Background: Both growth hormone (GH) and FGF21 are fasting-inducible hormones involved in modulation of lipolysis.
Results: GH injection caused a rapid elevation of circulating FGF21 in mice, and such an effect was blocked by the lipolysis inhibitor niacin. FGF21 knock-out mice exhibited greater potency in GH-induced lipolysis.
Conclusion: GH and FGF21 form a negative feedback loop to fine-tune lipolysis in adipocytes.
Significance: This study provides a novel insight on hormonal control of lipolysis.
Keywords: Fibroblast Growth Factor (FGF), Growth Hormone, Hepatocyte, Lipolysis, Metabolic Regulation, FFA, FGF21, Metabolic Hormone
Abstract
Fibroblast growth factor (FGF) 21 and growth hormone (GH) are metabolic hormones that play important roles in regulating glucose and lipid metabolism. Both hormones are induced in response to fasting and exert their actions on adipocytes to regulate lipolysis. However, the molecular interaction between these two hormones remains unclear. Here we demonstrate the existence of a feedback loop between GH and FGF21 on the regulation of lipolysis in adipocytes. A single bolus injection of GH into C57 mice acutely increases both mRNA and protein expression of FGF21 in the liver, thereby leading to a marked elevation of serum FGF21 concentrations. Such a stimulatory effect of GH on hepatic FGF21 production is abrogated by pretreatment of mice with the lipolysis inhibitor niacin. Direct incubation of either liver explants or human HepG2 hepatocytes with GH has no effect on FGF21 expression. On the other hand, FGF21 production in HepG2 cells is significantly induced by incubation with the conditioned medium harvested from GH-treated adipose tissue explants, which contains high concentrations of free fatty acids (FFA). Further analysis shows that FFA released by GH-induced lipolysis stimulates hepatic FGF21 expression by activation of the transcription factor PPARα. In FGF21-null mice, both the magnitude and duration of GH-induced lipolysis are significantly higher than those in their wild type littermates. Taken together, these findings suggest that GH-induced hepatic FGF21 production is mediated by FFA released from adipose tissues, and elevated FGF21 in turn acts as a negative feedback signal to terminate GH-stimulated lipolysis in adipocytes.
Introduction
Fibroblast growth hormone 21 (FGF21)4 is an atypical member of the fibroblast growth factors (FGFs) family mainly secreted by the liver (1–4). Unlike the classical members of the FGFs family, FGF21 does not have mitogenic activities, but acts as an endocrine factor involved in the regulation of energy homeostasis, insulin sensitivity, glucose and lipid metabolism. In rodents, elevation of circulating FGF21 by either systemic infusion of the recombinant protein or transgenic expression counteracts obesity-related metabolic disorders, including hyperglycemia, dyslipidemia, insulin resistance, and fatty liver disease (4–9). A similar beneficial effect of recombinant FGF21 on glucose and lipid profiles has also been observed in rhesus monkeys with diabetes (10). While liver is the main site for FGF21 production, adipocytes appear to be the major targets for FGF21 actions. In adipocytes, FGF21 increases insulin-independent glucose uptake by inducing the expression of the glucose transporter GLUT1, and also modulates the expression of genes involved in lipolysis (11, 12). FGF21 does not have heparin binding property, and requires β-klotho as a co-receptor to augment its binding to FGF receptors (13). However, the detailed receptor and postreceptor signaling pathways underlying the metabolic actions of FGF21 remain unclear.
Similar to other metabolic hormones, circulating FGF21 levels are regulated by nutritional states and display circadian rhythm in both rodents and humans (14, 15). During fasting, hepatic FGF21 expression was markedly induced through a mechanism that involves the activation of peroxisome proliferator-activated receptor α (PPARα). A number of studies have demonstrated a critical role of FGF21 in mediating ketogenesis during adaptive starvation response (4, 16, 17). FGF21 expression is induced by thermogenic activation as well as several metabolic hormones, including thyroid hormone and insulin. Several transcription factors, including E4-binding protein 4 (E4BP4) and retinoic acid receptor-related receptor α (RORα), have been shown to transactivate the FGF21 gene in hepatocytes (18–22).
Growth hormone (GH) is released from the anterior pituitary gland and plays important roles in the regulation of multiple physiological processes including growth and metabolism (23, 24). Circulating GH is induced by fasting and exhibits diurnal changes, a phenomenon reminiscence of FGF21 (25, 26). Furthermore, GH is also an important regulator of lipolysis and ketogenesis in both humans and rodents. In response to fasting, GH is released and acts on adipose tissue to promote lipolysis, thereby leading to the elevation of FFA in the bloodstream (27–30). A previous study on FGF21 transgenic mice demonstrated that FGF21 blunts GH signaling by reducing the active form of signal transducer and activator of transcription-5 (STAT-5), which is the major mediator of GH action, which in turn leads to reduced expression of IGF-1 (31). In addition, GH resistance in patients with anorexia nervosa, a state of chronic nutritional deprivation, is also suggested to be mediated by elevated FGF21 levels (32). However, the functional relationship between these two hormones on the regulation of lipid metabolism has not been explored so far.
In this study, we tested the acute effect of GH administration on FGF21 production in mice. Our results revealed a rapid elevation of hepatic FGF21 expression and circulating FGF21 concentrations upon GH stimulation. Therefore, we further investigated the mechanism underlying GH-induced hepatic FGF21 expression and also explored the temporal association between these two hormones in the regulation of lipolysis using FGF21 knock-out mice.
EXPERIMENTAL PROCEDURES
Animal Studies
C57BL/6J male mice, 12 weeks of age, were obtained from the laboratory animal unit of the University of Hong Kong. FGF21 knock-out male mice in C57BL/6J background were described previously (11). The mice were housed in a room under controlled temperature (23 ± 1 °C) with free access to water and standard chow. Mice were fasted for 8 h, followed by intraperitoneal injection of GH (1.5 mg/kg body weight), niacin (100 mg/kg body weight), GH and niacin, or PBS as control. Serum was collected at different time points after treatment. All animal experimental protocols were approved by the Animal Ethics Committee of the University of Hong Kong.
Liver Perfusion with Collagenase and Cell Isolation
Parenchymal cells (PC) and non- parenchymal cells (NPC) were isolated by collagenase perfusion method (24). Briefly, C57 mice were anesthetized, and liver perfusion was performed with 0.02% collagenase type IV (Sigma) in Hanks' balanced salt solution through portal vein at a rate of 8 ml/min. Afterward, cells were dispersed in RPMI 1640 medium. Cell suspensions were then filtered through 100 μm nylon cell strainer (BD Falcon) to remove tissue debris and cellular aggregates. The filtrate was centrifuged at 50 × g for 1 min for collection of hepatocyte-enriched PC in the pellet, and the supernatant was then centrifuged at 600 × g for 10 min for collection of NPC.
Explant Culture of Mouse Liver and Adipose Tissue
After perfusion with collagenase as described above, perfused liver was isolated and minced into 2 mm per piece. Fifteen to twenty pieces of explants were incubated in Williams' E medium (WEM, Invitrogen, France) supplemented with 5% fetal bovine serum (FBS; Dutscher, Issy-les-Moulineaux, France) for 4 h. Medium was then replaced with WEM containing 0.5% FBS with different treatments (500 ng/ml GH, 500 μm fenofibrate or PBS as control) and incubated for 6 h in a humidified incubator (Sanyo, Osaka, Japan) at 37 °C. Both liver explants and medium were then harvested and analyzed for FGF21 mRNA expression and protein concentration, respectively.
Epididymal white adipose tissue (WAT) dissected from C57 mice was minced into ∼2 mg per piece. WAT explants were cultured in phenol red free DMEM (Invitrogen) containing 0.5% fatty acids free BSA (Sigma) and 5 mm d-glucose for 30 min, followed by treatments with GH (500 ng/ml), GH and niacin (2 mm), or isopreterenol (10 μm) for 24 h. The conditioned medium was then harvested for culturing human HepG2 cells.
Cell Culture and in Vitro Assays
Human HepG2 hepatocytes were cultured in DMEM containing 1% FBS for 16 h, followed by treatments with GH (500 ng/ml), fenofibrate (500 μm) or PBS as control. Alternatively, cells were cultured with conditioned medium from WAT explants culture prepared above in the presence or absence of GW6471 (10 μm) for 24 h. Cells and medium were then harvested and analyzed for FGF21 mRNA expression and protein concentration, respectively.
Biochemical Analysis and Immunoassay
FFA and glycerol levels in serum and conditioned medium were measured using Free Fatty Acid, Half Micro Test (Roche), and free glycerol reagent (Sigma), respectively. Tissues were homogenized in Radio Immuno Precipitation Assay (RIPA) lysis buffer using tissue lyser (Biospec, Mexico). Supernatant was collected after centrifugation, and protein concentrations were quantified by a bicinchoninic acid (BCA) protein assay kit (Pierce). Concentrations of FGF21 in serum, medium and tissues were measured using an immunoassay as described previously (14).
Real-time PCR
Total RNA was extracted from various tissues using TRIzol reagent (Invitrogen), and was subjected to reverse transcription using the Superscript first-strand cDNA synthesis system (Promega) according to the manufacturer's instruction. The relative gene abundance was quantified by real-time PCR using GreenERTM qPCR Supermix (Invitrogen, Carlsbad, CA) as described previously (34). The reactions were performed in an ABI 7000 sequence detection system. The sequences of primers are listed as supplemental Table S1. The relative gene expression was analyzed using the 2(−ΔΔCt) method and normalized against 18 S rRNA.
Statistical Analysis
All analyses were performed with the Statistical Package for Social Sciences version 14.0 (SPSS, Chicago. IL). Data are expressed as means ± S.D. Statistical significance was determined by one-way ANOVA. In all statistical comparisons, a p value <0.05 was used to indicate a statistically significant difference.
RESULTS
GH Acutely Induces Production of FGF21 in Mice
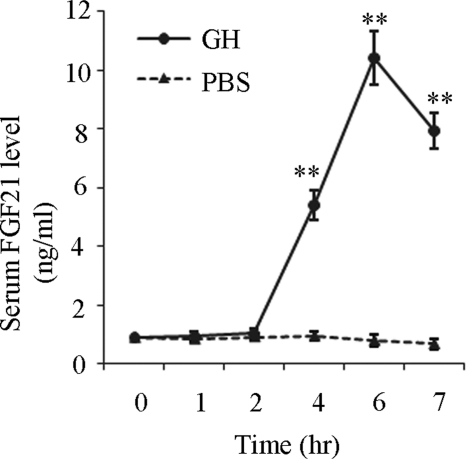
To investigate the effects of GH on FGF21 production, serum concentrations of FGF21 in C57 male mice were analyzed at various time points after receiving a single intraperitoneal injection of GH (1.5 mg/kg body weight). Circulating GH level was increased by ∼2.5-fold at 2 h after GH injection. Notably, GH induced an elevation of serum FGF21 in a time-dependent manner (Fig. 1). Compared with control mice treated with vehicle, serum FGF21 levels significantly increased by 5.6-fold after 4 h and by 10.4-fold after 6 h of GH treatment, respectively. Serum FGF21 levels were declined after 7 h of GH treatment.
FIGURE 1.
Effect of GH on the circulating concentrations of FGF21 in mouse. Sera were harvested at different time points after mice were treated with GH (1.5 mg/kg body weight) or PBS, and then subjected to FGF21 measurement using ELISA. **, p < 0.01 versus PBS group (n = 6).
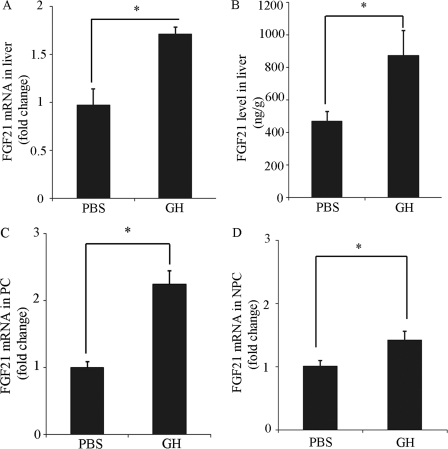
As liver is the major production site for circulating FGF21 (12), we next investigated the effect of GH on FGF21 mRNA expression and protein concentrations in this tissue using real-time PCR analysis and ELISA. The results demonstrated that both the FGF21 mRNA abundance and protein levels in the liver were significantly elevated by ∼2-fold after GH treatment (Fig. 2, A and B). Further analysis in different cell populations of the liver showed that the FGF21 mRNA abundance in hepatocyte-enriched PC fraction was elevated by 2.3-fold following GH treatment (Fig. 2C). By contrast, there was only a modest increase (1.4-fold) in the NPC fraction (Fig. 2D), suggesting that the hepatocyte is perhaps the major type of cells for FGF21 expression.
FIGURE 2.
Effect of GH on gene expression and protein levels of FGF21 in mouse liver. Livers were harvested 6 h after mice were treated with GH (1.5 mg/kg body weight) or PBS (as a vehicle control), followed by determination of FGF21 mRNA expression and protein levels using real-time PCR and ELISA. (A) relative FGF21 mRNA abundance in liver. (B) FGF21 protein concentrations in liver. Relative FGF21 mRNA abundance in (C) parenchymal cells (PC) and (D) non-parenchymal cells (NPC). *, p < 0.05 versus PBS group (n = 6).
Direct GH Stimulation Has No Effect on FGF21 Production in Liver Explants and HepG2 Cells
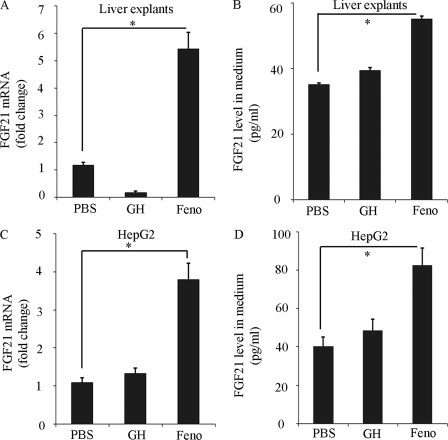
To investigate whether or not GH-induced FGF21 mRNA expression is due to its direct action on the liver, liver explants from C57 mice were treated with GH or fenofibrate for 6 h, followed by real-time PCR analysis. Unexpectedly, direct stimulation of liver explants with GH had no effect on either FGF21 mRNA expression or its protein secretion (Fig. 3, A and B). On the other hand, the PPARα agonist fenofibrate potently increased both FGF21 mRNA expression and its protein secretion in the liver explants. Consistent with ex vivo findings in liver explants, GH stimulation had little effect on either FGF21 mRNA expression (Fig. 3C) or its protein release (Fig. 3D) in HepG2 cells, despite the fact that fenofibrate significantly induced FGF21 production. Taken together, these findings suggest that GH-induced FGF21 production in mice is not due to its direct action on the liver.
FIGURE 3.
Effect of GH on FGF21 gene expression and protein secretion in liver explants and human HepG2 hepatocytes. Liver explants from C57 male mice or HepG2 cells were treated with GH (500 ng/ml), fenofibrate (Feno, 500 μm) or PBS for 6 h and subjected to (A and C) real-time PCR analysis for FGF21 gene expression. The concentrations of FGF21 protein released in the conditioned medium were analyzed by ELISA (B and D). *, p < 0.05 versus PBS group (n = 5).
GH-induced FGF21 Production Is Dependent on Lipolysis
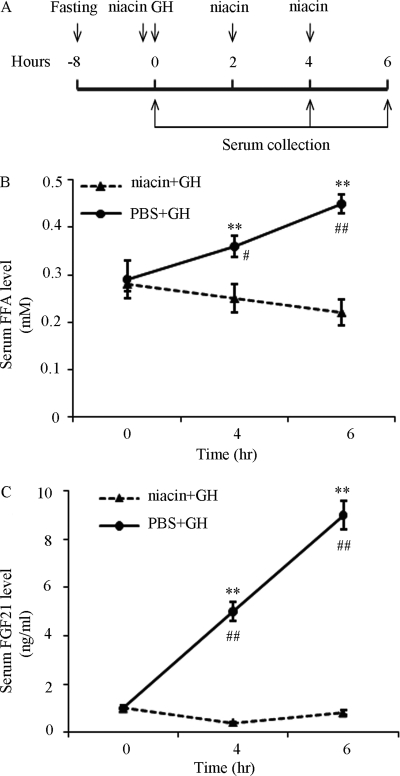
GH is a potent activator of lipolysis and increases the release of FFA in adipose tissues (29, 34, 35). Notably, FFA has been shown to induce hepatic FGF21 expression through the activation of PPARα (14, 17, 36). Therefore, we tested our hypothesis that GH induces FGF21 production through an indirect mechanism dependent on lipolysis. To this end, we evaluated the effect of the lipolysis inhibitor niacin (37) on GH-induced elevation of circulating FGF21 in mice (Fig. 4A). As expected, systemic administration of GH resulted in an elevation of circulating FFA level in mice, whereas such a stimulatory effect of GH on lipolysis was abrogated by pretreatment with niacin (Fig. 4B). Furthermore, GH-induced elevation of circulating FGF21 was abrogated by co-treatment with niacin (Fig. 4C).
FIGURE 4.
Effect of the lipolysis inhibitor niacin on GH-induced elevation of serum FGF21 in mice. Sera were collected at different time points after GH treatment (1.5 mg/kg body weight) in the presence or absence of niacin (100 mg/kg body weight) as shown in (A) and then subjected to the analysis for FFA (B) and FGF21 levels (C) by using FFA Half Micro Test and ELISA, respectively. **, p < 0.01 versus niacin+GH group at the same time point, and #, p < 0.05; ##, p < 0.01 versus PBS+GH group at 0 h, respectively (n = 6).
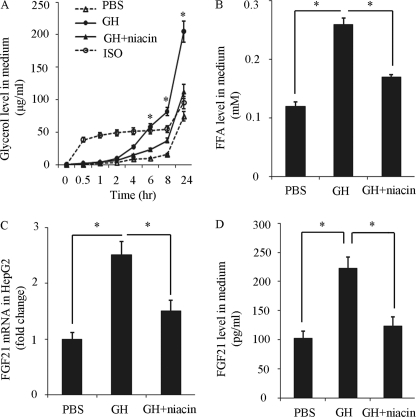
To further verify that FFA released from adipose tissues mediates GH-induced hepatic expression of FGF21, epididymal WAT explants were treated with GH in the presence or absence of niacin, and the conditioned medium was collected for incubation with HepG2 cells. Before incubation, glycerol and FFA levels in the conditioned medium were measured to confirm the action of GH on lipolysis. The result showed that glycerol levels in medium from WAT explants began to increase significantly at 6 h after GH treatment, and became most pronounced at 24 h after GH treatment, whereas the increase was prevented by pretreatment of niacin (Fig. 5A). Likewise, the FFA levels in the conditioned medium from the WAT explants also increased markedly after 24 h treatment with GH (Fig. 5B). After incubation with the conditioned medium from GH-stimulated WAT explants, both FGF21 mRNA expression and protein release in HepG2 cells were significantly increased, whereas such changes were not obvious in HepG2 cells incubated with the conditioned medium from GH-stimulated WAT explants in the presence of niacin (Fig. 5, C and D). Taken together, these findings suggest that FFA released from adipose tissue is required for GH-induced FGF21 expression in hepatocytes.
FIGURE 5.
The conditioned medium from GH-treated adipose tissue explants induces hepatic FGF21 expression in human HepG2 hepatocytes. Conditioned medium from white adipose tissue (WAT) explants culture were collected at different time points after treatment with GH (500 ng/ml), GH + niacin (2 mm), isopeterenol (10 μm, as a positive control) or PBS, and then subjected to analysis for glycerol (A) and FFA (B) (24-h treatment levels). The conditioned medium collected at 24 h after various treatments were then used to treat human HepG2 hepatocytes for another 24 h. The FGF21 mRNA expression (C) and protein secretion (D) were measured by real-time PCR and ELISA, respectively. *, p < 0.05 versus PBS group or GH + niacin group, respectively (n = 6).
PPARα Is Responsible for FGF21 Gene Induction by GH-mediated Lipolysis in Hepatocytes
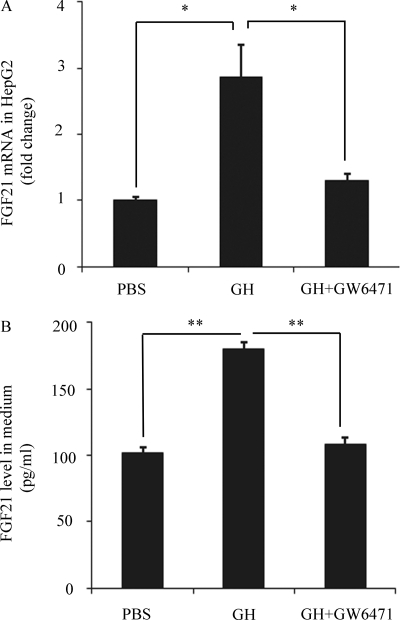
To investigate whether or not activation of PPARα is required for the induction of GH-mediated lipolysis on hepatic FGF21 expression, we evaluated the effect of the PPARα antagonist (GW6471) on GH-induced elevation of FGF21 production via lipolysis in HepG2 cells. This analysis showed that the induction of both FGF21 mRNA expression and its protein secretion by the conditioned medium from GH-stimulated WAT explants was abrogated by co-treatment with the PPARα antagonist (Fig. 6, A and B), to a level equivalent to those incubated with conditioned medium from untreated WAT explants. These findings suggest that PPARα plays an important role in the induction of hepatic FGF21 expression by GH-mediated lipolysis.
FIGURE 6.
Effect of the PPARα antagonist GW-6471 on induction of hepatic FGF21 production by the conditioned medium from GH-treated WAT explants. HepG2 cells were treated with conditioned medium from WAT explants treated with GH or PBS as in Fig. 5, in the presence or absence of the PPARα antagonist (GW6471, 10 μm) for 24 h. FGF21 mRNA expression (A) and its protein release in the conditioned medium (B) were measured as in Fig. 5. *, p < 0.05; **, p < 0.01 versus GH group (n = 5).
FGF21 Deficiency Potentiates GH-mediated Lipolysis in Mice
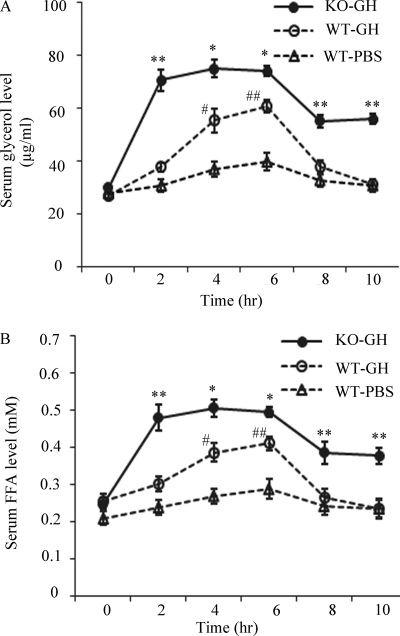
To investigate the physiological relevance of GH-induced FGF21 induction, we next investigated the impact of FGF21 deficiency on GH-induced lipolysis in mice. The GH induced lipolysis, as determined by the elevation of glycerol and FFA in serum, was much more pronounced in FGF21 knock-out mice as compared with that in wild type littermates (Fig. 7, A and B). In FGF21 knock-out mice, the circulating level of glycerol was significantly increased by 2.4-fold at 2 h after GH injection, and remained steady until 6 h. At 10 h after GH injection, the level of circulating glycerol was still 1.9-fold higher than that at the baseline. By contrast, both the magnitude and duration of GH-stimulated elevation of circulating glycerol in wild type mice were much lower than those in FGF21 knock-out mice. In wild type mice, the circulating glycerol level returned to a baseline level after 8 h of GH injection. A similar pattern of changes in circulating FFA was also observed in FGF21 knock-out mice as compared with their wild type littermates (Fig. 7B). Taken together, these findings suggest that FGF21 deficiency potentiates GH-induced lipolysis in mice.
FIGURE 7.
The impact of FGF21 deficiency on GH-induced lipolysis in mice. Sera were collected at different time points after injection of GH (1.5 mg/kg body weight) or PBS in FGF21 knock-out (KO) mice or wild type (WT) littermates, and then subjected to analysis for glycerol (A) and FFA levels (B). *, p < 0.05; **, p < 0.01 versus WT-PBS group or WT-GH group, respectively, and #, p < 0.05; ##, p < 0.01 versus WT-PBS group (n = 5).
DISCUSSION
FGF21 and GH are two important endocrine regulators of lipid metabolism. Both hormones are induced by fasting and exert their actions on adipocytes to modulate lipolysis (12, 16, 28). In the present study, we provide both ex vivo and animal evidences demonstrating that GH is a potent inducer of FGF21 production, and elevated FGF21 in turn serves as a negative signal to terminate GH-induced lipolysis in adipocytes. These findings suggest that FGF21 and GH act in concert to control the amplitude and duration of lipolysis during adaptive response to fasting.
The liver is a major target of GH, where this hormone often induces the expression of its target genes (such as IGF-I) by activating the STAT family (24, 28). Once activated, STAT proteins translocate to the nucleus and bind to response elements in the regulatory regions of its target genes for transactivation (23). However, several lines of evidence from the present study suggest that GH-induced FGF21 production is not due to the direct actions of GH on hepatocytes, but is attributed to its lipolytic activity in adipocytes, which in turn releases FFA to induce FGF21 expression in the liver. First, direct treatment of either liver explants or human HepG2 hepocytes with GH has no obvious effect on FGF21 expression and secretion, whereas the conditioned medium from GH-treated WAT recapitulates the effects of GH injection on induction of FGF21 production in mice. Second, GH-induced elevation of FGF21 production is abrogated by the lipolysis inhibitor niacin. In line with our findings, the lipolytic product FFA has been shown to induce FGF21 expression in both cultured hepatocytes and humans (14, 22, 36), by activation of PPARα (36). FFA has been postulated to account for the nocturnal rise of circulating FGF21 in man (14). In the present study, we found that the induction of hepatic FGF21 production by the conditioned medium of GH-treated WAT is diminished by pretreatment with the PPARα antagonist GW6471, further supporting the notion that FFA mediates GH-induced FGF21 production by activation of PPARα in hepatocytes.
Our finding that GH induces elevation of circulating FGF21 through FFA can explain the circadian rhythm of these metabolic factors in humans. The circadian patterns of circulating GH, FFA, and FGF21 are similar, with their peak levels at midnight during deep sleep and nadir levels at approximately noontime (14, 25, 27). Noticeably, an investigation of young healthy subjects reported that the nocturnal GH peaks preceded the rise of FFA by 2 h, suggesting that GH secretion may account for the FFA oscillation (38). On the other hand, the nocturnal rise of FGF21 is secondary to the midnight elevation of FFA in man (14). Therefore, these findings suggest that enhanced GH secretion during midnight results in an increased supply of lipid fuels by increasing FFA, thereby promoting the nocturnal rise of FGF21, which in turn terminates GH-induced lipolysis during the circadian cycle. Therefore, our results raise the possibility that GH and FGF21 may serve as a pair of Yin-Yang regulators that tightly control the circulating levels of FFA in man.
GH is a well-established lipolytic hormone in both animals and humans (24, 34). In healthy individuals, baseline FFA values often more than double with peak values recorded 2–3 h after a single bolus injection of GH (39). Under physiological conditions, GH-induced lipolysis in adipose tissue may be a major contributor to the diurnal supply of lipid fuels (29, 34, 35). On the other hand, conflicting data related to the roles of FGF21 on lipolysis has been reported (5, 9, 36). An early study in 3T3-L1 adipocytes demonstrated that chronic treatment of recombinant FGF21 increases lipolysis (33). By contrast, data obtained from both human adipocytes and in mice showed the inhibitory effects of FGF21 on lipolysis (12). A recent study on FGF21 knock-out mice suggests that FGF21 stimulates lipolysis in the white adipose tissue during feeding but inhibits it during fasting (11). In the present study, we found that the lack of FGF21 results in a marked potentiation of GH-induced lipolysis, thus supporting an anti-lipolytic role of FGF21 under this condition. A recent report showing that transgenic overexpression of FGF21 may induce GH resistance by blocking GH-induced IGF-I production in hepatocytes (31). Furthermore, in patients with anorexia nervosa (a state of chronic nutritional deprivation), circulating FGF21 is positively correlated with GH, but inversely with IGF-I, suggesting that elevated FGF21 is an important contributor to GH resistance in humans (32). Our findings would suggest that, in addition to an effect on GH signaling, FGF-21 can also lead to GH resistance through counteracting the action of GH on lipolysis.
The suppressive effects of FGF21 on GH-induced lipolysis may account for the beneficial effects of FGF21 on alleviation of insulin resistance and hyperglycemia observed in animal models (7, 9). GH induces insulin resistance mainly by promoting lipolysis, thereby releasing FFA from adipocytes to liver and muscle where it impedes insulin signaling and enhances gluconeogenesis (29, 36). The definitive evidence of the role of FFA in GH-mediated insulin resistance was obtained in clinical studies in which the effects of exogenously administered GH on insulin resistance were abrogated by the lipolysis inhibitor acipimox (35, 40). In both rodents and rhesus monkey, recombinant FGF21 has been shown to attenuate obesity-induced hyperinsulinemia, hyperglycemia and insulin resistance (5, 10). However, FGF21 has no direct effect on insulin signaling in either hepatocytes or skeletal muscle cells. Taken together, these findings suggest that the insulin-sensitizing effect of FGF21 is attributed to its ability in inhibiting the excessive elevation of circulating FFA induced by the lipolytic hormones such as GH.
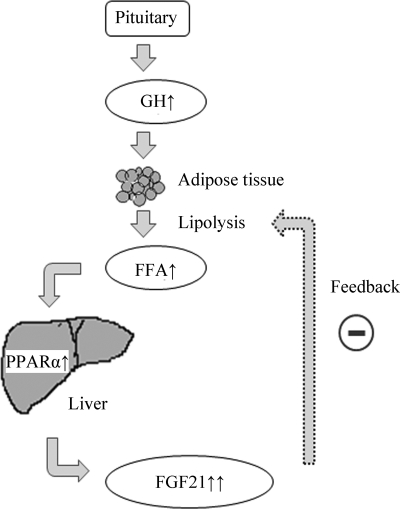
In summary, the present study has identified a feedback loop between GH and FGF21 on modulation of lipolysis in adipocytes (Fig. 8). GH stimulates lipolysis, leading to the release of FFA from adipocytes to enhance hepatic FGF21 production through the activation of PPARα. Elevated FGF21 in turn functions as a negative feedback signal to terminate GH-induced lipolysis. Such a feedback regulation may represent an important mechanism in fine-tuning lipolysis and lipid homeostasis, and may also explain in part the multiple beneficial effects of FGF21 on obesity-related metabolic disorders.
FIGURE 8.
A proposed feedback model whereby GH and FGF21 fine-tune lipolysis in adipocytes. During fasting, the secretion of growth hormone from the somatotropes in anterior pituitary is increased, leading to the induction of lipolysis in white adipose tissue. The lipolysis products including FFA induce FGF21 gene expression and its protein secretion in the liver, which in turn serves as a negative feedback signal to terminate lipolysis initiated by GH in adipocytes.
Supplementary Material
This work was supported by the Collaborative Research Fund (HKU3/CRF/09), from the Research Grant Council of Hong Kong, seeding fund for basic research and the matching fund for the National Basic Research Program of China (Project no: 2011CB504004) from the University of Hong Kong.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table S1.
- FGF
- fibroblast growth hormone
- PPARα
- peroxisome proliferator-activated receptor α
- FFA
- free fatty acid
- WAT
- white adipose tissue
- GH
- growth hormone.
REFERENCES
- 1. Nishimura T., Nakatake Y., Konishi M., Itoh N. (2000) Biochim. Biophys. Acta 1492, 203–206 [DOI] [PubMed] [Google Scholar]
- 2. Kharitonenkov A., Shanafelt A. B. (2009) Curr. Opin. Investig. Drugs. 10, 359–364 [PubMed] [Google Scholar]
- 3. Kharitonenkov A., Shiyanova T. L., Koester A., Ford A. M., Micanovic R., Galbreath E. J., Sandusky G. E., Hammond L. J., Moyers J. S., Owens R. A., Gromada J., Brozinick J. T., Hawkins E. D., Wroblewski V. J., Li D. S., Mehrbod F., Jaskunas S. R., Shanafelt A. B. (2005) J. Clin. Invest. 115, 1627–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Badman M. K., Pissios P., Kennedy A. R., Koukos G,., Flier J. S., Maratos-Flier E. (2007) Cell Metab. 5, 426–437 [DOI] [PubMed] [Google Scholar]
- 5. Coskun T., Bina H. A., Schneider M. A., Dunbar J. D., Hu C. C., Chen Y., Moller D. E., Kharitonenkov A. (2008) Endocrinology 149, 6018–6027 [DOI] [PubMed] [Google Scholar]
- 6. Xu J., Lloyd D. J., Hale C., Stanislaus S., Chen M., Sivits G., Vonderfecht S., Hecht R., Li Y. S., Lindberg R. A., Chen J. L., Jung D. Y., Zhang Z., Ko H. J., Kim J. K., Véniant M. M. (2009) Diabetes 58, 250–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xu J., Stanislaus S., Chinookoswong N., Lau Y. Y., Hager T., Patel J., Ge H., Weiszmann J., Lu S. C., Graham M., Busby J., Hecht R., Li Y. S., Li Y., Lindberg R. A., Véniant M. M. (2009) Am. J. Physiol. Endocrinol. Metab. 297, E1105–E1114 [DOI] [PubMed] [Google Scholar]
- 8. Murata Y., Konishi M., Itoh N. (2011) J. Nutr. Metab. 2011, 981315–981323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cuevas-Ramos D., Almeda-Valdes P., Aguilar-Salinas C. A., Cuevas-Ramos G., Cuevas-Sosa A. A., Gomez-Perez F. J. (2009) Curr. Diabetes Rev. 5, 216–220 [DOI] [PubMed] [Google Scholar]
- 10. Kharitonenkov A., Wroblewski V. J., Koester A., Chen Y. F., Clutinger C. K., Tigno X. T., Hansen B. C., Shanafelt A. B., Etgen G. J. (2007) Endocrinology 148, 774–781 [DOI] [PubMed] [Google Scholar]
- 11. Hotta Y., Nakamura H., Konishi M., Murata Y., Takagi H., Matsumura S., Inoue K., Fushiki T., Itoh N. (2009) Endocrinology 50, 4625–4633 [DOI] [PubMed] [Google Scholar]
- 12. Arner P., Pettersson A., Mitchell P. J., Dunbar J. D., Kharitonenkov A., Rydén M. (2008) FEBS Lett. 582, 1725–1730 [DOI] [PubMed] [Google Scholar]
- 13. Yie J., Hecht R., Patel J., Stevens J., Wang W., Hawkins N., Steavenson S., Smith S., Winters D., Fisher S., Cai L., Belouski E., Chen C., Michaels M. L., Li Y. S., Lindberg R., Wang M., Véniant M., Xu J. (2009) FEBS Lett. 583, 19–24 [DOI] [PubMed] [Google Scholar]
- 14. Yu H., Xia F., Lam K. S., Wang Y., Bao Y., Zhang J., Gu Y., Zhou P., Lu J., Jia W., Xu A. (2011) Clin. Chem. 57, 691–700 [DOI] [PubMed] [Google Scholar]
- 15. Oishi K., Uchida D., Ishida N. (2008) FEBS Lett. 582, 3639–3642 [DOI] [PubMed] [Google Scholar]
- 16. Inagaki T., Dutchak P., Zhao G., Ding X., Gautron L., Parameswara V., Li Y., Goetz R., Mohammadi M., Esser V., Elmquist J. K., Gerard R. D., Burgess S. C., Hammer R. E., Mangelsdorf D. J., Kliewer S. A. (2007) Cell Metab. 5, 415–425 [DOI] [PubMed] [Google Scholar]
- 17. Gälman C., Lundåsen T., Kharitonenkov A., Bina H. A., Eriksson M., Hafström I., Dahlin M., Amark P., Angelin B., Rudling M. (2008) Cell Metab. 8, 169–174 [DOI] [PubMed] [Google Scholar]
- 18. Hondares E., Iglesias R., Giralt A., Gonzalez F. J., Giralt M., Mampel T., Villarroya F. (2011) J. Biol. Chem. 286, 12983–12990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tong X., Muchnik M., Chen Z., Patel M., Wu N., Joshi S., Rui L., Lazar M. A., Yin L. (2010) J. Biol. Chem. 285, 36401–36409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang Y., Solt L. A., Burris T. P. (2010) J. Biol. Chem. 285, 15668–15673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Adams A. C., Astapova I., Fisher F. M., Badman M. K., Kurgansky K. E., Flier J. S., Hollenberg A. N., Maratos-Flier E. (2010) J. Biol. Chem. 285, 14078–14082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mai K., Bobbert T., Groth C., Assmann A., Meinus S., Kraatz J., Andres J., Arafat A. M., Pfeiffer A. F., Möhlig M., Spranger J. (2010) Am. J. Physiol. Endocrinol. Metab. 299, E126–E130 [DOI] [PubMed] [Google Scholar]
- 23. Herrington J., Smit L. S., Schwartz J., Carter-Su C. (2000) Oncogene 19, 2585–2597 [DOI] [PubMed] [Google Scholar]
- 24. LeRoith D., Yakar S. (2007) Nat. Clin. Pract. Endocrinol. Metab. 3, 302–310 [DOI] [PubMed] [Google Scholar]
- 25. Weitzman E. D. (1976) Annu. Rev. Med. 27, 225–243 [DOI] [PubMed] [Google Scholar]
- 26. Friend K., Iranmanesh A., Login I. S., Veldhuis J. D. (1997) Eur. J. Endocrinol. 137, 377–386 [DOI] [PubMed] [Google Scholar]
- 27. Møller N., Jørgensen J. O., Schmitz O., Møller J., Christiansen J., Alberti K. G., Orskov H. (1990) Am. J. Physiol. 258, E86–E91 [DOI] [PubMed] [Google Scholar]
- 28. Vijayakumar A., Novosyadlyy R., Wu Y., Yakar S., LeRoith D. (2010) Growth Horm. IGF Res. 20, 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nielsen S., Møller N., Christiansen J. S., Jørgensen J. O. (2001) Diabetes 50, 2301–2308 [DOI] [PubMed] [Google Scholar]
- 30. Gravhølt C. H., Schmitz O., Simonsen L., Bülow J., Christiansen J. S., Møller N. (1999) Am. J. Physiol. 277, E848–E854 [DOI] [PubMed] [Google Scholar]
- 31. Inagaki T., Lin V. Y., Goetz R., Mohammadi M., Mangelsdorf D. J., Kliewer S. A. (2008) Cell Metab. 8, 77–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fazeli P. K., Misra M., Goldstein M., Miller K. K., Klibanski A. (2010) J. Clin. Endocrinol. Metab. 95, 369–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang X., Yeung D. C., Karpisek M., Stejskal D., Zhou Z. G., Liu F., Wong R. L., Chow W. S., Tso A. W., Lam K. S., Xu A. (2008) Diabetes 57, 1246–1253 [DOI] [PubMed] [Google Scholar]
- 34. Segerlantz M., Bramnert M., Manhem P., Laurila E., Groop L. C. (2003) European J. Endocrinol. 149, 511–519 [DOI] [PubMed] [Google Scholar]
- 35. Segerlantz M., Bramnert M., Manhem P., Laurila E., Groop L. C. (2001) J. Clin. Endocrinol. Metab. 86, 5813–5818 [DOI] [PubMed] [Google Scholar]
- 36. Mai K., Andres J., Biedasek K., Weicht J., Bobbert T., Sabath M., Meinus S., Reinecke F., Möhlig M., Weickert M. O., Clemenz M., Pfeiffer A. F., Kintscher U., Spuler S., Spranger J. (2009) Diabetes 58, 1532–1538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Walters R. W., Shukla A. K., Kovacs J. J., Violin J. D., DeWire S. M., Lam C. M., Chen J. R., Muehlbauer M. J., Whalen E. J., Lefkowitz R. J. (2009) J. Clin. Invest. 119, 1312–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rosenthal M. J., Woodside W. F. (1988) Metabolism. 37, 645–648 [DOI] [PubMed] [Google Scholar]
- 39. Moller N., Vendelbo M. H., Kampmann U., Christensen B., Madsen M., Norrelund H., Jorgensen J. O. (2009) Clin. Nutr. 28, 597–603 [DOI] [PubMed] [Google Scholar]
- 40. Nørrelund H., Nair K. S., Nielsen S., Frystyk J., Ivarsen P., Jørgensen J. O., Christiansen J. S., Møller N. (2003) J. Clin. Endocrinol. Metab. 88, 4371–4378 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.