Abstract
Sustained JNK activation plays a critical role in hepatotoxicity by acetaminophen or GalN/TNF-α. To address the importance of JNK translocation to mitochondria that accompanies sustained activation in these models, we assessed the importance of the expression of a potential initial target of JNK in the outer membrane of mitochondria, namely Sab (SH3 domain-binding protein that preferentially associates with Btk), also known as Sh3bp5 (SH3 domain-binding protein 5). Silencing the expression of Sab in the liver using adenoviral shRNA inhibited sustained JNK activation and mitochondrial targeting of JNK and the upstream MKK4 (MAPK kinase 4), accompanied by striking protection against liver injury in vivo and in cultured hepatocytes in both toxicity models. We conclude that mitochondrial Sab may serve as a platform for the MAPK pathway enzymes and that the interaction of stress-activated JNK with Sab is required for sustained JNK activation and toxicity.
Keywords: Drug Metabolism, Glutathione, Serine/threonine Protein Kinase, Signal Transduction, Toxicology, Drug Action, Drug Toxicity, Liver
Introduction
JNKs are a family of serine/threonine kinases belonging to the MAPK family. JNK is activated through phosphorylation by MKK4/7 (MAPK kinase 4/7), which is in turn activated upstream by ASK1 (apoptosis signal-regulating kinase 1) and other MAP3Ks. In most cells, including hepatocytes, two JNK isoforms, JNK1 and JNK2, are expressed (1). JNK plays an important role in stress response and is activated by stresses such as reactive oxygen species (ROS)2 and UV light and by cytokines such as TNF-α (2, 3). Once activated, JNK regulates many metabolic and survival pathways but also mediates cell death (1, 4). The ability of JNK to mediate both cell survival and cell death pathways is often determined by the duration of JNK activation (3, 5). Transient JNK activation is associated with cell stress responses such as JunD phosphorylation that can protect cells, whereas sustained JNK activation promotes both apoptotic and necrotic cell death pathways (2, 3, 6). Sustained JNK is important in mediating apoptosis and necrosis caused by a wide range of agents, including ROS, TNF, UV light, various drugs, and toxins (1, 4). In the liver, prolonged JNK activation has been shown to mediate injury caused by acetaminophen (APAP), ischemia, and TNF-α (7–10).
JNK-dependent cell death is accompanied in many cases by translocation to mitochondria (8, 11, 12), and cell death is preceded by loss of mitochondrial function, suggesting that the direct interaction of JNK with mitochondria is important. JNK binding to isolated brain mitochondria has also been shown to shut down mitochondrial metabolism by leading to down-regulation of pyruvate dehydrogenase activity (13). We demonstrated previously that, during APAP-induced liver injury, APAP treatment results in sustained JNK activation and translocation to mitochondria (8, 14), which induces mitochondrial permeability pore transition, leading to hepatocyte death. JNK translocation to mitochondria may also promote mitochondrial ROS generation, which is important in sustaining JNK activation, particularly when mitochondria are rendered susceptible by toxins such as APAP and anisomycin, thus sustaining JNK activation in a self-amplifying loop (8, 9, 15).
Although there is supportive evidence that JNK interaction with mitochondria may regulate mitochondrial function, the mechanism of regulation remains unclear. Several proteins in mitochondria have been shown to bind JNK and could mediate its effects. Bcl-xL (B-cell lymphoma-extra large), Mcl-1 (myeloid cell leukemia sequence 1), and Sab (SH3 domain-binding protein that preferentially associates with Btk) have been identified to potentially bind and/or be phosphorylated by JNK (16–19). The phosphorylation of Bcl-xL by JNK inactivates Bcl-xL, rendering mitochondria more sensitive to mitochondrial outer membrane permeability or mitochondrial permeability pore transition (17, 18). On the other hand, JNK1 appears to phosphorylate Mcl-1, which stabilizes the anti-apoptotic protein in the liver (16). The importance of JNK binding to Sab on mitochondria and its effect on mitochondrial function have not been extensively explored. Sab (also known as Sh3bp5 (SH3 domain-binding protein 5)) is a mitochondrial protein that was first identified by the yeast two-hybrid system as a binding target of phosphorylated JNK (p-JNK) (19). Sab is a scaffold protein containing a kinase interaction motif, similar to that observed in c-Jun, which is important in binding JNK (20). Ser-321 of Sab has been shown to be phosphorylated by JNK and by SAPK3 (stress-activated protein kinase 3) (19, 21). A recent study in HeLa cells revealed that anisomycin-induced JNK activation directly leads to increased mitochondrial ROS generation, which requires JNK binding to Sab (22). Taken together, these data suggest that Sab may be the key mitochondrial protein to which activated JNK binds, leading to effects on mitochondrial function. Therefore, in this work, we tested the hypothesis that JNK-mediated toxicity depends on the binding of p-JNK to Sab using two important JNK-dependent liver injury models, APAP- and GalN/TNF-α-induced liver injury.
EXPERIMENTAL PROCEDURES
Reagents
Antisera to JNK, p-JNK, ASK1, MKK4, MKK7, p-MKK4, p-MKK7, actin, prohibitin-1, Bap37, and cytochrome oxidase IV were from Cell Signaling Technology. Antisera to Sab, Bax (Bcl-2-associated X protein), GAPDH, GRP-78, and cytochrome c were from Santa Cruz Biotechnology. 3-Nitrotyrosine antiserum was from Abcam. The following reagents were used: APAP, d-GalN (Sigma), and mouse recombinant TNF-α (Calbiochem).
Animals
Male C57BL/6NHsd mice (6–8 weeks of age) were obtained from Harlan Bioproducts for Science Inc. (Indianapolis, IN). APAP was dissolved in warm PBS (55 °C) and cooled to 37 °C before intraperitoneal injection into overnight fasted mice at a dose of 300 mg/kg. Mice were pretreated with 800 mg/kg d-GalN dissolved in PBS by intraperitoneal injection 30 min prior to intraperitoneal injection with mouse recombinant TNF-α (12 μg/kg) in pyrogen-free PBS. For adenoviral injection, the animals received 1 × 109 IU/25 g of body weight via tail veins. Serum alanine aminotransferase (ALT) was measured at the University of Southern California Pathology Reference Laboratory.
Cell Isolation and Culture
Primary mouse hepatocytes (PMHs) were isolated and cultured as described previously (23). Three hours after plating of isolated hepatocytes, APAP (5 mm) dissolved in fresh prewarmed DMEM/F-12 culture medium was added. After 15 h of treatment, cells were double-stained with Hoechst 33258 (8 μg/ml; Invitrogen) and SYTOX Green (1 μmol/liter; Invitrogen). Quantitation of total and apoptotic cells was performed by counting a minimum of 1000 cells in 10 different fields. Necrotic cells (SYTOX Green-positive) were determined by counting the same field as described previously (24). In other experiments, hepatocytes from shRNA-treated mice 7 days after adenoviral injections were incubated with actinomycin D (ActD; 0.5 μg/ml)/TNF-α (20 ng/ml) and, after 6 h, stained with Hoechst 33258, and apoptotic cells were counted (25).
Isolation of Liver Mitochondria and Cytoplasm
Mitochondria were isolated from mouse livers by differential centrifugation as described previously (26). Livers were homogenized in H-medium (210 mm mannitol, 70 mm sucrose, 2 mm HEPES, 0.05% (w/v) bovine serum albumin, and protease and phosphatase inhibitors). The homogenate was centrifuged at 800 × g for 10 min, the pellet was removed, and the centrifugation process was repeated. The resulting supernatant was centrifuged at 8500 × g for 15 min. The pellet, which represents the mitochondrial fraction, was washed with H-medium, and centrifugation was repeated. The mitochondria were resuspended in H-medium for Western blot analysis. The supernatant (cytoplasmic fraction) was centrifuged at 100,000 × g for separation of the endoplasmic reticulum pellet (microsomes) and supernatant cytosol.
Western Blot Analysis
Aliquots of cytoplasmic or mitochondrial extracts were fractionated by electrophoresis on 7.5, 10, or 12% SDS-polyacrylamide gel (Bio-Rad). Subsequently, proteins were transferred to nitrocellulose membrane, and blots were blocked with 5% (w/v) nonfat milk dissolved in Tris-buffered saline with Tween 20. The blots were then incubated with the desired primary and secondary antibodies. Finally, the proteins were detected by luminol ECL reagent (Thermo Scientific). All gels shown are representative samples from at least three experiments.
Measurements of Respiration in Isolated Mitochondria
Mice were treated with APAP, and at the times indicated, the livers were removed, and mitochondrial fractions were separated by differential centrifugation. Respiratory control ratio (state III/state IV) measurements were performed by monitoring oxygen consumption in the presence of mitochondrial substrates using a Clark-type electrode as described previously (26).
Adenoviral shRNA Preparation
A BLOCK-iT adenoviral RNAi expression system (Invitrogen) was used as described (27) to generate expression constructs for shRNAs targeting lacZ (shlacZ) and mouse sab (shsab). The oligonucleotide sequences used to synthesize the shlacZ construct were 5′-CACCGCTACACAAATCAGCGATTTCGAAAAATCGCTGATTTGTGTAG-3′ and 5′-AAAACTACACAAATCAGCGATTTTTCGAAATCGCTGATTTGTGTAGC-3′. The oligonucleotide sequences used for the shsab construct were 5′-CACCGGATGACAAGCGGCAGTTTGACGAATCAAACTGCCGCTTGTCATCC-3′ and 5′-AAAAGGATGACAAGCGGCAGTTTGATTCGTCAAACTGCCGCTTGTCATCC-3′. Mice (25 g) were injected with 1 × 109 infectious units of adenovirus expressing shRNA after purification using Vivapure AdenoPACK (Sartorius Stedim Biotech S.A.).
GSH Measurements
Seven days after adenoviral injection, mice were injected with APAP intraperitoneally. 1 or 2 h following APAP treatment, total liver homogenate and mitochondrial GSH were measured using the recycling assay. Liver homogenate or mitochondria were mixed with 10% trichloroacetic acid and subjected to the GSH recycling assay (28). GSH refers to total GSH (GSH + GSSG).
Histological Analysis
Livers were removed, fixed with 10% buffered formalin, embedded in paraffin, and cut into 5-μm-thick sections. All specimens were stained with hematoxylin/eosin and evaluated under a light microscope. Immunohistochemistry for nitrotyrosine-protein adducts was performed according to the Leica BOND Stainer protocol, followed by incubation with primary antibody to 3-nitrotyrosine.
Statistical Analysis
Statistical analyses were performed using Student's t test. p < 0.05 was defined as statistically significant.
Animal Care
All animals received care under the institutional guidelines. These studies were approved by the Institutional Animal Care and Utilization Committee.
RESULTS
Expression and Subcellular Localization of Sab in Mouse Liver and Co-immunoprecipitation of Sab and p-JNK
At first, we determined the expression and subcellular localization of Sab in mouse liver. Sab was present in hepatic mitochondria (supplemental Fig. S1A). The addition of proteinase K at increasing concentrations to isolated mitochondria digested Sab in parallel with the voltage-dependent anion channel, an outer membrane protein (supplemental Fig. S1B). In contrast, intermembrane cytochrome c and matrix ornithine transcarbamylase were protected from digestion. Therefore, Sab resides in the outer membrane of mitochondria. Next, we examined the association of p-JNK and Sab by co-immunoprecipitation and observed that immunoprecipitation with mouse anti-Sab antibody brought down p-JNK and with rabbit anti-p-JNK antibody brought down Sab from protein extracts of mitochondrial fractions after APAP treatment of PMHs (supplemental Fig. S1C). Similar results were seen with anisomycin in PMHs (data not shown).
Effect of Sab Knockdown on APAP-induced JNK Activation and Translocation to Mitochondria in Vivo
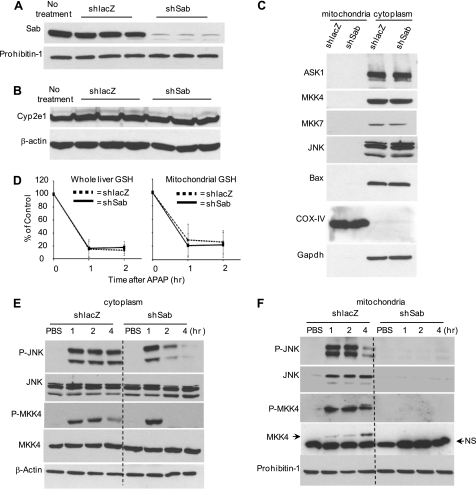
Adenoviral shlacZ was used as a control and had no effect on Sab expression. In contrast, adenoviral shsab markedly decreased mitochondrial Sab (Fig. 1A). However, no decrease in Cyp2e1 (cytochrome P450 2E1) was observed (Fig. 1B). Cyp2e1 is responsible for the bulk of activation of APAP to N-acetyl-p-benzoquinone imine (NAPQI), the toxic metabolite (29, 30). Also, much higher titers of adenovirus than used in our studies have been associated with decreased expression of Cyp2e1 and protection against APAP (31), but this was not the case under our experimental conditions. Furthermore, silencing Sab did not alter basal expression of MAPK ASK1, MKK4, MKK7, and JNK levels or Bax levels (Fig. 1C). APAP-induced glutathione depletion in whole liver and mitochondria was not affected by the adenoviral pretreatments (Fig. 1D). The rate and extent of GSH depletion are a widely accepted surrogate for NAPQI production. Therefore, silencing Sab expression did not affect APAP conversion to NAPQI. Furthermore, comparison of covalent binding of NAPQI in a Western blot of liver homogenates 2 h after APAP treatment showed no differences in shlacZ- and shsab-pretreated mice (supplemental Fig. S2).
FIGURE 1.
Effect of silencing Sab on APAP-induced signaling activation in hepatotoxicity. A, immunoblot assessing efficiency of knockdown of Sab in mouse liver mitochondria. B, effect of adenoviral treatment on Cyp2e1 expression. Adenoviral shlacZ or adenoviral shsab was given intravenously as described under “Experimental Procedures,” and livers were removed after 7 days for immunoblotting. Each lane represents one mouse. C, effect of silencing Sab on basal expression of MAPK(s) and Bax. Mice were injected with adenoviral shlacZ or adenoviral shsab as described in under “Experimental Procedures.” Seven days after adenoviral injection, livers were taken, and the cytoplasm (post-mitochondrial fraction) was isolated by differential centrifugation. Western blot analysis was performed using antisera against ASK1, MKK4, MKK7, total JNK, and Bax. Cytochrome oxidase IV (COX-IV) and GAPDH were used as loading controls and subcellular markers. Data are representative of three experiments. D, GSH assay comparing the GSH levels of total liver homogenates or mitochondria after in vivo treatment with APAP (300 mg/kg) in warm PBS with or without Sab expression. The GSH assay is described under “Experimental Procedures.” Dashed line (♦), shlacZ-injected mice; solid line (■), shsab-injected mice (n = 3 or 4 per group). E and F, immunoblots assessing JNK and MKK4 activation in the cytoplasm (E) and translocation to mitochondria (F) after in vivo treatment with APAP (300 mg/kg) in warm PBS with or without Sab expression. NS, nonspecific band. All immunoblots are representative of three to five experiments.
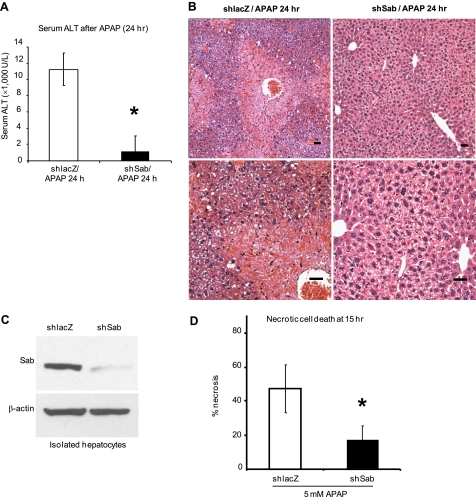
We next examined the effect of Sab silencing on JNK activation and translocation to mitochondria and APAP hepatotoxicity. JNK activation or association with mitochondria was not seen in PBS-treated mice. Cytoplasmic JNK in shlacZ controls and Sab-silenced mice was equally activated at 1 h after APAP treatment. Sustained activation of JNK and upstream MKK4 was seen in the cytoplasm of shlacZ controls up to 4 h, but JNK and MKK4 activation was not sustained after 1 h in Sab-silenced mice (Fig. 1E). At 1 and 2 h after APAP treatment, p-JNK was associated with mitochondria in shlacZ controls, which decreased at 4 h. In comparison, p-JNK binding to mitochondria was markedly inhibited when Sab was silenced (Fig. 1F). No translocation of ASK1, MKK7, or p-MKK7 to mitochondria was observed after APAP treatment of controls (data not shown). Lack of antisera to p-ASK1 precluded its assessment. Thus, the sustained activation of MKK4 and JNK and the association of p-JNK with mitochondria required Sab. In APAP-treated mice, prior knockdown of Sab markedly protected against APAP toxicity as reflected in serum ALT and histological hemorrhagic necrosis at 24 h (Fig. 2, A and B). Adenovirus (shlacZ or shsab)-treated mice without APAP treatment had normal ALT levels and histology (data not shown).
FIGURE 2.
Effect of silencing Sab on APAP-induced hepatotoxicity. A, serum ALT of shlacZ- or shsab-pretreated mice at 24 h following APAP treatment. Error bars represent S.D. *, p = 0.01 versus shlacZ group (Student's t test; n = 4 per group). B, representative histology of hematoxylin/eosin-stained liver tissue of mice from A. Scale bars = 100 μm. C and D, effect of decreased Sab expression on APAP-induced necrosis of PMHs isolated from adenoviral shlacZ- or adenoviral shsab-treated mice 7 days after adenoviral injection. C, Sab expression in isolated hepatocytes assessed by Western blotting. D, cultured hepatocytes from these mice were incubated with 5 mm APAP dissolved in warm medium. Fifteen hours after APAP addition, hepatocytes were stained with SYTOX Green, and necrotic cells were counted. *, p < 0.05 (n = three experiments).
To verify that the effects of Sab knockdown directly protect hepatocytes, we treated mice with adenoviral shlacZ or adenoviral shsab and then isolated and cultured the hepatocytes. Sab knockdown (Fig. 2C) markedly protected the cultured hepatocytes against APAP (5 mm)-induced necrosis (Fig. 2D). This indicates that the protection against acetaminophen is due to knockdown of Sab in hepatocytes.
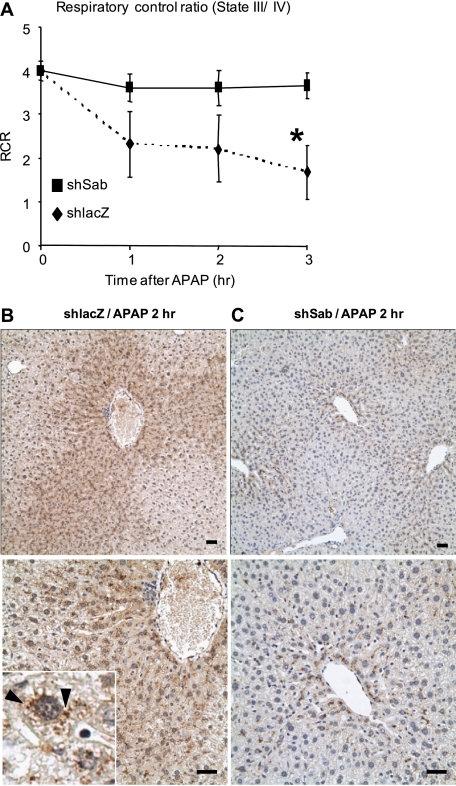
We also assessed the electron transport chain respiratory activity and coupling efficiency in mitochondria at various times after APAP treatment in vivo. Isolated liver mitochondria from shlacZ-pretreated mice showed the expected APAP-induced progressive severe loss of mitochondrial respiratory control ratio (state III/state IV) due mainly to decreased respiration. After APAP treatment, mitochondrial function in Sab-silenced mice was largely unaffected (Fig. 3A). Nitrotyrosine formation is believed to be important in APAP toxicity by promoting mitochondrial permeability transition (32). In shlacZ controls, APAP caused marked centrilobular nitrotyrosine staining (Fig. 3B). This staining was punctate (Fig. 3B, inset), consistent with the known predominance of nitrotyrosine staining in mitochondria (32). In contrast, when Sab was silenced, only minimal nitrotyrosine staining was seen (Fig. 3C). Therefore, APAP-induced nitrotyrosine formation in mitochondria requires the interaction of p-JNK with Sab.
FIGURE 3.
Effect of silencing Sab on mitochondrial respiratory control ratio (state III/state IV) and nitrotyrosine formation in APAP-induced hepatotoxicity. A, respiratory control ratio (RCR) at various times after APAP treatment in shlacZ (dashed line (♦))- or shsab (solid line (■))-pretreated mice. Details are provided under “Experimental Procedures.” Error bars represent S.D. *, p = 0.002 versus shsab group (Student's t test; n = 3 or 5 per group). B and C, representative immunohistochemical staining of nitrotyrosine liver tissue from shlacZ (B)- and shsab (C)-pretreated mice after treatment with APAP (n = 3 per group). Scale bars = 100 μm. The inset shows punctate nitrotyrosine staining (arrowhead) after APAP treatment in shlacZ-pretreated mice.
Effect of Decreased Sab Expression on GalN/TNF-α-induced Hepatotoxicity
We determined the effect of decreased Sab expression on GalN/TNF-α-induced hepatotoxicity. JNK is known to play a key role in liver injury in this model (33, 34). We confirmed this by showing that treatment with a JNK inhibitor prevented hepatotoxicity in this model (supplemental Fig. S3).
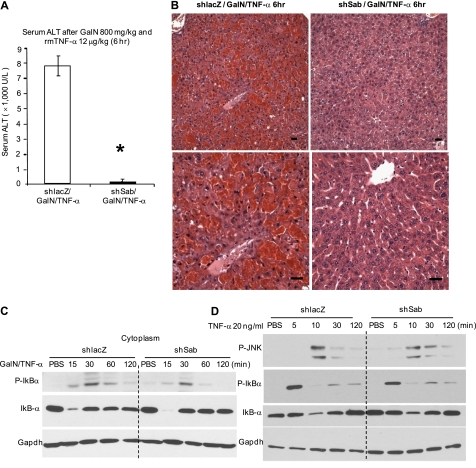
Mice received GalN 30 min before TNF-α, and serum and liver histology were examined 6 h later. Marked liver injury was seen in shlacZ-pretreated mice, accompanied by a marked ALT increase (Fig. 4A). In contrast, shsab-pretreated mice were strikingly protected, with a minimal ALT increase and negligible histological injury (Fig. 4B).
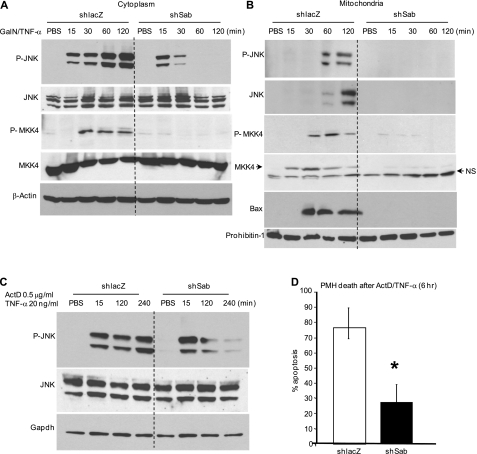
FIGURE 4.
Effect of decreased Sab expression on GalN/TNF-α-induced hepatotoxicity in vivo and initial TNF receptor signaling in vivo and in PMHs. A, serum ALT of shlacZ- or shsab-pretreated mice at 6 h following TNF-α treatment. Error bars represent S.D. rmTNF-α, recombinant mouse TNF-α. *, p = 0.008 versus shlacZ group (Student's t test; n = 4 per group). B, representative histology of hematoxylin/eosin staining of mouse liver tissue. Scale bars = 100 μm. C and D, effect of Sab knockdown on upstream TNF receptor signaling in vivo and in PMHs. Mice were injected with adenoviral shlacZ or adenoviral shsab as described under “Experimental Procedures.” C, 7 days after adenoviral injection, mice were injected with GalN/TNF-α intraperitoneally, and livers were removed and fractionated. Immunoblotting of liver cytoplasm was performed at the indicated times after GalN/TNF-α treatment in vivo. D, hepatocytes from adenovirus-treated mice were cultured and incubated with TNF-α alone for the indicated times. Immunoblotting of PMH extracts was performed using antisera against p-JNK, p-IκBα, IκBα, and GAPDH (loading control). Data are representative of three experiments.
Next, we assessed the effect of Sab silencing on TNF-α/TNF receptor signaling. TNF-α induced a transient increase in p-IκBα and a decrease in IκBα, which were unaffected by silencing Sab in the GalN-pretreated mice in vivo (Fig. 4C). The lack of effect of silencing of Sab on TNF-α signaling was confirmed in PMHs exposed to TNF-α alone, which exhibited no inhibition of p-IκBα formation or loss of IκBα (Fig. 4D). Of note, p-JNK transiently associated with mitochondria after exposure to TNF-α alone (supplemental Fig. S4). GalN/TNF-α similarly activated cytoplasmic JNK at 15 min in the livers of shlacZ- and shsab-pretreated animals, further indicating intact TNF receptor signaling (Fig. 5A). Sustained JNK and MKK4 activation was seen in shlacZ controls after GalN/TNF-α treatment in vivo, but sustained JNK and MKK4 activation was not seen when Sab was silenced (Fig. 5A). p-JNK at 1 and 2 h and p-MKK4 at 0.5, 1, and 2 h after TNF-α injection were associated with mitochondria in shlacZ controls (Fig. 5B). In comparison, when Sab was silenced, p-JNK and p-MKK4 binding to mitochondria was markedly inhibited (Fig. 5B). As in the APAP model, ASK1, MKK7, and p-MKK7 were not associated with mitochondria (data not shown). Translocation of Bax to mitochondria, which participates in TNF-α-induced apoptosis (35), was inhibited when sustained JNK activation was prevented by silencing Sab. To confirm that the role of Sab is relevant to the protection of hepatocytes from death due to sustained JNK activation, we exposed cultured hepatocytes from shlacZ- or shsab-pretreated mice to ActD plus TNF-α. shsab-silenced hepatocytes were protected from sustained JNK activation (Fig. 5C) and apoptosis (Fig. 5D).
FIGURE 5.
Effect of decreased Sab expression on TNF-α-induced signaling activation in hepatotoxicity. A and B, immunoblots assessing JNK and MKK4 activation in the cytoplasm (A) and translocation to mitochondria (B) after in vivo treatment with GalN/TNF-α with or without Sab expression. NS, nonspecific band. Results are representative of three to five experiments. C, effect of decreased Sab expression on ActD/TNF-α-induced JNK activation of PMHs. Seven days after adenoviral injection, hepatocytes were isolated. Cultured hepatocytes from these mice were incubated with PBS or ActD/TNF-α, and whole cell extracts were examined by Western blotting for p-JNK and JNK at the indicated times. GAPDH was used as a loading control. D, PMHs from shlacZ- or shsab-pretreated mice were incubated with ActD/TNF-α for 6 h, and hepatocytes were stained with Hoechst 3325. Apoptotic cells (shrunken/fragmented nuclei) were counted as described under “Experimental Procedures.” *, p < 0.05 versus shlacZ group (n = three experiments).
DISCUSSION
JNK plays a key role in cell death and hepatotoxicity (1, 2, 9). In the context of TNF-α-induced toxicity, it has been noted that sustained JNK activation, which occurs when NF-κB is inhibited, promotes apoptosis (5, 33). Among the mechanisms for the action of JNK are activation of Bax, inactivation of Bcl-xL, degradation of cellular caspase-8 (FLICE)-like inhibitory protein (cFLIP), and increased mitochondrial oxidative stress (3, 18, 36). In the case of APAP hepatotoxicity, we have previously described a critical role of JNK, independent of TNF-α signaling (7, 8). Current evidence supports the role of mitochondrial ROS release in response to the toxic metabolite of APAP, NAPQI, leading to sustained JNK activation at least partially due to activation of the extramitochondrial MAPK cascade initiated by ASK1 activation (37). However, other mechanisms of MAPK activation such as endoplasmic reticulum stress may contribute. Once sustained activation of JNK occurs in response to APAP, its translocation to mitochondria is seen. We have previously shown that the addition of activated JNK to isolated mitochondria from APAP-treated mice directly leads to worsening mitochondrial function (8). Most importantly, inhibition of JNK or silencing the expression of JNK1 and JNK2 markedly protects mice from APAP toxicity (8). In the GalN/TNF-α model, sustained JNK activation is also known to depend on continued mitochondrial ROS release (33, 34). Thus, JNK plays a key role in hepatotoxicity, and sustained activation of JNK leads to its translocation to mitochondria. To understand the mechanism of toxicity induced by JNK and the importance of translocation to mitochondria, we set out to identify the protein in mitochondria with which JNK associates.
In searching the literature, we identified previous work in other contexts in cell culture models in which JNK activated by anisomycin was associated with a mitochondrial protein known as Sab (19, 20). While in the process of preparing this manuscript, a report appeared showing that, in HeLa cells, anisomycin-induced cell death required the binding of p-JNK to Sab, and silencing of p-JNK or Sab was protective; it was demonstrated that the addition of activated JNK to mitochondria from anisomycin-treated HeLa cells promoted increased ROS production (22). Our initial studies in HepG2 cells revealed similar findings: JNK activation by anisomycin led to JNK-dependent mitochondrial depolarization, and silencing Sab expression protected against depolarization (supplemental Fig. S5).
In this study, we addressed the role of Sab in liver injury. We confirmed that Sab is present exclusively in the mitochondrial fraction of mouse liver and co-immunoprecipitated with p-JNK after APAP treatment of PMHs. Having established that Sab is in the outer membrane and that it binds p-JNK, we examined the role of Sab in two models of acute liver injury in which hepatotoxicity is dependent on JNK and in which inhibition of JNK protects against toxicity, namely the APAP and GalN/TNF-α models. In both models in vivo and in PMHs, silencing the expression of Sab provided dramatic protection against liver toxicity. In shlacZ controls in both models, sustained JNK activation was seen, accompanied by mitochondrial translocation of p-JNK. In the APAP model, initial JNK activation was unaffected by silencing Sab, but association of p-JNK with mitochondria was prevented. However, subsequent JNK activation was not sustained in the shsab-pretreated mice. Similar results were seen in the GalN/TNF-α model. Although the initial JNK activation in response to TNF-α was unaffected, the delayed and sustained activation of JNK was not observed in Sab-silenced mice. In addition, in both models, the upstream kinase of JNK, MKK4, exhibited sustained activation and also translocated to mitochondria, supporting the role of binding to Sab in facilitating sustained activation of the MAPK pathway.
Our current findings, along with our previous work (8) and that of others (22), support the hypothesis that the lack of sustained JNK activation in the absence of Sab reflects the inhibition of JNK-mediated mitochondrial ROS production needed for sustained JNK activation. Furthermore, Sab may provide a platform for the MAPK module to assemble and generate sustained JNK activation. Although we could find no evidence of translocation of ASK1, MKK7, or p-MKK7 to mitochondria in either model, MKK4 and p-MKK4 translocation to mitochondria was observed and was prevented when Sab was silenced. In the case of APAP, based on our previous work showing that the addition of p-JNK directly to mitochondria from APAP-treated mice impairs mitochondrial function (8), p-JNK binding to mitochondrial Sab may directly affect mitochondrial function, leading to further increased ROS production, sustaining JNK activation in a feed-forward vicious cycle, ultimately leading to the mitochondrial permeability pore transition pore opening, which causes cellular demise by necrosis. Our findings showing that silencing Sab markedly inhibited APAP-induced mitochondrial nitrotyrosine formation strongly support the role of JNK binding to Sab in enhanced and sustained mitochondrial ROS and reactive nitrogen species production. Although the source of NO in this model is controversial, the formation of nitrotyrosine in mitochondria points to increased mitochondrial O2˙̄ as a target of NO, which leads to peroxynitrite formation. In addition, the recent finding of Sab-dependent JNK-mediated inhibition of complexes I and III in HeLa cells further supports this hypothesis (22). In contrast to APAP-induced necrosis, GalN/TNF-α- or ActD/TNF-α-induced cell death is mainly apoptotic and involves caspases and Bcl-2 family members such as Bax (which are dispensable in APAP necrosis) (38, 39). Nevertheless sustained JNK activation is known to depend on mitochondrial ROS in TNF-α-induced apoptosis (33, 34, 36). Thus, the mode of cell death is different in the two models: necrotic cell death due to cell stress-induced JNK-dependent loss of function in mitochondria made more vulnerable by APAP metabolite versus apoptosis due to TNF-α-induced signaling (caspase, tBid (truncated BH3-interacting domain death agonist), Bax) to mitochondria, leading to outer membrane permeabilization under the condition of NF-κB inhibition. However, in both models, inhibition of JNK protects, and the binding of p-JNK to outer mitochondrial membrane Sab is necessary for sustained JNK activation to occur, which is a requirement for cell death in both models (see overall model in supplemental Fig. S6).
At present, several important unanswered questions derive from our work. The mechanism of how the interaction of p-JNK and Sab leads to sustained JNK activation requires further work. Is this a direct effect on mitochondria, leading to increased ROS production as suggested in other studies (8, 22), and/or is this a consequence of Sab serving as a platform for the MAPK pathway module, leading to indirect effects of sustained activated JNK released into the cytoplasm on other pathways that then affect mitochondria? Both are likely to be important, and the relative contribution of each may be model-dependent. Are the signaling mechanisms we describe in mouse hepatocytes relevant to human hepatocytes? Is phosphorylation of Sab by p-JNK an important aspect of the downstream effects of Sab on mitochondria? The answer to these important questions will require additional work. Nevertheless, irrespective of these uncertainties, we have demonstrated that Sab plays a key role as the binding partner of JNK and other MAPKs on mitochondria and that the inhibition of this interaction prevents sustained JNK activation and offers a new target to protect against chemical-induced necrosis and death receptor-induced apoptosis.
Supplementary Material
Acknowledgments
The assistance of the Cell Culture Core, the Cell and Tissue Imaging Core, and the Analytical/Instrumentation Core of the University of Southern California Research Center for Liver Diseases (supported by National Institutes of Health Grant P30 DK48522) is greatly appreciated.
This work was supported, in whole or in part, by National Institutes of Health Grant R01 DK067215 (to N. K.), a training supplement to Grant DK067215 to promote reentry into research careers (to S. W.), and K01 Award AA016911 (to D. H.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental “Methods” and Figs. S1–S6.
- ROS
- reactive oxygen species
- APAP
- acetaminophen
- p-JNK
- phosphorylated JNK
- ALT
- serum alanine aminotransferase
- PMH
- primary mouse hepatocyte
- ActD
- actinomycin D
- shlacZ
- lacZ shRNA
- shsab
- sab shRNA
- NAPQI
- N-acetyl-p-benzoquinone imine.
REFERENCES
- 1. Johnson G. L., Nakamura K. (2007) Biochim. Biophys. Acta 1773, 1341–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Czaja M. J. (2007) Semin. Liver Dis. 27, 378–389 [DOI] [PubMed] [Google Scholar]
- 3. Han D., Ybanez M. D., Ahmadi S., Yeh K., Kaplowitz N. (2009) Antioxid. Redox Signal. 11, 2245–2263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weston C. R., Davis R. J. (2007) Curr. Opin. Cell Biol. 19, 142–149 [DOI] [PubMed] [Google Scholar]
- 5. Liu H., Lo C. R., Czaja M. J. (2002) Hepatology 35, 772–778 [DOI] [PubMed] [Google Scholar]
- 6. Lamb J. A., Ventura J. J., Hess P., Flavell R. A., Davis R. J. (2003) Mol. Cell 11, 1479–1489 [DOI] [PubMed] [Google Scholar]
- 7. Gunawan B. K., Liu Z. X., Han D., Hanawa N., Gaarde W. A., Kaplowitz N. (2006) Gastroenterology 131, 165–178 [DOI] [PubMed] [Google Scholar]
- 8. Hanawa N., Shinohara M., Saberi B., Gaarde W. A., Han D., Kaplowitz N. (2008) J. Biol. Chem. 283, 13565–13577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Han D., Shinohara M., Ybanez M. D., Saberi B., Kaplowitz N. (2010) Handb. Exp. Pharmacol. 196, 267–310 [DOI] [PubMed] [Google Scholar]
- 10. Bradham C. A., Stachlewitz R. F., Gao W., Qian T., Jayadev S., Jenkins G., Hannun Y., Lemasters J. J., Thurman R. G., Brenner D. A. (1997) Hepatology 25, 1128–1135 [DOI] [PubMed] [Google Scholar]
- 11. Aoki H., Kang P. M., Hampe J., Yoshimura K., Noma T., Matsuzaki M., Izumo S. (2002) J. Biol. Chem. 277, 10244–10250 [DOI] [PubMed] [Google Scholar]
- 12. Chauhan D., Li G., Hideshima T., Podar K., Mitsiades C., Mitsiades N., Munshi N., Kharbanda S., Anderson K. C. (2003) J. Biol. Chem. 278, 17593–17596 [DOI] [PubMed] [Google Scholar]
- 13. Zhou Q., Lam P. Y., Han D., Cadenas E. (2008) J. Neurochem. 104, 325–335 [DOI] [PubMed] [Google Scholar]
- 14. Shinohara M., Ybanez M. D., Win S., Than T. A., Jain S., Gaarde W. A., Han D., Kaplowitz N. (2010) J. Biol. Chem. 285, 8244–8255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shen H. M., Liu Z. G. (2006) Free Radic. Biol. Med. 40, 928–939 [DOI] [PubMed] [Google Scholar]
- 16. Kodama Y., Taura K., Miura K., Schnabl B., Osawa Y., Brenner D. A. (2009) Gastroenterology 136, 1423–1434 [DOI] [PubMed] [Google Scholar]
- 17. Fan M., Goodwin M., Vu T., Brantley-Finley C., Gaarde W. A., Chambers T. C. (2000) J. Biol. Chem. 275, 29980–29985 [DOI] [PubMed] [Google Scholar]
- 18. Kharbanda S., Saxena S., Yoshida K., Pandey P., Kaneki M., Wang Q., Cheng K., Chen Y. N., Campbell A., Sudha T., Yuan Z. M., Narula J., Weichselbaum R., Nalin C., Kufe D. (2000) J. Biol. Chem. 275, 322–327 [DOI] [PubMed] [Google Scholar]
- 19. Wiltshire C., Matsushita M., Tsukada S., Gillespie D. A., May G. H. (2002) Biochem. J. 367, 577–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wiltshire C., Gillespie D. A., May G. H. (2004) Biochem. Soc. Trans. 32, 1075–1077 [DOI] [PubMed] [Google Scholar]
- 21. Court N. W., Kuo I., Quigley O., Bogoyevitch M. A. (2004) Biochem. Biophys. Res. Commun. 319, 130–137 [DOI] [PubMed] [Google Scholar]
- 22. Chambers J. W., LoGrasso P. V. (2011) J. Biol. Chem. 286, 16052–16062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Han D., Hanawa N., Saberi B., Kaplowitz N. (2006) Free Radic. Biol. Med. 41, 627–639 [DOI] [PubMed] [Google Scholar]
- 24. Nagai H., Matsumaru K., Feng G., Kaplowitz N. (2002) Hepatology 36, 55–64 [DOI] [PubMed] [Google Scholar]
- 25. Feng G., Kaplowitz N. (2002) Am. J. Physiol. Gastrointest. Liver Physiol. 282, G825–G834 [DOI] [PubMed] [Google Scholar]
- 26. Han D., Williams E., Cadenas E. (2001) Biochem. J. 353, 411–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Than T. A., Lou H., Ji C., Win S., Kaplowitz N. (2011) J. Biol. Chem. 286, 22047–22054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tietze F. (1969) Anal. Biochem. 27, 502–522 [DOI] [PubMed] [Google Scholar]
- 29. Dahlin D. C., Miwa G. T., Lu A. Y., Nelson S. D. (1984) Proc. Natl. Acad. Sci. U.S.A. 81, 1327–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kaplowitz N. (2005) Nat. Rev. Drug Discov. 4, 489–499 [DOI] [PubMed] [Google Scholar]
- 31. Getachew Y., James L., Lee W. M., Thiele D. L., Miller B. C. (2010) Biochem. Pharmacol. 79, 1363–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cover C., Mansouri A., Knight T. R., Bajt M. L., Lemasters J. J., Pessayre D., Jaeschke H. (2005) J. Pharmacol. Exp. Ther. 315, 879–887 [DOI] [PubMed] [Google Scholar]
- 33. Kamata H., Honda S., Maeda S., Chang L., Hirata H., Karin M. (2005) Cell 120, 649–661 [DOI] [PubMed] [Google Scholar]
- 34. Osawa Y., Nagaki M., Banno Y., Yamada Y., Imose M., Nozawa Y., Moriwaki H., Nakashima S. (2001) J. Cell Physiol. 187, 374–385 [DOI] [PubMed] [Google Scholar]
- 35. Degenhardt K., Sundararajan R., Lindsten T., Thompson C., White E. (2002) J. Biol. Chem. 277, 14127–14134 [DOI] [PubMed] [Google Scholar]
- 36. Chang L., Kamata H., Solinas G., Luo J. L., Maeda S., Venuprasad K., Liu Y. C., Karin M. (2006) Cell 124, 601–613 [DOI] [PubMed] [Google Scholar]
- 37. Nakagawa H., Maeda S., Hikiba Y., Ohmae T., Shibata W., Yanai A., Sakamoto K., Ogura K., Noguchi T., Karin M., Ichijo H., Omata M. (2008) Gastroenterology 135, 1311–1321 [DOI] [PubMed] [Google Scholar]
- 38. Wang Y., Singh R., Lefkowitch J. H., Rigoli R. M., Czaja M. J. (2006) J. Biol. Chem. 281, 15258–15267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kaufmann T., Jost P. J., Pellegrini M., Puthalakath H., Gugasyan R., Gerondakis S., Cretney E., Smyth M. J., Silke J., Hakem R., Bouillet P., Mak T. W., Dixit V. M., Strasser A. (2009) Immunity. 30, 56–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.