon anti-IL-8 Ab incubation. Cystamine-pretreated cells showed almost complete inhibition of AGE-induced cell death. Cystamine might be inhibiting not only IL-8-mediated biological response, but also AGE-induced responses, which go through the AGE-RAGE interaction, such as IL-8-IL-8R. AGE might be inducing other biological responses, such as an increase in ROI, which may be partially inducing cell death through mitochondrial potential change. Almost 8% of cell death was observed upon cystamine treatment. Because it blocks transglutaminase, cystamine might inhibit other growth factors in the cell medium from IL-8-mediated cell growth.
Considering the deleterious effects mediated by AGE in aged and diabetic persons, the detailed mechanism of action would help to design therapeutic drugs for effective therapy against age-related diseases such as neurodegenerative diseases and diabetes. We report here for the first time that the early events mediated by AGE signaling lead to cell death proceeding through release of IL-8. IL-8-mediated cellular responses lead to activation of calcineurin, nuclear translocation of NF-AT, and expression of FasL. As an early response, AGE degranulates several proteolytic enzymes and cytokines, and these molecules induce inflammatory responses. The previous studies that dealt with the deleterious effects of AGE in cells might be answered through our findings. Thus, this study has immense importance to help understand the mechanism of action by which AGE induces its deleterious effects, such as neurodegeneration in physiological conditions, especially in diabetic and aged persons.
Acknowledgment
We thank Prof. T. Ramasarma, Distinguished Chair Professor, CDFD, for valuable suggestions.
This work was supported by a grant from the Defense Research and Development Organization (DRDO), Government of India and a core grant from the Centre for DNA Fingerprinting and Diagnostics (CDFD), a fellowship from the Indian Council for Medical Research (ICMR), Government of India (to N. R.), and a fellowship from the Council for Scientific and Industrial Research (CSIR), Government of India (to S. M).
- AGE
- advanced glycation end product
- RAGE
- receptor for AGE
- AP-1
- activator protein 1
- CsA
- cyclosporine A
- HSA
- human serum albumin
- ICAM
- intercellular cell adhesion molecule
- IκBα
- inhibitory subunit of NF-κB
- NF-κB
- nuclear transcription factor κB
- NF-AT
- nuclear transcription factor of activated T-cells
- MGO
- methyl glyoxal
- MDC
- monodansyl cadaverine
- ROI
- reactive oxygen intermediates
- TG
- transglutaminase
- Ac
- acetyl
- pNA
- para-nitroaniline
- Ab
- antibody
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- BAPTA-AM
- 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetra(acetoxymethyl) ester.
REFERENCES
- 1. Franceschi C., Bonafè M., Valensin S., Olivieri F., De Luca M., Ottaviani E., De Benedictis G. (2000) Ann. N.Y. Acad. Sci. 908, 244–254 [DOI] [PubMed] [Google Scholar]
- 2. Khuhawar M. Y., Kandhro A. J., Khand F. D. (2006) Anal. Lett. 39, 2205–2215 [Google Scholar]
- 3. Lapolla A., Flamini R., Dalla Vedova A., Senesi A., Reitano R., Fedele D., Basso E., Seraglia R., Traldi P. (2003) Clin. Chem. Lab. Med. 41, 1166–1173 [DOI] [PubMed] [Google Scholar]
- 4. Odani H., Shinzato T., Matsumoto Y., Usami J., Maeda K. (1999) Biochem. Biophys. Res. Commun. 256, 89–93 [DOI] [PubMed] [Google Scholar]
- 5. Singh R., Barden A., Mori T., Beilin L. (2001) Diabetologia 44, 129–146 [DOI] [PubMed] [Google Scholar]
- 6. Yamagishi S., Nakamura K., Matsui T. (2006) Curr. Drug Discov. Technol. 3, 83–88 [DOI] [PubMed] [Google Scholar]
- 7. Schmidt A. M., Hori O., Chen J. X., Li J. F., Crandall J., Zhang J., Cao R., Yan S. D., Brett J., Stern D. (1995) J. Clin. Invest. 96, 1395–1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bose J. S., Gangan V., Prakash R., Jain S. K., Manna S. K. (2009) J. Med. Chem. 52, 3184–3190 [DOI] [PubMed] [Google Scholar]
- 9. Yeh Y. C., Lai H. C., Ting C. T., Lee W. L., Wang L. C., Wang K. Y., Lai H. C., Liu T. J. (2007) Biochem. Pharmacol. 74, 969–980 [DOI] [PubMed] [Google Scholar]
- 10. Eom Y. W., Kim M. A., Park S. S., Goo M. J., Kwon H. J., Sohn S., Kim W. H., Yoon G., Choi K. S. (2005) Oncogene 24, 4765–4777 [DOI] [PubMed] [Google Scholar]
- 11. Barré B., Perkins N. D. (2007) EMBO J. 26, 4841–4855 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12. Sreenivasan Y., Sarkar A., Manna S. K. (2003) Oncogene 22, 4356–4369 [DOI] [PubMed] [Google Scholar]
- 13. Manna S. K., Aggarwal B. B. (1999) J. Immunol. 162, 1510–1518 [PubMed] [Google Scholar]
- 14. Manna S. K., Manna P., Sarkar A. (2007) Cell Death Differ. 14, 158–170 [DOI] [PubMed] [Google Scholar]
- 15. Clarke D. L., Sutcliffe A., Deacon K., Bradbury D., Corbett L., Knox A. J. (2008) J. Immunol. 181, 3503–3514 [DOI] [PubMed] [Google Scholar]
- 16. Manna S. K., Ramesh G. T. (2005) J. Biol. Chem. 280, 7010–7021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jeitner T. M., Delikatny E. J., Ahlqvist J., Capper H., Cooper A. J. (2005) Biochem. Pharmacol. 69, 961–970 [DOI] [PubMed] [Google Scholar]
- 18. Karpuj M. V., Becher M. W., Springer J. E., Chabas D., Youssef S., Pedotti R., Mitchell D., Steinman L. (2002) Nat. Med. 8, 143–149 [DOI] [PubMed] [Google Scholar]
- 19. Klee C. B., Ren H., Wang X. (1998) J. Biol. Chem. 273, 13367–13370 [DOI] [PubMed] [Google Scholar]
- 20. Rusnak F., Mertz P. (2000) Physiol. Rev. 80, 1483–1521 [DOI] [PubMed] [Google Scholar]
- 21. Kingsbury T. J., Cunningham K. W. (2000) Genes Dev. 14, 1595–1604 [PMC free article] [PubMed] [Google Scholar]
- 22. Abbasi S., Su B., Kellems R. E., Yang J., Xia Y. (2005) J. Biol. Chem. 280, 36737–36746 [DOI] [PubMed] [Google Scholar]
- 23. Liu S., Liu P., Borras A., Chatila T., Speck S. H. (1997) EMBO J. 16, 143–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Srivastava R. K., Sasaki C. Y., Hardwick J. M., Longo D. L. (1999) J. Exp. Med. 190, 253–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Molkentin J. D., Lu J. R., Antos C. L., Markham B., Richardson J., Robbins J., Grant S. R., Olson E. N. (1998) Cell 93, 215–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Raghavendra P. B., Sreenivasan Y., Ramesh G. T., Manna S. K. (2007) Apoptosis 12, 307–318 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27. Zhu J., Shibasaki F., Price R., Guillemot J. C., Yano T., Dötsch V., Wagner G., Ferrara P., McKeon F. (1998) Cell 93, 851–861 [DOI] [PubMed] [Google Scholar]
- 28. Crabtree G. R., Olson E. N. (2002) Cell 109, (suppl.) S67–S79 [DOI] [PubMed] [Google Scholar]
- 29. Maiuri M. C., Zalckvar E., Kimchi A., Kroemer G. (2007) Nat. Rev. Mol. Cell Biol. 8, 741–752 [DOI] [PubMed] [Google Scholar]
- 30. Mizushima N., Levine B., Cuervo A. M., Klionsky D. J. (2008) Nature 451, 1069–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Komatsu M., Ueno T., Waguri S., Uchiyama Y., Kominami E., Tanaka K. (2007) Cell Death Differ. 14, 887–894 [DOI] [PubMed] [Google Scholar]
- 32. Manna S. K., Bose J. S., Gangan V., Raviprakash N., Navaneetha T., Raghavendra P. B., Babajan B., Kumar C. S., Jain S. K. (2010) J. Biol. Chem. 285, 22318–22327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Patschan S., Goligorsky M. S. (2008) Autophagy 4, 521–523 [DOI] [PubMed] [Google Scholar]
- 34. Manna S. K., Sarkar A., Sreenivasan Y. (2006) Eur. J. Immunol. 36, 754–769 [DOI] [PubMed] [Google Scholar]
- 35. Bose J. S., Gangan V., Jain S. K., Manna S. K. (2009) J. Cell Physiol. 218, 653–662 [DOI] [PubMed] [Google Scholar]
- 36. Sarkar A., Sreenivasan Y., Ramesh G. T., Manna S. K. (2004) J. Biol. Chem. 279, 33768–33781 [DOI] [PubMed] [Google Scholar]
- 37. Biederbick A., Kern H. F., Elsässer H. P. (1995) Eur. J. Cell Biol. 66, 3–14 [PubMed] [Google Scholar]
- 38. Seglen P. O., Gordon P. B. (1982) Proc. Natl. Acad. Sci. U.S.A. 79, 1889–1892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yamamoto A., Tagawa Y., Yoshimori T., Moriyama Y., Masaki R., Tashiro Y. (1998) Cell Struct. Funct. 23, 33–42 [DOI] [PubMed] [Google Scholar]
- 40. Gangadharan C., Thoh M., Manna S. K. (2010) Breast Cancer Res. Treat. 120, 671–683 [DOI] [PubMed] [Google Scholar]
- 41. Brew K., Dinakarpandian D., Nagase H. (2000) Biochim. Biophys. Acta 1477, 267–283 [DOI] [PubMed] [Google Scholar]
- 42. Wang E., Pennington J. G., Goldenring J. R., Hunziker W., Dunn K. W. (2001) J. Cell Sci. 114, 3309–3321 [DOI] [PubMed] [Google Scholar]
- 43. Lesort M., Lee M., Tucholski J., Johnson G. V. (2003) J. Biol. Chem. 278, 3825–3830 [DOI] [PubMed] [Google Scholar]