Abstract
Autotaxin (ATX) is a secreted lysophospholipase D that generates the bioactive lipid mediator lysophosphatidic acid (LPA). We and others have reported that ATX binds to integrins, but the function of ATX-integrin interactions is unknown. The recently reported crystal structure of ATX suggests a role for the solvent-exposed surface of the N-terminal tandem somatomedin B-like domains in binding to platelet integrin αIIbβ3. The opposite face of the somatomedin B-like domain interacts with the catalytic phosphodiesterase (PDE) domain to form a hydrophobic channel through which lysophospholipid substrates enter and leave the active site. Based on this structure, we hypothesize that integrin-bound ATX can access cell surface substrates and deliver LPA to cell surface receptors. To test this hypothesis, we investigated the integrin selectivity and signaling pathways that promote ATX binding to platelets. We report that both platelet β1 and β3 integrins interact in an activation-dependent manner with ATX via the SMB2 domain. ATX increases thrombin-stimulated LPA production by washed platelets ∼10-fold. When incubated under conditions to promote integrin activation, ATX generates LPA from CHO cells primed with bee venom phospholipase A2, and ATX-mediated LPA production is enhanced more than 2-fold by CHO cell overexpression of integrin β3. The effects of ATX on platelet and cell-associated LPA production, but not hydrolysis of small molecule or detergent-solubilized substrates, are attenuated by point mutations in the SMB2 that impair integrin binding. Integrin binding therefore localizes ATX activity to the cell surface, providing a mechanism to generate LPA in the vicinity of its receptors.
Keywords: Autotaxin, Lysophosphatidic Acid
Introduction
Lysophosphatidic acid (1-acyl 2-hydroxylglycerol 3-phosphate (LPA))3 is a bioactive lipid that binds to cell surface G-protein-coupled receptors to regulate cell growth, differentiation, apoptosis, and development (1). In the cardiovascular system, LPA alters endothelial barrier function (2–4), modulates the phenotype of vascular smooth muscle cells (5), and is a weak platelet agonist (4, 6). Genetic and pharmacological approaches identify a role for LPA signaling in experimental models of vascular injury and atherosclerosis and suggest that responses of vascular cells to LPA observed in vitro are important in vivo (7–9). LPA, predominantly bound to serum albumin, is present in plasma at 0.5–1.0 μm (10, 11). Plasma LPA is generated by hydrolysis of circulating lysophosphatidylcholine (LPC) catalyzed by the secreted lysophospholipase D, autotaxin (ATX) (12). ATX activity is required to sustain plasma LPA levels in the face of rapid elimination of this lipid from the circulation (13, 14). ATX potently stimulates the growth and migration of many cell types and is required for vascular development in mice. These functions clearly involve the production and actions of LPA; however, mechanisms regulating ATX activity to produce localized or cell-specific signaling responses are not well understood.
ATX belongs to a family of enzymes with ectonucleotide pyrophosphatase/phosphodiesterase activity (15). Among these ectonucleotide pyrophosphatase/phosphodiesterase activities, ATX (designated ENPP2) is unique in that it retains the characteristic activity of this class of enzymes against water-soluble nucleotide substrates but is also a lysophospholipase D (12, 16). ATX is a multidomain protein with tandem N-terminal somatomedin B-like (SMB1,2) domains, a catalytic phosphodiesterase domain (PDE), and a nuclease-like domain (15). The recently reported crystal structure of ATX reveals tight and extensive interactions between the PDE and nuclease-like domains and weaker interactions between the two SMB domains and the PDE domain. An extended hydrophobic substrate binding channel leading to the active site is formed in large part by the interface between the SMB and PDE domains. This channel appears to have arisen by deletion of sequences that are present in other ectonucleotide pyrophosphatase/phosphodiesterase activity proteins and, based on mutational studies and co-crystal structures, accounts for the unique selectivity of ATX for lipid versus small molecule substrates (17, 18). The introduction of mutations that block access to the channel reduces the ability of ATX to promote cell migration. These observations led to a proposed model in which the channel provides a conduit for cell surface localized generation of LPA and possibly also delivery of ATX-generated LPA to its G-protein-coupled receptors (18, 19).
Several lines of evidence implicate platelets as important participants in LPA production in the circulation in some settings. LPA levels in serum prepared from platelet-rich plasma are ∼5–10-fold higher than in platelet-poor plasma (20–22), which suggests that activated platelets play an active role in LPA production during clotting. Experimental induction of thrombocytopenia in rats, using an anti-platelet antibody, decreases the production of LPA in serum by almost 50% (20). Similarly, treatment of mice with a small molecule inhibitor of the platelet fibrinogen receptor, integrin αIIbβ3, that causes thrombocytopenia significantly decreases circulating LPA (23). Although platelets themselves are clearly not a major source of ATX in the plasma, these observations raise the possibility that activated platelets or perhaps microparticles formed by activated platelets are a source of LPC substrate for ATX. Recent work identified acyl-protein thioesterase 1 (APT1) as being released during thrombin activation of platelets and is essential for platelet LPA production. The PLA1 activity of APT1 generates sn-2 LPC on the outer surface of the membrane bilayer, which is proposed to undergo acyl migration to sn-1 LPC, and serves as a substrate for ATX (24). This process would necessitate that ATX localizes to the platelet surface. However, the mechanisms involved in cell localization of ATX and their importance for ATX-catalyzed production of LPA are presently unclear.
Activated integrins have previously been suggested to interact with ATX. ATX associates with activated platelets and accumulates in arterial thrombi through interactions with platelet integrin αIIb β3 (11), and evidence for binding of ATX to additional integrin receptors on lymphocytes (25) and oligodendrocytes (26) has also been presented. Integrin activation could therefore serve as a mechanism to localize the recruitment ATX and spatially and temporally regulate LPA production. In this study we investigate integrin selectivity of ATX binding to platelets and mammalian cells and the regulation of this process by cell signaling pathways. Using antibody and small molecule inhibitors of the ATX integrin interaction and newly developed ATX mutants with impaired integrin binding, we demonstrate that recruitment of ATX to the surface of platelets and mammalian cells is a mechanism for localized LPA production.
EXPERIMENTAL PROCEDURES
Isolation of Recombinant ATX and ATX-derived Fragments
cDNAs encoding wild type ATX, catalytically inactive ATX-T210A, an N-terminal ATX fragment containing amino acids 53–143, and various site-directed mutants were generated using standard methods inserted into a variant of pSecTag (Invitrogen) that was engineered for compatibility with the Gateway cloning system. Proteins were expressed by liposome-mediated transfection of these constructs in suspension cultures of Chinese hamster ovary (CHO) cells. Recombinant proteins were purified from filtration-concentrated CHO cell culture medium by nickel chelation chromatography as described previously (11).
Preparation of Platelets
Platelets were prepared using minor modifications of previously described methods (11). Citrated human blood was centrifuged at 450 × g for 5 min to yield platelet-rich plasma, to which 134 nm prostaglandin I2 was added. Platelets were separated from plasma by filtration of platelet-rich plasma, using a column of Sepharose 2B (Sigma) equilibrated in HEPES-buffered modified Tyrode's buffer (138 mm NaCl, 5.6 mm dextrose, 2.7 mm KCl, 10 mm HEPES, 12 mm NaHCO3, 0.36 mm NaH2PO4, pH 7.35) with 0.35% fatty acid-free bovine serum albumin (BSA; US Biological). For adhesion assays, platelet-rich plasma was incubated with 7 μm calcein AM for 30 min at 37 °C prior to gel filtration. Unless otherwise indicated, gel-filtered platelets were diluted to 200,000/μl in Tyrode's buffer containing fatty acid-free (ethanol and charcoal-extracted) BSA (Sigma).
Static Platelet/Cell Adhesion Assay
Recombinant wild type ATX, ATX variants, or fibrinogen (American Diagnostica) diluted in Tris buffer (50 mm Tris, 100 mm NaCl, pH 7.4) were incubated in wells of a black polystyrene 96-well plate (Nunc) overnight at 4 °C. The wells were subsequently incubated for 1 h at room temperature with Tyrode's buffer containing BSA to block nonspecific binding sites. Calcein-labeled platelets or CHO cells (see above) were incubated for 1 h at 37 °C; nonadherent platelets were removed by washing Tyrode's containing BSA and 2 mm Ca2+ and 1 mm Mg2+, or Mn2+, as indicated. The number of adherent platelets was determined by measuring fluorescence (excitation and emission = 494/517 nm) and reference to a standard curve generated by fluorescence measurements, using independently quantitated numbers of platelets. For analysis of platelet spreading and morphology, platelets were diluted to 20,000/μl, and adhesion was performed on LabTek2 coverslips. Adherent platelets were fixed with 4% paraformaldehyde, permeabilized, and stained with TRITC-phalloidin.
Radioiodination of ATX
ATX was radiolabeled using iodine-125 (125I) monochloride. 400–500 μg of ATX was incubated with 2 m glycine and 0.231 μmol of iodine monochloride/mg protein for 5 min on ice. 125I-ATX was dialyzed overnight at 4 °C against PBS. Ninety five to 99% of the 125I associated with the ATX was precipitated by trichloroacetic acid. 125I-ATX was combined with platelets in the absence or presence of agonists and incubated at room temperature. Platelets were centrifuged at 16,000 × g for 30 s through 30% sucrose, and the amount of radioactivity associated with the platelet pellet determined (PerkinElmer Life Sciences, Packard Cobar II Auto-Gamma).
CHO Cells
CHO cells stably expressing human β3 integrin or αIIbβ3 were a generous gift from Zhenyu Li (University of Kentucky). For binding experiments, these cells were labeled with calcein using minor modifications of the method described for the platelets above. For measurements of LPA production by ATX, CHO cells were incubated with bee venom PLA2 (Sigma) at concentrations of 0–10 μg/ml for up to 60 min.
Determination of lyso-PLD activity was performed using detergent-solubilized substrates. ATX activity against 18:0 LPC was determined using a spectrophotometric coupled enzymatic assay with minor adaptations for use in a microplate reader (12).
Measurement of LPA and LPC
The lyso-PLD activity of ATX was measured in the absence and presence of platelets, stably transfected CHO cells, and purified integrins. We also measured LPC in some experiments. Lipids were extracted using acidified organic solvents as described previously. When measuring changes in endogenous lipids, the unnatural lipids 17:0 LPA or 17:0 LPC were added as an internal standard. In cases where production of C17 LPA by ATX-catalyzed hydrolysis of C17 LPC was measured, C17 sphingosine 1-phosphate was substituted as the recovery standard. The organic phase from these extractions was recovered, evaporated to dryness, and reconstituted in 100 μl of methanol. Lipids (generally 10 μl of each sample) were separated by reverse phase HPLC on an Agilent Zorbax C8 column and quantified by tandem mass spectrometry using an ABI 4000 Q-Trap hybrid linear ion trap triple quadrupole mass spectrometer. The mass spectrometer was operated in selective reaction monitoring mode to measure lipid species-specific precursor product ion pairs, with quantification accomplished by reference to calibration curves generated using synthetic standards obtained from Avanti Polar lipids that were independently quantitated by phosphorous analysis as described previously (13).
RESULTS
Synergistic Effect of Platelet Activation and ATX on LPA Generation
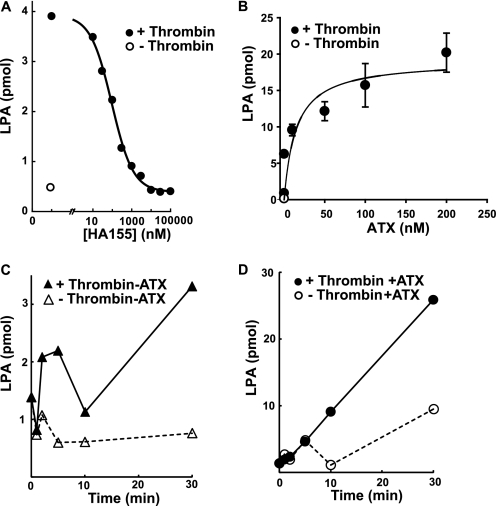
The central role of ATX in production of circulating bioactive LPA has been established by a combination of genetic and pharmacological approaches. Very little is known about physiological mechanisms that may be responsible for regulating LPA production by ATX. Several observations suggest that platelets may serve as a useful model system to study regulation of ATX activity. Isolated platelets produce LPA after agonist stimulation in a process that appears to involve platelet release of APT1 and APT1-catalyzed generation of LPC, which presumably serves as a substrate for ATX. To confirm the role of ATX in platelet LPA generation, we tested the effects of a recently described potent small molecular inhibitor of ATX (13). A maximally effective dose of thrombin (0.5 units/ml) increased the LPA content of isolated platelets ∼6-fold after 30 min. The thrombin-mediated increase in platelet-derived LPA was completely attenuated in a dose-dependent manner by the ATX inhibitor HA155 (Fig. 1A). Platelets contain immunologically detectable ATX (11), so these results indicate that ATX likely accounts for the previously reported ability of isolated human platelets to generate LPA in response to agonists (20, 28). Remarkably, thrombin-stimulated LPA generation by isolated platelets was dramatically increased when recombinant ATX was added to these incubations. The increase in LPA production was dependent on the concentration of added ATX with a half-maximal effect observed at concentrations of ∼100 nm (Fig. 1B), which is in the range of the concentration of ATX reported in human plasma (29). When incubated with a maximally effective concentration of ATX, LPA generation by platelets was sustained at times up to 20 min (Fig. 1, C and D). The time course of LPA in the absence of added ATX or in response to a maximally effective concentration of ATX in the absence of agonist was biphasic, which may reflect the previously reported ability of platelets to degrade LPA (Fig. 1, C and D) (30). The low concentration of delipidated BSA in these incubations contained trace levels of lysophospholipids, including LPC, that were not sufficient to sustain the quantities of LPA being formed. LPC was present in unactivated platelets (∼0.2 nmol/106 platelets) and, presumably as a result of the recently described PLA1 activity of APT1 (24), increased ∼2-fold after stimulation with thrombin for 30 min in the presence of the ATX inhibitor. Taken together, our observations of sustained, amplified LPA production by thrombin-stimulated platelets in the presence of ATX suggest under these conditions ATX is acting on platelet-generated LPC, where substrate supply to the enzyme is not a rate-limiting determinant of LPA production.
FIGURE 1.
Synthesis of LPA by thrombin-stimulated platelets is ATX-dependent and enhanced by exogenous ATX. A, gel-filtered platelets were treated with vehicle (○) or 0.5 units/ml thrombin (●) in the presence of the indicated concentrations of the ATX inhibitor HA155 for 30 min, and LPA was quantitated as described under “Experimental Procedures.” B, LPA production by gel-filtered platelets was determined in the presence of vehicle (○) or 0.5 units/ml thrombin (●) in the presence of the indicated concentrations of purified ATX and LPA quantitated as described under “Experimental Procedures.” C and D, LPA production by gel-filtered platelets was determined at the indicated times in incubations containing no added ATX and vehicle (△) or 0.5 units/ml thrombin (▴) or 100 nm ATX and vehicle (○) or 0.5 units/ml thrombin (●). Data shown are means of duplicate determinations (A, C, and D) or means ± S.E. of triplicate determinations (B) from representative experiments that have been repeated using multiple independent preparations of platelets.
Signaling Pathways Regulating Binding of Platelet Integrins to ATX
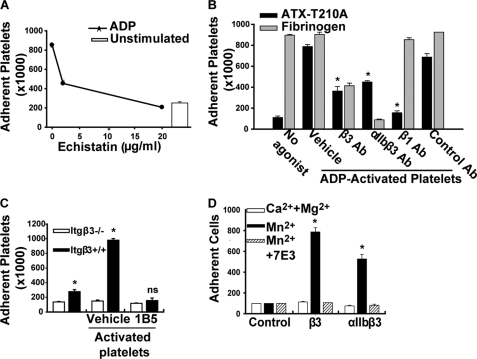
The ability of ATX to interact with integrin receptors could provide a mechanism for localizing the enzyme along the platelet surface. We therefore characterized ATX-platelet integrin interactions in more detail. LPA is a weak platelet agonist. To avoid confounding effects of ATX-generated LPA, we used a catalytically inactive mutant ATX-T210A to investigate agonist-dependent signaling pathways that regulate binding to platelets. Our earlier studies demonstrated that an antibody inhibitor of β3 integrins blocked platelet adhesion to ATX (11). In agreement, echistatin, an RGD-containing peptide that competitively inhibits ligand binding to β1 and β3 integrins, dose-dependently reduced platelet adhesion to ATX-T210A to levels observed in unstimulated platelets (Fig. 2A). To investigate the possibility that ATX-T210A can bind non-β3 integrins on activated platelets, we used integrin function-blocking antibodies and compared responses to that of 7E3, an antibody inhibitor of β3 integrins that reduced adhesion by 79 ± 6% (n = 8). Monoclonal antibodies to integrin αIIbβ3 (10E5) and to β1 integrins (P4C10) also reduced adhesion of platelets to ATX-T210A by 65 ± 13% (n = 4) and 87 ± 6% (n = 2), respectively (Fig. 2B). The specificity of P4C10 was confirmed by the lack of effect on fibrinogen binding to platelet β3 integrins. LM609, a function-blocking αVβ3 antibody, had variable effects on the adhesion of platelets from three different donors to ATX-T210A, ranging from 1 to 62% inhibition (median inhibition 22%), which may reflect low and variable levels of platelet αVβ3 expression in platelets from different donors. Binding to ATX-T210A was substantially, but not completely, impaired in platelets from mice lacking β3 integrin (Itgβ3−/−). Murine platelet adhesion was inhibited by 1B5, a hamster monoclonal antibody to αIIbβ3 (Fig. 2C). Taken together, our results indicate that the association of ATX with platelets requires integrin activation and occurs through β3 and β1 integrins.
FIGURE 2.
Platelet adhesion to ATX requires β1 and β3 integrins. A, platelet adhesion to microtiter wells with immobilized ATX-T210A without (open bar) or with 10 μm ADP (●) and the indicated concentrations of echistatin, an RGD-containing disintegrin. B, results obtained with platelets from one donor incubated with wells containing immobilized ATX (dark bars) or fibrinogen (gray bars; immobilized at 10 μg/ml) in the presence of 10 μm ADP and 20 μg/ml of a function-blocking integrin β3 antibody (7E3), a function-blocking αIIbβ3 antibody (10E5), a function-blocking integrin β1 antibody (P4C10), or a nonfunction-blocking integrin antibody (23C6). C, platelets were isolated from wild type (Itgβ+/+) or Itgβ3−/− mice and incubated (200,000/μl) with wells containing immobilized ATX in the presence of 150 μm PAR4-activating peptide. Results are representative of those obtained in three separate experiments. *, p < 0.05 versus Itgβ+/+ platelets, two-way ANOVA; ns = not significant). D, calcein-labeled CHO cells (300 cells/μl) (control) or CHO cells that were stably expressing β3 or αIIbβ3 were incubated with immobilized ATX in the presence of 2 mm CaCl2 and 1 mm MgCl2 (open bars), 50 μm Mn2 (black bars), or 50 μm Mn2 and 20 mg/ml of the function-blocking integrin β3 antibody 7E3 (hatched bars). (*, p < 0.05 versus control CHO cells, ANOVA.) Results are presented as the number of adherent platelets or CHO cells (mean ± S.D.) from triplicate determinations.
To determine whether β3 integrins were sufficient for supporting cell adhesion to ATX, we examined the effect of expressing the integrin β3 subunit alone or in combination with αIIb on CHO cell adhesion to ATX-T210A. Although the parental CHO cell line did not adhere significantly to ATX, CHO cells expressing β3 integrins acquired the ability to interact with ATX-T210A in the presence of the integrin activator Mn2+ (Fig. 2D). The interaction of CHO cells with ATX-T210A was specifically mediated by β3 integrins because it was completely blocked by 7E3 (Fig. 2D). Because integrin β3 associates with the widely expressed vitronectin α subunit CHO cells (which do not express the platelet-specific αIIb subunit), these observations also establish that ATX-T210A binds to αVβ3, indicating that association with this integrin may account for ATX binding to other cell types.
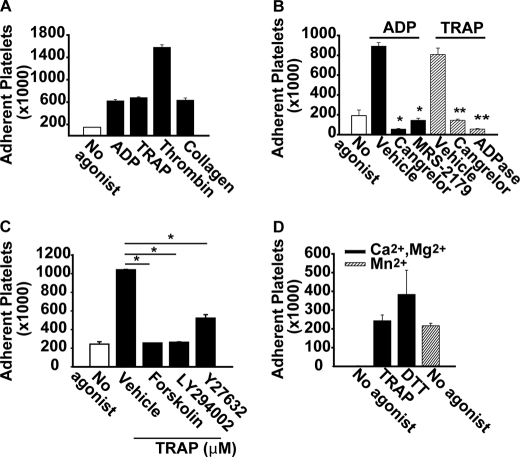
Platelet integrins classically require agonist activation to recognize protein ligands. We therefore verified the agonist requirements for ATX-T210A binding to platelets. Agonists that act through G-protein-coupled receptors, including ADP, the PAR1 thrombin receptor-activating peptide (TRAP), and thrombin, as well as a non-G-protein-coupled receptor pathway (collagen), all promoted platelet adhesion to ATX-T210A (Fig. 3A). ADP-promoted platelet adhesion to ATX-T210A was attenuated by the P2Y12 receptor antagonist cangrelor and the P2Y1 antagonist MRS-2719 (Fig. 3B). Cangrelor and ADPase (apyrase) also inhibited adhesion of TRAP-stimulated platelets to ATX (Fig. 3B), identifying a role for released ADP in PAR1-stimulated platelet adhesion to ATX-T210A (Fig. 3B). In keeping with known mechanisms of integrin activation, ADP-stimulated platelet adhesion to ATX was inhibited by pretreatment of platelets with forskolin to elevate intraplatelet cAMP and was partially reduced by inhibitors of protein kinase A (PKI(14–22)), PI3K (LY294002), or Rho kinase (Y27632) (Fig. 3C). In addition to classic platelet agonists, the direct integrin activator Mn2+ and treatment of platelets with the reducing agent DTT, which stimulates the adhesive function of several integrins, resulted in agonist-independent adhesion to ATX (Fig. 3D).
FIGURE 3.
Platelet binding to ATX requires agonist activation of integrins. A, gel-filtered calcein-labeled platelets (200,000/μl in Tyrode's buffer with 2 mm CaCl2 and 1 mm MgCl2) were incubated with microtiter wells containing ATX-T210A (immobilized at 5 μg/ml) in the absence of agonist (open bar) or in the presence of 10 μm ADP, 15 μm TRAP, 0.5 units/ml thrombin, or 10 μg/ml collagen for 1 h at 37 °C. After washing the wells three times, platelet adhesion was quantitated by determining fluorescence associated with the wells. B, platelets were incubated in microtiter wells containing immobilized ATX in the absence of agonist (open bar) or presence of ADP (closed bars) or TRAP (hatched bars). Where indicated, 0.5 μm cangrelor (P2Y12 antagonist), 10 μm MRS-2179 (P2Y1 antagonist), or 1 units/ml apyrase (ADPase) was included in the assay. *, p < 0.05 versus ADP/vehicle, ANOVA; **, p < 0.05 versus TRAP/vehicle, ANOVA. C, platelets were incubated with wells with immobilized ATX in the absence of agonist or presence of ADP and with either 100 μm forskolin, 20 μm LY294002 (PI3K inhibitor), or 2.5 μm Y27632 (Rho kinase inhibitor). *, p < 0.05 versus vehicle, ANOVA. D, platelets (in Tyrode's buffer with the indicated divalent cations) were incubated in microtiter wells containing ATX with vehicle (no agonist), 15 μm TRAP, 2.5 μm DTT, or 50 μm Mn2+. Results are presented as the number of adherent platelets (mean ± S.D.) from at least three independent experiments performed in triplicate.
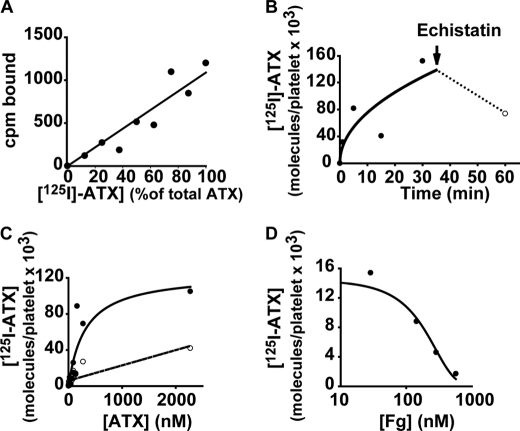
The preceding observations of ATX binding to integrins on platelets and mammalian cells employed microtiter plate assays with immobilized ATX. We next investigated binding of soluble 125I-labeled ATX to platelets in suspension. When 125I-ATX and unlabeled ATX were incubated at various ratios while maintaining the total ATX concentration constant, a linear relationship was observed between the percent 125I-ATX bound and the percent added (Fig. 4A), indicating that radiolabeling of ATX did not affect the protein's ability to recognize platelets. Binding of 125I-ATX to activated platelets was time-dependent and saturable (Fig. 4, B and C). Scatchard analysis of data obtained using an excess of unlabeled ATX to define nonspecific binding revealed an affinity of ∼300 nm and a maximal number of ∼75,000 ATX-binding sites/platelet. Addition of excess echistatin partially displaced bound 125I-ATX (Fig. 4B), suggesting that at least some of the ATX remained on the platelet surface after 30 min. Inclusion of fibrinogen in the reaction dose-dependently blocked 125I-ATX binding to platelets with a KI of 220 nm (Fig. 4D).
FIGURE 4.
Affinity and stoichiometry of 125I-ATX binding to activated platelets. A, 15 μm TRAP-activated platelets (200,000/μl) were incubated with a total of 73 nm ATX, composed of varying amounts of 125I-labeled ATX and unlabeled ATX, and platelet-bound ATX was determined as described under “Experimental Procedures.” B, TRAP-activated platelets were incubated with 125I-ATX for the indicated times. At 30 min, echistatin (20 μg/ml) was added, and incubations were continued for an additional 30 min to determine reversibility. C, TRAP-activated platelets (●) or resting platelets (○) were incubated with the indicated concentrations of ATX for 30 min. D, TRAP-activated platelets were incubated with 125I-ATX in the presence of the indicated concentrations of fibrinogen (Fg). Data are means of duplicate determinations.
lyso-PLD-independent Effects of ATX on Platelet Aggregation and Adhesion
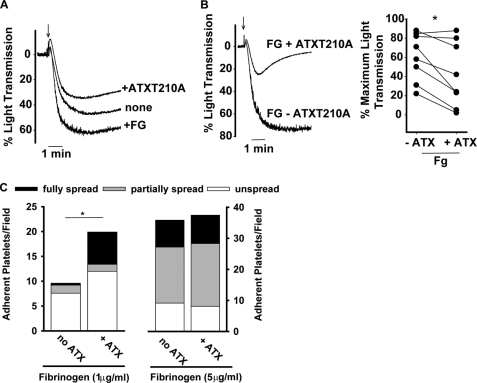
ATX is a monomeric protein in solution (16) and would not be expected to support platelet aggregation, which requires cross-linking of at least two binding sites on adjacent platelets. Under conditions where ADP-induced aggregation of washed platelets was enhanced by addition of exogenous fibrinogen, inclusion of soluble ATX-T210A did not further enhance platelet aggregation (Fig. 5A). In fact, the maximal extent of aggregation of platelets was moderately lower in the presence of ATX-T210A (Fig. 5, A and B, left panel). The ability of ATX-T210A to reduce platelet aggregation varied between platelet preparations from different donors (Fig. 5B, right panel), but the extent of aggregation was consistently lower in the presence of ATX-T210A. Together, these observations and the fact that fibrinogen competes for soluble ATX binding (Fig. 3D) are consistent with a model in which ATX and fibrinogen interact with a mutually accessible binding site(s) on platelets but only fibrinogen supports platelet aggregation.
FIGURE 5.
Effects of ATX on fibrinogen-dependent platelet aggregation and adhesion. A, gel-filtered platelets (200,000/μl) were stirred in the absence or presence of 200 μg/ml fibrinogen (+FG) or 50 μg/ml ATX-T210A (+ATX). Platelet aggregation was stimulated by the addition of 15 μm TRAP at the time indicated by the arrow. B, gel-filtered platelets (200,000/μl) stirred in the presence of 200 μg/ml fibrinogen without ATX (FG − ATX) or with 50 μg/ml ATX (FG + ATX). 15 μm TRAP was added at the time indicated by the arrow. Left panel, representative light transmission tracing from single donor; right panel, cumulative maximal percent aggregation from different donors (*, p < 0.05, paired t test). C, platelets (20,000/μl) were incubated with wells precoated with either 1 or 5 μg/ml fibrinogen in the presence or absence of 5 μg/ml ATX. Adherent platelets were stained with TRITC-phalloidin, and the total number of platelets/field in 4–5 fields was counted. Where indicated, the number of platelets adherent in the presence of soluble ATX was also determined. The platelets in each field were scored as fully spread (defined by the presence of cortical actin reorganization), partially spread (presence of filopodia), or not spread (neither cortical actin or filopodia). *, p < 0.05, t test.
ATX exerts a counter-adhesive effect on oligodendrocytes that is consistent with inhibition of integrin-dependent adhesion and signaling (26). ATX may also be capable of hydrolyzing nucleotides along the platelet surface and thereby impact signaling events. The inhibitory effects of ATX on platelet aggregation were independent of catalytic activity because inactive ATX-T210A also inhibited aggregation. Nonetheless, we sought to determine whether ATX has direct inhibitory effects on platelet function by determining whether ATX would alter adhesion to fibrinogen immobilized at submaximal concentrations (low density; 1 μg/ml). In the absence of agonists, platelets adhere to low density fibrinogen, and the adhesion triggers platelet activation (31, 32). In agreement with our aggregation studies, soluble ATX blocked platelet adhesion to immobilized fibrinogen by 70% (from 9.6 ± 2.0 to 2.8 ± platelets/high powered field). However, more platelets adhered to surfaces containing both immobilized fibrinogen and ATX than to surfaces containing fibrinogen alone (20 ± 5 versus 9.6 ± 2 platelets platelets/high powered field; Fig. 5C, left). In addition, more platelets spread on surfaces with immobilized fibrinogen and ATX than spread on surfaces containing immobilized fibrinogen alone (Fig. 5C, left). These results suggest that fibrinogen binding may promote “outside-in” signaling necessary for platelets to recognize ATX. When fibrinogen was immobilized at higher density concentrations (5 μg/ml) that are known to promote maximal platelet activation and adhesion (31, 32), the inclusion of immobilized ATX did not inhibit adhesion or spreading (Fig. 5C, right). Thus, rather than negatively affecting the adhesive function of platelet integrins, as has been observed in experiments with oligodendrocytes, ATX moderately facilitates platelet adhesion to fibrinogen.
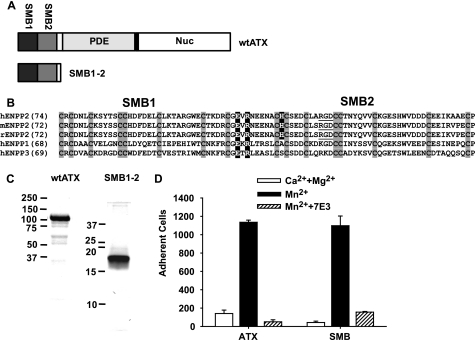
Tandem SMB Domains Are the Sole ATX–binding Sites for β3 Integrins
We next sought to identify the motifs in ATX responsible for interacting with platelets. ATX contains an arginine, glycine, aspartic acid (RGD) sequence motif within the second SMB-like domain (Fig. 6, A and B). Because this motif confers integrin binding to other proteins, including the structurally related SMB-like domain of vitronectin, we surmised that it would perform a similar function in ATX. Surprisingly, the ATX crystal structure revealed that the RGD motif amino acids are incompletely solvent-exposed. In agreement, we found that nonconservative substitutions of the RGD residues did not abolish integrin binding.4 However, in the course of these studies, we found that mutation of a charged, surface-exposed residue at the N terminus of the ATX SMB2 domain significantly reduced binding of ATX to platelet integrins, identifying an important role for the SMB2 domain in this process (17). To determine whether the SMB2 domain is the sole integrin-binding site on ATX and begin to explore interactions of ATX with integrins on other cells, we compared binding of an ATX fragment containing the tandem SMB domains to CHO cells expressing human integrin β3. Binding of these cells to the SMB domain fragment was promoted by the integrin activator Mn2+ and inhibited by 7E3 in an identical manner to that of wild type ATX, indicating that the SMB domains (and not the PDE and nuclease-like domains) account for ATX binding to β3 integrins (Fig. 6, C and D).
FIGURE 6.
N-terminal tandem SMB domain is the sole ATX integrin-binding site. A, domain structure of ATX and the N-terminal tandem SMB domain fragment. Nuc, nuclease-like domain B, sequence alignment of the SMB1 and SMB2 domains of human, rat, and mouse ENPP2 (ATX) and human ENPP1 and ENPP3 with the RGD sequence underlined, cysteine residues boxed in gray, and charged residues targeted for mutation boxed in black. C, analysis of purified recombinant wild type ATX (wtATX) and the N-terminal tandem SMB domain fragment (SMB1–2) by SDS-PAGE and staining with Coomassie Blue. D, CHO cells stably expressing the human β3 integrin subunit were incubated with immobilized ATX or the N-terminal SMB1–2 fragment in the presence of 2 mm CaCl2 and 1 mm MgCl2 (open bars), 50 μm MnCl2 (black bars), or 50 μm MnCl2 and 20 μg/ml of the function-blocking integrin β3 antibody 7E3 (hatched bars). Data shown are means ± S.D. of triplicate determinations.
ATX Binding to Platelet Integrins Enables Agonist-stimulated LPA Production
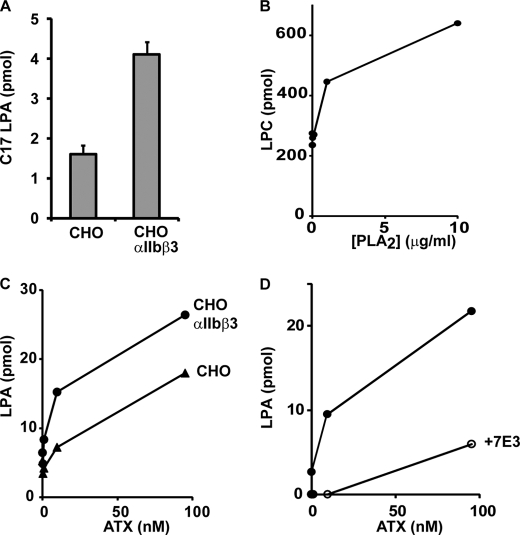
Having established the properties of ATX binding to β3 integrins, we used platelets and CHO cell systems to test the hypothesis that integrin-mediated recruitment of ATX to the outer face of the plasma membrane enables localized LPA production. To investigate this possibility, we measured the rate of hydrolysis of BSA-bound C17-LPC in the presence of either the parental CHO cells or the αIIIbβ3-expressing CHO cells using Mn2+ as the integrin activator. Under conditions where, based on our measurements of the affinity of platelet αIIbβ3 for ATX, all of the added ATX would be bound to the integrin, we observed an ∼2-fold increase in ATX activity in the presence of integrin-expressing cells (Fig. 7A). These results suggest that integrin binding increases ATX activity against exogenous substrates. Our experiments with activated platelets indicated that integrin-bound ATX could act on endogenously generated lysophospholipid substrates. Although like resting platelets, parental, and αIIbβ3-expressing CHO cells contained readily detectable LPC, we did not observe significant production of LPA when they were incubated with ATX (data not shown). However, we found that preincubation of these cells with bee venom PLA2 increased cell-associated LPC levels ∼2-fold (Fig. 7B), which presumably reflects the previously described ability of this enzyme to promote LPC-dependent responses when added exogenously to mammalian cells (35). Under these conditions, ATX elicited concentration-dependent increases in cell-associated LPA, and at lower concentrations of ATX, LPA production was increased ∼4-fold by overexpression of αIIbβ3 (Fig. 7C). The effect of ATX was substantially attenuated when the integrin-blocking antibody 7E3 was included in these incubations (Fig. 7D). Although the parental CHO cell line failed to bind to ATX appreciably in the static adhesion assay (Fig. 2), these results suggest that relatively low levels of integrin binding are sufficient to sustain significant production of LPA by cell-associated ATX.
FIGURE 7.
ATX activity against exogenously provided and cell-associated substrates is increased by integrin binding. A, control or αIIbβ3 expressing CHO cells were incubated with 100 nm ATX in Tyrode's buffer containing 1 mm MgCl2, 50 μm MnCl2, 100 μm C17 LPC, and 0.35% BSA. Production of C17 LPA was measured after a 1-h incubation at 37 °C. B, CHO cells were incubated with increasing concentrations of bee venom PLA2, and total cell-associated levels of 15 abundant LPC species were determined. C, control or αIIbβ3-expressing CHO cells were preincubated with bee venom PLA2 and then incubated with the indicated concentrations of ATX in the Tyrode's buffer used for the experiment shown in A, omitting the C17 LPC, and production of LPA was determined as described under “Experimental Procedures.” D, αIIbβ3-expressing CHO cells were preincubated with bee venom PLA2 and then incubated with the indicated concentrations of ATX in the Tyrode's buffer in the presence or absence of 10 μg/ml of the integrin blocking antibody 7E3 and production of LPA determined as described under “Experimental Procedures.” The data shown are means ± S.D. of triplicate determinations (A) or duplicate determinations (B–D).
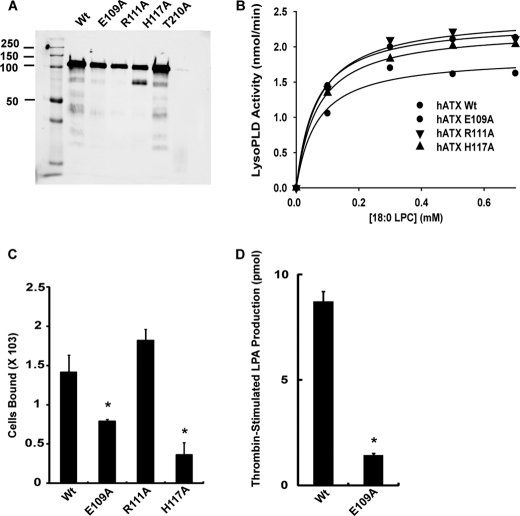
The crystal structures reported are of rodent ATX orthologs (17, 18). We made variants of human ATX with nonconservative substitutions of charged surface-exposed residues in the SMB2 domain, including H117A, which corresponds to H119A of the rodent sequence and was previously shown to exhibit decreased binding to platelet β3 integrins (Fig. 8A and also see Fig. 6 for sequence details) (17). We examined catalytic activity of these variants against mixed micelles of Triton X-100 and 18:0 LPC. The apparent Km and Vmax values for LPC of these SMB domain mutants were very similar to that of wild type ATX, indicating that mutation of the SMB2 domain does not impair catalytic activity against detergent-solubilized substrates (Fig. 8B). Activity of these variants against the small molecule substrate bis-paranitrophenol phosphate was also indistinguishable from wild type ATX (data not shown). We examined binding of these ATX SMB2 domain variants to β3 integrin-expressing CHO cells. Binding of these cells to ATX H117A (as expected) and ATX E109A but not ATXR117A was significantly lower than to wild type ATX (Fig. 8C). We then compared the ability of wild type ATX and ATX E109A to increase LPA production when incubated with thrombin-stimulated platelets. Although wild type ATX produced a robust increase in LPA production, ATX E109A did not, indicating that association with β3 integrins is important for this process (Fig. 8D).
FIGURE 8.
ATX SMB2 mutants with impaired binding to β3 integrins display reduced LPA generating capacity. A, SDS-PAGE analysis of purified wild type ATX and the indicated variants. B, lyso-PLD activity of wild type ATX and the indicated variants determined using Triton X-100 solubilized 18:0 LPC. C, binding of β3 integrin-expressing CHO cells to wild type ATX and the indicated ATX variants was determined as described under “Experimental Procedures” (mean ± S.D. of triplicate determinants). D, effect of wild type ATX and ATX E019A on LPA production by thrombin-stimulated platelets was determined as described under “Experimental Procedures” (mean ± S.D. of triplicate determinations).
DISCUSSION
LPA is produced and degraded by multiple pathways and also serves as an intermediate in the de novo synthesis of phospholipids. In this study, we describe a mechanism by which the metabolic and signaling functions of LPA can be delimited by localizing LPA production to the cell surface through recruitment to activated integrin receptors. We used human platelets as a model system to dissect the regulation and specificity of integrin binding to ATX. We found that a disparate variety of platelet agonists promote ATX binding to platelets. The ability of agonists to stimulate ATX binding appears to be strongly dependent on ADP signaling through the P2Y12 purinergic receptor, as evidenced by the ability of cangrelor and apyrase to inhibit TRAP-induced adhesion to immobilized ATX. The finding that forskolin treatment abolishes platelet adhesion to ATX is also consistent with a role for P2Y12 signaling through Gαi to decrease platelet cAMP levels as an important component of the signaling pathway response. Stimulation of platelet adhesion to ATX by Mn2+ in the absence of agonists implicates integrins in this process, and studies using integrin-blocking antibodies and CHO cells expressing recombinant integrins indicate a role for β3 integrins (αIIbβ3 and αVβ3) and to a lesser extent β1 integrins in this process. The involvement of integrins in platelet binding to ATX is consistent with the density of ATX-binding sites/platelet observed with radiolabeled soluble ATX binding experiments. These results raise the possibility that, because αVβ3 and β1 integrins are broadly expressed, cell surface recruitment of ATX through integrin binding may be a widespread phenomenon.
Consistent with this idea, binding of ATX to lymphocytes and oligodendrocytes has been reported previously (25, 26). In the latter system, ATX binding was shown to disrupt cell adhesion through a process that was postulated to involve antagonism of an unidentified adhesive receptor (26). We found that ATX impedes fibrinogen-dependent platelet aggregation. This is likely because ATX and fibrinogen compete for binding to the same integrin receptors but as ATX is a monovalent integrin ligand it cannot support platelet aggregation. Consistent with this idea, we found that fibrinogen competitively inhibited binding of soluble radiolabeled ATX to platelets. However, when ATX and fibrinogen were immobilized together at submaximal concentrations, we found that ATX modestly enhanced, rather than inhibited, adhesion. Thus, unlike ATX binding to oligodendrocytes, which appears to trigger signals that inhibit other adhesive events, our results are consistent with a model in which fibrinogen and ATX compete for the same binding sites on platelets. Our prior observation that ATX is recruited to thrombus in vivo suggests that the interaction of ATX with activated platelet integrins we have characterized here in vitro occurs physiologically (11). In in vitro assays, ATX displays nucleotide pyrophosphatase/phosphodiesterase activity. It is possible that localization of ATX along the platelet surface provides a mechanism for degradation of released ATP (a platelet inhibitor) and ADP (a platelet activator). To date, however, we have been unable to detect an impact of ATX on circulating nucleotides (27).
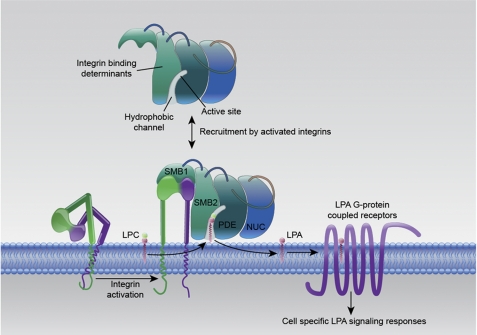
Efforts to define the physiological role of LPA signaling in platelet function have been confounded by observations of heterogeneity in human platelet responses to LPA and the fact that, although LPA is a weak agonist for human platelets from most donors, it does not activate rodent platelets (4, 6). However, several lines of evidence implicate platelets as important participants in the production of LPA in whole blood ex vivo and in mouse models (20, 23). We found that a recently described potent small molecule ATX inhibitor completely attenuated production of LPA by thrombin-stimulated washed platelets. The delipidated BSA used in these incubations contained trace levels of LPC that were less than 1% of the quantity of LPA being generated, so clearly these results indicate that endogenous ATX acting on endogenously formed LPC is responsible for producing LPA in this system. Addition of purified ATX to washed platelets at concentrations in the range of ∼100 nm, which are in line with those present in plasma, resulted in dramatically enhanced sustained production of LPA by thrombin-stimulated washed platelets. We also found that ATX could hydrolyze CHO cell-associated lysophospholipids and that this activity was substantially enhanced when cells expressing integrin β3 were used as the source of substrate. In such experiments, a concentration in the range of 1–10 nm ATX achieved half of the maximal LPA levels. Small differences in levels to achieve maximal LPA levels (Fig. 7, B and C) may be attributed to the slightly different levels of purity between different preparations of recombinant ATX, which results in slight differences in quantification of protein in different preparations. We identified the N-terminal ATX tandem SMB domain as sole site of integrin binding, and we generated point mutants in the SMB1 domain of ATX that exhibited parallel reductions in binding to β3 integrins and the ability to generate LPA when incubated with thrombin-stimulated platelets. Taken together, these results indicate that integrin binding to ATX brings the enzyme into close proximity with cell surface substrates that would generate LPA in the vicinity of its receptors (Fig. 9). Intriguingly, the ATX hydrophobic substrate binding channel through which cell surface substrates and products must enter and leave the active site is formed from the interface between the SMB and PDE domains (17, 18), raising the possibility that integrin binding might alter catalytic activity of the enzyme.
FIGURE 9.
Recruitment of ATX to the cell surface by activated integrins enables localized production of LPA by hydrolysis of cell surface lysophospholipid substrates.
Although ATX can clearly generate LPA in blood plasma through hydrolysis of high concentrations of circulating LPC, and this activity is important for maintaining plasma LPA levels in the ≥100 μm range in the face of rapid elimination (13, 14), very little is known about the extracellular concentration of LPA in tissues. Furthermore, although a role for circulating levels of the structurally related lipid mediator sphingosine 1-phosphate in control of the permeability of lymphatic and vascular endothelium is well supported by substantial data, the function of plasma LPA remains enigmatic (33). Our results suggest that the functions of circulating and cell-associated ATX are distinct. Recruitment of circulating ATX to activated platelets or the surface of vascular endothelial and vascular smooth muscle cells could lead to cell-specific LPA generation and LPA signaling with important roles in vascular inflammation and injury responses (33).
Inhibition of LPA/ATX signaling is an attractive therapeutic target in cardiovascular disease in inflammation and cancer. To date, all of the inhibitors of ATX activity described, including several recently reported highly potent compounds, target the catalytic site of the enzyme to inhibit substrate binding or catalysis (13, 34). The ATX-integrin interaction reported here could be exploited to generate agents that selectively target ATX activity against cell-associated substrates. Indeed, antibody and small molecule inhibitors of integrin-ligand interactions have already been developed as anti-thrombotic and anti-cancer agents, and one compound of this type has been reported to alter LPA signaling involved in breast cancer metastasis in a mouse model (23). The possibility that some of the efficacy of these agents derives from inhibition of ATX recruitment to integrins warrants further examination.
Acknowledgments
We thank Tracy Drennan, Juliana Odetunde, and Marcielle de Beer for technical assistance and advice and Zhenyu Li, Barry Coller, and The Medicines Company for providing reagents.
This work was supported, in whole or in part, by National Institutes of Health Grants GM050388 and RR026884 (to A. J. M.), HL078663 (to S. S. S.), GM094155 (to C. V. K.), and P20RR021954 and F30 HL099272 (to Z. F.). This work was also supported by the resources and use of the facilities at the Lexington Veterans Affairs Medical Center.
A. J. Morris, unpublished observations.
- LPA
- lysophosphatidic acid
- ATX
- autotaxin
- LPC
- lysophosphatidylcholine
- PLA2
- phospholipase A2
- ANOVA
- analysis of variance
- SMB
- somatomedin B-like
- TRITC
- tetramethylrhodamine isothiocyanate
- TRAP
- thrombin receptor activating peptide.
REFERENCES
- 1. Choi J. W., Herr D. R., Noguchi K., Yung Y. C., Lee C. W., Mutoh T., Lin M. E., Teo S. T., Park K. E., Mosley A. N., Chun J. (2010) Annu. Rev. Pharmacol. Toxicol. 50, 157–186 [DOI] [PubMed] [Google Scholar]
- 2. Panetti T. S., Hannah D. F., Avraamides C., Gaughan J. P., Marcinkiewicz C., Huttenlocher A., Mosher D. F. (2004) J. Thromb. Haemost. 2, 1654–1656 [DOI] [PubMed] [Google Scholar]
- 3. Rizza C., Leitinger N., Yue J., Fischer D. J., Wang D. A., Shih P. T., Lee H., Tigyi G., Berliner J. A. (1999) Lab. Invest. 79, 1227–1235 [PubMed] [Google Scholar]
- 4. Siess W., Zangl K. J., Essler M., Bauer M., Brandl R., Corrinth C., Bittman R., Tigyi G., Aepfelbacher M. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 6931–6936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hayashi K., Takahashi M., Nishida W., Yoshida K., Ohkawa Y., Kitabatake A., Aoki J., Arai H., Sobue K. (2001) Circ. Res. 89, 251–258 [DOI] [PubMed] [Google Scholar]
- 6. Pamuklar Z., Lee J. S., Cheng H. Y., Panchatcharam M., Steinhubl S., Morris A. J., Charnigo R., Smyth S. S. (2008) Arterioscler. Thromb. Vasc. Biol. 28, 555–561 [DOI] [PubMed] [Google Scholar]
- 7. Subramanian P., Karshovska E., Reinhard P., Megens R. T., Zhou Z., Akhtar S., Schumann U., Li X., van Zandvoort M., Ludin C., Weber C., Schober A. (2010) Circ. Res. 107, 96–105 [DOI] [PubMed] [Google Scholar]
- 8. Panchatcharam M., Miriyala S., Yang F., Rojas M., End C., Vallant C., Dong A., Lynch K., Chun J., Morris A. J., Smyth S. S. (2008) Circ. Res. 103, 662–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhou Z., Subramanian P., Sevilmis G., Globke B., Soehnlein O., Karshovska E., Megens R., Heyll K., Chun J., Saulnier-Blache J. S., Reinholz M., van Zandvoort M., Weber C., Schober A. (2011) Cell Metab. 13, 592–600 [DOI] [PubMed] [Google Scholar]
- 10. Baker D. L., Desiderio D. M., Miller D. D., Tolley B., Tigyi G. J. (2001) Anal. Biochem. 292, 287–295 [DOI] [PubMed] [Google Scholar]
- 11. Pamuklar Z., Federico L., Liu S., Umezu-Goto M., Dong A., Panchatcharam M., Fulkerson Z., Fulerson Z., Berdyshev E., Natarajan V., Fang X., van Meeteren L. A., Moolenaar W. H., Mills G. B., Morris A. J., Smyth S. S. (2009) J. Biol. Chem. 284, 7385–7394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Umezu-Goto M., Kishi Y., Taira A., Hama K., Dohmae N., Takio K., Yamori T., Mills G. B., Inoue K., Aoki J., Arai H. (2002) J. Cell Biol. 158, 227–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Albers H. M., Dong A., van Meeteren L. A., Egan D. A., Sunkara M., van Tilburg E. W., Schuurman K., van Tellingen O., Morris A. J., Smyth S. S., Moolenaar W. H., Ovaa H. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 7257–7262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tomsig J. L., Snyder A. H., Berdyshev E. V., Skobeleva A., Mataya C., Natarajan V., Brindley D. N., Lynch K. R. (2009) Biochem. J. 419, 611–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stefan C., Jansen S., Bollen M. (2005) Trends Biochem. Sci. 30, 542–550 [DOI] [PubMed] [Google Scholar]
- 16. Tokumura A., Majima E., Kariya Y., Tominaga K., Kogure K., Yasuda K., Fukuzawa K. (2002) J. Biol. Chem. 277, 39436–39442 [DOI] [PubMed] [Google Scholar]
- 17. Hausmann J., Kamtekar S., Christodoulou E., Day J. E., Wu T., Fulkerson Z., Albers H. M., van Meeteren L. A., Houben A. J., van Zeijl L., Jansen S., Andries M., Hall T., Pegg L. E., Benson T. E., Kasiem M., Harlos K., Kooi C. W., Smyth S. S., Ovaa H., Bollen M., Morris A. J., Moolenaar W. H., Perrakis A. (2011) Nat. Struct. Mol. Biol. 18, 198–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nishimasu H., Okudaira S., Hama K., Mihara E., Dohmae N., Inoue A., Ishitani R., Takagi J., Aoki J., Nureki O. (2011) Nat. Struct. Mol. Biol. 18, 205–212 [DOI] [PubMed] [Google Scholar]
- 19. Tabchy A., Tigyi G., Mills G. B. (2011) Nat. Struct. Mol. Biol. 18, 117–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Aoki J., Taira A., Takanezawa Y., Kishi Y., Hama K., Kishimoto T., Mizuno K., Saku K., Taguchi R., Arai H. (2002) J. Biol. Chem. 277, 48737–48744 [DOI] [PubMed] [Google Scholar]
- 21. Tigyi G., Miledi R. (1992) J. Biol. Chem. 267, 21360–21367 [PubMed] [Google Scholar]
- 22. Eichholtz T., Jalink K., Fahrenfort I., Moolenaar W. H. (1993) Biochem. J. 291, 677–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Boucharaba A., Serre C. M., Grès S., Saulnier-Blache J. S., Bordet J. C., Guglielmi J., Clézardin P., Peyruchaud O. (2004) J. Clin. Invest. 114, 1714–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bolen A. L., Naren A. P., Yarlagadda S., Beranova-Giorgianni S., Chen L., Norman D., Baker D. L., Rowland M. M., Best M. D., Sano T., Tsukahara T., Liliom K., Igarashi Y., Tigyi G. (2011) J. Lipid Res. 52, 958–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kanda H., Newton R., Klein R., Morita Y., Gunn M. D., Rosen S. D. (2008) Nat. Immunol. 9, 415–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fox M. A., Colello R. J., Macklin W. B., Fuss B. (2003) Mol. Cell. Neurosci. 23, 507–519 [DOI] [PubMed] [Google Scholar]
- 27. Federico L., Pamuklar Z., Smyth S. S., Morris A. J. (2008) Curr. Drug Targets 9, 698–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sano T., Baker D., Virag T., Wada A., Yatomi Y., Kobayashi T., Igarashi Y., Tigyi G. (2002) J. Biol. Chem. 277, 21197–21206 [DOI] [PubMed] [Google Scholar]
- 29. Nakamura K., Igarashi K., Ide K., Ohkawa R., Okubo S., Yokota H., Masuda A., Oshima N., Takeuchi T., Nangaku M., Okudaira S., Arai H., Ikeda H., Aoki J., Yatomi Y. (2008) Clin. Chim. Acta 388, 51–58 [DOI] [PubMed] [Google Scholar]
- 30. Smyth S. S., Sciorra V. A., Sigal Y. J., Pamuklar Z., Wang Z., Xu Y., Prestwich G. D., Morris A. J. (2003) J. Biol. Chem. 278, 43214–43223 [DOI] [PubMed] [Google Scholar]
- 31. Coller B. S., Kutok J. L., Scudder L. E., Galanakis D. K., West S. M., Rudomen G. S., Springer K. T. (1993) J. Clin. Invest. 92, 2796–2806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jirousková M., Jaiswal J. K., Coller B. S. (2007) Blood 109, 5260–5269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Smyth S. S., Cheng H. Y., Miriyala S., Panchatcharam M., Morris A. J. (2008) Biochim. Biophys. Acta 1781, 563–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gierse J., Thorarensen A., Beltey K., Bradshaw-Pierce E., Cortes-Burgos L., Hall T., Johnston A., Murphy M., Nemirovskiy O., Ogawa S., Pegg L., Pelc M., Prinsen M., Schnute M., Wendling J., Wene S., Weinberg R., Wittwer A., Zweifel B., Masferrer J. (2010) J. Pharmacol. Exp. Ther. 334, 310–317 [DOI] [PubMed] [Google Scholar]
- 35. Perrin-Cocon L., Agaugué S., Coutant F., Masurel A., Bezzine S., Lambeau G., André P., Lotteau V. (2004) Eur. J. Immunol. 34, 2293–2302 [DOI] [PMC free article] [PubMed] [Google Scholar]