Background: The Cockayne syndrome chromatin remodeler CSB and p53 physically interact.
Results: CSB facilitates p53-chromatin association when p53 levels are low, and p53 inhibits CSB-chromatin association when p53 levels are elevated.
Conclusion: Chromatin association of CSB and p53 is reciprocally regulated.
Significance: Reciprocal chromatin association may be a mechanism by which CSB and p53 coordinate their activities to regulate a common set of processes.
Keywords: Chromatin, Chromatin Remodeling, p53, Protein-DNA Interaction, Protein-Protein Interactions, CSB, Cockayne Syndrome, Chromatin Scanning, Genome Integrity
Abstract
The Cockayne syndrome complementation group B (CSB) protein is an ATP-dependent chromatin remodeler with an essential function in transcription-coupled DNA repair, and mutations in the CSB gene are associated with Cockayne syndrome. The p53 tumor suppressor has been known to interact with CSB, and both proteins have been implicated in overlapping biological processes, such as DNA repair and aging. The significance of the interaction between CSB and p53 has remained unclear, however. Here, we show that the chromatin association of CSB and p53 is inversely related. Using in vitro binding and chromatin immunoprecipitation approaches, we demonstrate that CSB facilitates the sequence-independent association of p53 with chromatin when p53 concentrations are low and that this is achieved by the interaction of CSB with the C-terminal region of p53. Remarkably, p53 prevents CSB from binding to nucleosomes when p53 concentrations are elevated. Examining the enzymatic properties of CSB revealed that p53 excludes CSB from nucleosomes by occluding a nucleosome interaction surface on CSB. Together, our results suggest that the reciprocal regulation of chromatin access by CSB and p53 could be part of a mechanism by which these two proteins coordinate their activities to regulate DNA repair, cell survival, and aging.
Introduction
Cockayne syndrome is a recessive disease associated with features of premature aging, sun sensitivity, and numerous developmental abnormalities. More than 70% of Cockayne syndrome patients have mutations in the genomic locus encoding the Cockayne syndrome complementation group B (CSB)2 protein; however, the underlying molecular mechanisms that lead to Cockayne syndrome are poorly understood. CSB belongs to the SWI2/SNF2 ATP-dependent chromatin remodeler family (1–4). These remodelers play fundamental and often essential roles in all nuclear processes that utilize chromatin, and the CSB protein has been implicated to function in a variety of nuclear process, including transcription, chromatin remodeling, and genome integrity maintenance (5–12). Most notably, CSB has an essential role in transcription-coupled DNA repair, a process that removes bulky DNA lesions that stall transcription, such as those created by UV irradiation (13). CSB is, in fact, the first known protein recruited to sites of lesion-stalled transcription, a prerequisite for the subsequent recruitment of the nucleotide excision repair machinery (6, 10, 14).
Although it is not known how the ATP-dependent chromatin-remodeling activity of CSB is utilized in the DNA repair process (6), ATP hydrolysis by CSB is an essential step of CSB recruitment, as the stable association of CSB with chromatin that occurs in response to lesion-stalled transcription is compromised by mutations that interfere with the ATPase activity of CSB (5). Current evidence supports a model whereby CSB uses energy from ATP hydrolysis to convert from a closed to an open conformation. By converting between these two conformations, CSB may sample chromatin for its substrates, such as DNA lesion-stalled transcription. In the presence of lesion-stalled transcription, the open conformation would be stabilized, leading to the stable association of CSB with chromatin at sites of DNA damage (5).
CSB is known to interact directly with the p53 tumor suppressor, but the biological ramifications of this interaction remain incompletely understood (11, 15). p53 plays a critical role in maintaining genome integrity and is activated by various mechanisms, including DNA damage (16, 17). Like CSB, p53 has been linked to the aging process (18). p53 appears to have both transcription-dependent and transcription-independent functions (19, 20). As a transcription factor, p53 functions in initiation and elongation. Moreover, p53 can function as a transcriptional activator or repressor. It is becoming increasingly apparent that that the cellular response to p53 is exquisitely tied to the degree of p53 activation and also to the cellular context (20). For instance, under conditions of normal cell growth, when stress levels are low, p53 promotes cell survival. This tumor-suppressive, prosurvival property appears to result from the concerted action of several independent mechanisms, including the promotion of DNA repair, antioxidant gene expression, and autophagy. On the other hand, when genome integrity is severely compromised and p53 is strongly activated, p53 appears to promote cell death, most likely to eliminate cells that might propagate mutations deleterious to the integrity of an organism. In the context of proliferating tissue, cells may be eliminated through the induction of apoptosis or senescence. In quiescent cells, cell death may be achieved, at least in part, through interference with mitochondrial biogenesis and energy metabolism (21), and, as opposed to p53 function in normal cells, these latter processes will generate reactive oxygen species. Thus, the biological response to p53 is intimately bound to the level and cellular context of p53 activation (19).
It has been proposed that p53 activation may inhibit CSB function in transcription elongation, and this may lead to RNA polymerase stalling on select genes encoding highly structured RNAs (11). Studies of the cellular response to hypoxic stress have led to the hypothesis that the interaction between CSB and p53 is part of a feedback mechanism in which CSB attenuates p53 activity, promoting cell survival (22). Like CSB, p53 has also been suggested to participate directly in DNA repair, and the p53 protein has been found to interact with several other DNA repair proteins as well as mismatched DNA (15, 23). In the context of transcription-coupled DNA repair, the function of the CSB-p53 interaction is unknown; however, cells from Li-Fraumeni patients, which have p53 mutations, display less efficient repair of UV-induced DNA lesions (15, 24, 25). The impact of CSB on p53 function has remained largely unexplored.
To elucidate further the functional significance of the CSB-p53 interaction, we examined the impact of CSB on the association of p53 with chromatin as well as the influence of p53 on the biochemical activities and chromatin association of CSB. Our results provide important insights into the mechanisms by which CSB and p53 might coordinate their activities to access chromatin and impact the efficiency of transcription and DNA repair as well as cell survival.
EXPERIMENTAL PROCEDURES
Construct Generation, Protein Expression, and GST Pulldown Assays
Constructs encoding wild-type p53 and p53Δ30 were generated by PCR amplification and cloned into pGEX-4T and pFastBac1 to express in bacteria and insect cells, respectively, and binding assays were performed as described previously (11). CSB expression constructs were as described previously (5). For protein expression in Sf9 cells, various FLAG-tagged proteins were purified by M2 affinity chromatography (26).
Nucleosome Assembly
DNA fragments of 152 and 202 bp, each containing two 20-bp GT phasing sequences located at positions 1–40 (gray box in Fig. 3a) (27), were assembled into mononucleosomes with HeLa cell core histones using step gradient salt dialysis (28). The DNA fragment containing p53-binding sequences was created by annealing two oligonucleotides containing the human MDM2 p53-response elements (ggtcaagttcagacacgttc and agttaagtcctgacttgtct) with an 18-bp spacing (cgaaactgcagtaaaagg) into the XhoI site, located at position 129, of the 202-bp DNA fragment (29). DNA fragments used for assembly were generated by PCR and body-labeled with [α-32P]dATP.
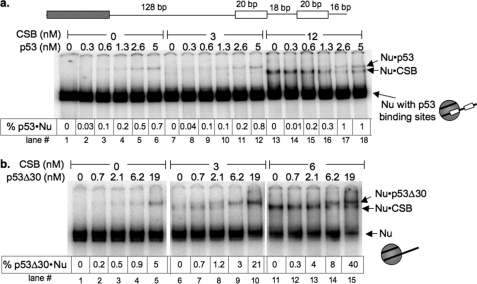
FIGURE 3.
CSB facilitates sequence-independent p53 binding to nucleosomal DNA through direct CSB-p53 interaction. a, a schematic representation of the DNA used for assembling mononucleosomes is shown. The gray box is the nucleosome phasing sequence, and the white boxes are the p53-binding elements. Varying amounts of p53 and CSB were incubated with ATP and radiolabeled 202-bp mononucleosomes containing p53-binding elements from the human MDM2 promoter. Electrophoretic mobility shifts were examined on a 5% native polyacrylamide gel. The percentages of nucleosomes bound by p53 (% p53·Nu) are as listed. b, varying amounts of p53Δ30 were incubated with radiolabeled 202-bp mononucleosomes and ATP in the presence or absence of CSB. Electrophoretic mobility shifts were examined on a 5% native polyacrylamide gel. The percentages of nucleosomes bound by p53Δ30 (% p53Δ30·Nu) are as listed. Binding assays were performed as schematized in Fig. 2a. Representations of the different mononucleosomes used in this study are to the right of each panel.
ATPase Assays
ATP hydrolysis reactions were carried out as described previously (30).
Gel Shift Assays
Proteins were mixed with mononucleosomes (<1 nm) at the indicated concentrations. Binding reactions were carried out in 12 mm Hepes (pH 7.9), 10 mm Tris-HCl (pH 7.5), 60 mm KCl, 8% glycerol, 4 mm MgCl2, 2 mm ATP·Mg, and 0.02% Nonidet P-40 at 30 °C. Reactions were loaded directly onto a 5% Polyacrylamide gel containing 0.5× Tris-borate-EDTA. The percentage of nucleosomes or DNA bound by p53 was quantitated using a PhosphorImager.
Cell Culture
CS1AN-Sv cells were maintained in DMEM/F-10 supplemented with 10% fetal bovine serum as described previously (10). To generate stable CS1AN-Sv cell lines expressing the CSB protein, cells were infected with lentivirus expressing the CSB protein from the phosphoglycerate kinase promoter of pLentiPGKNeo (31). Stable cell lines were selected with 600 μg/ml G418 (31).
Chromatin Immunoprecipitation
Cells were either untreated or treated with UV irradiation (100 J/m2 at 254 nm) applied from a Stratalinker UV cross-linker. Irradiated cells were allowed to recover for 1 h, after which ChIP assays were performed with anti-p53 monoclonal antibody DO-1 (26). Precipitated chromatin was analyzed by real-time PCR using a Bio-Rad MyiQ thermal cycler and SYBR Green. Primers were as described previously (32).
RESULTS
ATP Enhances CSB Binding to Nucleosomes in Vitro
Previously, we found that ATP hydrolysis by CSB leads to a conformational change of this remodeler that is a prerequisite for its stable association with chromatin inside a cell after UV irradiation (5). To determine whether ATP hydrolysis can influence CSB-chromatin association in vitro, we performed electrophoretic mobility shift assays using both 202-bp mononucleosomes, which have a 45-bp naked DNA overhang, and 152-bp core mononucleosomes, which have little naked DNA overhang. Varying amounts of purified recombinant CSB protein (Fig. 1a) were mixed with radiolabeled nucleosomes in the absence or presence of ATP, and CSB-nucleosome complexes were resolved on 5% native polyacrylamide gels (Fig. 1, b and c). At 3 nm CSB, ∼15% of the 202-bp nucleosome was bound by CSB in the presence of ATP and only 0.4% in the absence of ATP (Fig. 1b, lane 8 versus lane 4). Likewise, ∼3.3% of the 152-bp nucleosome was bound by CSB in the presence of ATP and only 0.46% was bound by CSB in the absence of ATP (Fig. 1c, lane 4 versus lane 8). These results indicate that ATP hydrolysis enhances CSB binding to nucleosomes in vitro and that CSB binds mononucleosomes with free DNA overhangs better than core mononucleosomes (Fig. 1, compare lane 8 in b with lane 4 in c). However, CSB-nucleosome interactions were also observed in the absence of ATP when higher concentrations of CSB protein (>10 nm) were used in the binding assays (data not shown). Therefore, in vitro, ATP hydrolysis by CSB can enhance its association with nucleosomes, but ATP hydrolysis is not absolutely required to generate the open chromatin-associating CSB conformation.
FIGURE 1.
ATP enhances CSB binding to nucleosomes in vitro. a, Coomassie Blue-stained gel of proteins used in this study. b, varying amounts of CSB were incubated with radiolabeled 202-bp mononucleosomes in the presence or absence of ATP. Electrophoretic mobility shifts were examined on a 5% native polyacrylamide gel. Representations of the different mononucleosomes used in this study are to the right of b and c. The percentages of nucleosomes bound by CSB (% CSB·Nu) are as listed. c, same as in b except that 152-bp mononucleosomes were used.
CSB Facilitates p53 Binding to Nucleosomes When p53 levels Are Low
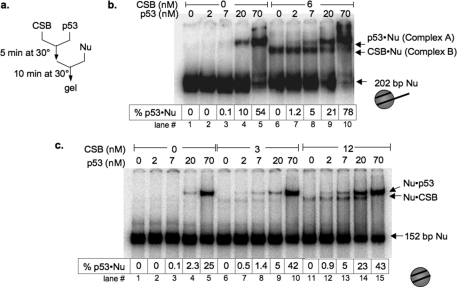
CSB is a member of the SWI2/SNF2 ATP-dependent chromatin remodeler family that has been shown to interact with p53 (6, 11, 15). This raised the possibility that CSB might facilitate p53 binding to nucleosomes. To test this hypothesis, we examined the influence of CSB on the sequence-independent interaction of p53 with 202-bp and 152-bp mononucleosomes, each devoid of p53-response elements (Fig. 2). When radiolabeled 202-bp mononucleosomes (Fig. 2b, lane 1) or 152-bp mononucleosomes (Fig. 2c, lane 1) were mixed with purified recombinant p53 or CSB in the presence of ATP, stable mobility-shifted nucleosomes were observed with 20 nm p53 (Fig. 2, b and c, lanes 4) or 3–6 nm CSB (Fig. 2, b and c, lanes 6), representing p53-nucleosome and CSB-nucleosome complexes, respectively (Fig. 2 and supplemental Fig. S1). When both CSB and p53 were mixed with nucleosomes, the formation of stable complexes with mobilities similar to p53-nucleosome and CSB-nucleosome complexes were also observed; however, interactions between p53 and nucleosomes could then be detected at 2 nm p53 (Fig. 2, b, lane 7, and c, lanes 7 and 12) compared with 20 nm in the absence of CSB. Examining the binding data extrapolated to fixed percentages of p53-nucleosome association (supplemental Table S1) revealed that at approximately equimolar CSB/p53 concentrations, CSB increased the association of p53 with nucleosomes by 10-fold (1% at 7 nm p53 versus 10% at 8 nm p53 and 6 nm CSB). Together, these results suggest that CSB facilitates p53 binding to nucleosomal DNA at low p53 concentrations. Additionally, p53 appeared to demonstrate slightly higher affinity for the 202-bp mononucleosome (Fig. 2, b and c, compare lanes 1–5).
FIGURE 2.
CSB facilitates p53 binding to nucleosomes in vitro. a, schematic representation of binding assays. b, varying amounts of p53 were incubated with radiolabeled 202-bp mononucleosomes and ATP in the presence or absence of CSB. Electrophoretic mobility shifts were examined on a 5% native polyacrylamide gel. Representations of the different mononucleosomes used in this study are to the right of b and c. The percentages of nucleosomes bound by p53 (% p53·Nu) are as listed. c, same as in b except that 152-bp mononucleosomes were used.
To confirm the identities of these mobility-shifted species, we included in these binding assays antibodies that specifically recognize p53 or HA-tagged CSB. As shown in supplemental Fig. S1A (lanes 5–7), anti-p53 antibody DO-1 specifically retarded the mobility of the slower migrating complex A, indicating that the slower migrating complex contained the p53 protein. On the other hand, we did not detect a change in the mobility of the faster migrating complex B (supplemental Fig. S1A, lanes 8–10), indicating that the faster migrating complex B did not contain p53. The mobility of the faster migrating complex B was altered, however, with an anti-HA antibody that recognizes HA-tagged CSB (supplemental Fig. S1B), indicating that this complex contained CSB. Importantly, the anti-HA antibody did not shift the slower migrating, p53-containing complex (supplemental Fig. S1B, lanes 6–10), arguing against the presence of CSB in that complex. Together, these results reveal that p53-nucleosome or CSB-nucleosome complexes were the only complexes detected and suggest that a trimeric complex composed of CSB, p53, and nucleosomes is either transient or unstable. Moreover, these data support the hypothesis that CSB facilitates the association of p53 with nucleosomes (Fig. 2 and supplemental Fig. S1).
CSB Does Not Enhance the Sequence-dependent Binding of p53 to Nucleosomes
In addition to displaying sequence-independent DNA binding, as a transcription factor, p53 binds to DNA in a sequence-specific manner. We therefore sought to determine whether CSB could facilitate the binding of p53 to its response element. To accomplish this, we incorporated the p53-response element of the human MDM2 promoter into one end of the 202-bp DNA fragment, and we used this DNA fragment to assemble nucleosomes (Fig. 3a). p53 demonstrated a much higher affinity for nucleosomes containing the p53-response element: 7 nm p53 was needed to bind 0.1% of total nucleosomes in the absence of a p53-binding sequence, but only 0.6 nm p53 was required to bind 0.1% of total nucleosomes when a p53-response element was present (Fig. 2b, lane 4; and Fig. 3a, lane 3). Importantly, inclusion of CSB in the binding assay did not further enhance the p53-nucleosome interaction in a sequence-dependent manner (Fig. 3a).
Direct CSB-p53 Interaction Is Important to Facilitate the Binding of p53 to Nucleosomes at Low p53 Concentrations
To determine whether a direct CSB-p53 interaction is important for the CSB-mediated enhancement of p53-nucleosome binding, we used a p53 protein devoid of its last 30 amino acids (p53Δ30) with compromised CSB-binding activity (Fig. 4b) (15). p53Δ30 displayed an apparent affinity for nucleosomes greater than that of full-length p53, as only 0.7 nm p53Δ30 was required to bind 0.1% total nucleosomes, but 7 nm full-length p53 was needed to bind a similar nucleosome fraction (Fig. 2b, lane 3, and Fig. 3b, lane 2). These results are in agreement with previous observations of p53Δ30 binding to naked DNA (33, 34). Importantly, CSB did not enhance the nucleosome association of p53Δ30 as dramatically as full-length p53: CSB enhanced 7 nm p53 binding to nucleosome by ∼50-fold but enhanced a similar amount of p53Δ30 binding to nucleosome by only 8-fold (Fig. 2b, compare lane 8 with lane 3, and Fig. 3b, compare lane 14 with lane 4). The low level of CSB-mediated enhancement observed for p53Δ30 is likely attributed to the residual binding of CSB to p53Δ30 (Fig. 4b). Therefore, a decrease in the association of CSB with p53 led to a concomitant decrease in the ability of CSB to facilitate p53-nucleosome association. Together, these results indicate that a direct CSB-p53 interaction is important for CSB to facilitate the binding of p53 to nucleosomes (Figs. 2b and 3b).
FIGURE 4.
Mapping the p53 interaction domains of CSB. a, schematic representations of the CSB functional domains and CSB deletion proteins used in this study. Noted are the seven conserved regions of the ATPase domain and the two putative nuclear localization signals (NLS). Amino acid positions of the constructs are as indicated. b, GST pulldown assays. Whole cell lysates from 293T cells transfected with constructs expressing wild-type CSB (lane 4) or the indicated CSB deletion mutants (lanes 5–9) were mixed with immobilized GST (lane 1), GST-p53Δ30 (lane 2), or GST-p53 (lane 3). Bound proteins were eluted and examined by SDS-PAGE. Shown are Western blot analyses using anti-HA and anti-FLAG antibodies. Wild-type CSB and CSBΔC were tagged with the HA epitope, and the other CSB truncations were tagged with FLAG. The amounts of lysate used in the binding assays were 60-fold of the input. CSB-N and CSB-C, N- and C-terminal regions of CSB, respectively; CSB-M, ATPase domain of CSB.
CSB Enhances the Association of p53 with Chromatin inside Cells at Low p53 Concentrations
To determine whether CSB could facilitate p53-nucleosome association in vivo, we examined the sequence-dependent and sequence-independent recruitment of p53 to chromatin using ChIP in the presence and absence of CSB. First, we examined the influence of CSB on the recruitment of p53 to three regions of the p21 gene: 1) the promoter region containing p53-response elements, 2) a promoter region devoid of p53-response elements, and 3) a region of intron 1 also devoid of p53-response elements (32). To accomplish this, we performed p53 ChIP assays with the CSB mutant cell line CS1AN-Sv (11) as well as a CS1AN-Sv cell line reconstituted with CSB. Functional CSB reconstitution was verified by rescue of UV sensitivity, and p53 levels were found to be similar in both cell lines (supplemental Fig. S2). As shown in Fig. 5a, during normal cell growth, when p53 levels are low, ChIP assays revealed no significant enrichment of p53 in CS1AN-Sv cells in these three regions of the p21 gene. Strikingly, however, introduction of CSB into this CSB mutant cell line led to enrichment of p53 in all three regions. To examine the influence of CSB on p53-chromatin association when p53 concentrations are elevated, we performed ChIP assays on UV-irradiated cells. As shown in Fig. 5b, when p53 concentrations were up-regulated, the association of p53 with chromatin was then independent of CSB.
FIGURE 5.
CSB facilitates p53-chromatin association in vivo. a, side-by-side ChIP assays were performed with the CSB mutant cell line CS1AN-Sv and CS1AN-Sv cells reconstituted with wild-type CSB. Three regions of the p21 gene were examined for enrichment of p53 association: two p21 promoter regions with or without p53-response elements and one intronic region (32). The amounts of recovered ChIP DNA are expressed as fraction of input DNA and presented as means ± S.E. (n = 3). b, the conditions were the same as described for a except that the cells were treated with a lethal dose of UV irradiation (100 J/m2). c, seven additional DNA regions were examined for p53 enrichment (32). Shown is percentage input above the background, calculated by subtracting amounts of background ChIP DNA from amounts of normalized p53 ChIP DNA and presented as means ± S.E. (n = 3).
To increase the coverage of our study, we examined the influence of CSB on the sequence-dependent and sequence-independent association of p53 at seven additional genomic regions (32). As shown in Fig. 5c, CSB enhanced the chromatin association of p53 at all regions examined during normal growth. When p53 levels were up-regulated, however, enrichment of p53 at all seven regions was independent of CSB.
High Levels of p53 Decrease CSB-Nucleosome Interactions
Although CSB facilitated p53 binding to nucleosomes at low p53 concentrations, notably, at higher p53 concentrations, CSB-nucleosome complexes became less apparent (Fig. 2, b and c), suggesting that high levels of p53 might decrease CSB-nucleosome association. To test this hypothesis, we used ATP hydrolysis assays to determine the Km of CSB for nucleosomes in the presence and absence of p53. The Km of CSB for nucleosomes is the concentration of nucleosomes necessary to achieve the half-maximal rate of ATP hydrolysis by CSB and is indicative of how tightly CSB interacts with nucleosomes (35).
As shown in Table 1, the Km of CSB for 202-bp mononucleosomes was ∼30 nm. However, when p53 was added to the reaction in a 20-fold molar excess, the Km of CSB for nucleosomes increased by ∼4-fold, supporting the notion that high p53 concentrations decrease CSB-nucleosome affinity.
TABLE 1.
Km for 202-bp mononucleosomes
|
Km |
||
|---|---|---|
| Without p53 | With 500 nm p53a | |
| nm | ||
| CSB (25 nm) | 30 ± 7 | 120 ± 20 |
| CSBΔN (40 nm) | 90 ± 20 | 500 ± 40 |
a The p53 concentration is based on a tetramer.
p53 Interferes with CSB-Chromatin Association by Occluding a Nucleosome Interaction Surface of CSB
To dissect the mechanism by which p53 interferes with CSB-nucleosome association, we identified the regions of CSB that interact with p53 by GST pulldown assays (Fig. 4). Immobilized GST-p53, GST-p53Δ30, or GST protein alone (Fig. 4b, lanes 1–3) was mixed with lysates from 293T cells expressing wild-type or mutant CSB proteins (Fig. 4, a and b, lanes 4–9). Only the GST-p53 fusion protein, but not the GST protein alone, bound to full-length CSB (Fig. 4b, CSBwt, compare lanes 10 and 12), confirming the interaction between CSB and p53 (11, 15). Removal of the C-terminal 30 amino acids of p53 diminished the ability of p53 to associate with CSB (Fig. 4b, compare lane 2 with lane 3 and lane 11 with lane 12). Similarly, p53Δ30 interacted more weakly with mutant CSB proteins that were missing their N- or C-terminal regions (Fig. 4b, compare lane 14 with lane 15 and lane 17 with lane 18). Examining the interaction of p53 with specific regions of CSB revealed that both the ATPase domain (CSB-M) and C-terminal region (CSB-C) of CSB could interact with p53 (Fig. 4b, lanes 24 and 27), and the ATPase domain of CSB appeared to bind p53 better than full-length CSB. Interestingly, removing the C-terminal 30 amino acids of p53 impaired the ability of p53 to interact with the C-terminal region of CSB (Fig. 4b, lane 26) but had no effect on the interaction of p53 with the ATPase domain of CSB (lane 23). On the other hand, the N-terminal region of CSB appeared to be dispensable for p53 interaction (Fig. 4b, lanes 20 and 21). CSB has been shown to interact with chromatin through both its central ATPase domain and C-terminal region, and evidence suggests that the N-terminal region of CSB can function as a negative regulatory domain for chromatin interaction by occluding the chromatin interaction surface (5). The data from the GST pulldown assays indicate that CSB also interacts with p53 through both its ATPase domain and C-terminal region and raise the hypothesis that p53 decreases the nucleosome association of CSB by occluding a chromatin interaction surface on CSB. This notion is supported by the observations that p53 increased the Km of CSBΔN for nucleosomes more than that of full-length CSB (Table 1).
DISCUSSION
The ATP-dependent chromatin remodeler CSB has been shown to interact with the tumor suppressor p53, and both proteins play important roles in a variety of overlapping nuclear processes (8, 11, 15, 22, 36). In this study, we have found that the association of CSB and p53 with chromatin is reciprocally regulated, and we have provided mechanistic insights into this regulation and how these two proteins may coordinate their activities.
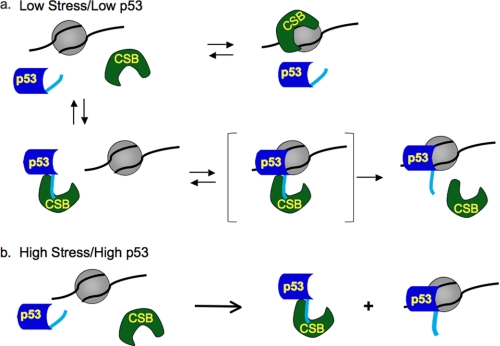
From our results, we present a model whereby CSB facilitates the sequence-independent chromatin association of p53 when p53 levels are low (Fig. 6a), and p53 interferes with CSB-chromatin association when p53 levels are up-regulated (Fig. 6b). This reciprocal relationship is primarily achieved through direct interactions involving the C-terminal 30 amino acids of p53 and the central ATPase domain and C-terminal region of CSB, which overlaps with the chromatin interaction surface of CSB (5, 11). In general, CSB interacts with chromatin dynamically (5, 37). Most chromatin interactions are nonproductive, but, occasionally, productive chromatin interactions stimulate the ATPase activity of CSB, promoting the transition of CSB from a closed to an open conformation (Fig. 6, green) (5). This open CSB protein would have an exposed chromatin interaction surface that could interact with the C-terminal region of p53 (Fig. 6, light blue) and facilitate the association of the p53 core domain with chromatin. The enhancement of p53-nucleosome binding by CSB is reminiscent of p53 activation by the C-terminus-binding monoclonal antibody PAb421 and by deletion or covalent modification of the C-terminal 30 amino acids of p53, which enhance p53-DNA binding in both sequence-dependent and sequence-independent manners (33, 34).
FIGURE 6.
Model for the reciprocally regulated chromatin association of p53 and CSB. a, the interaction of CSB with chromatin stimulates ATP hydrolysis by CSB and leads to the generation of an open CSB conformation (green) (5). The exposed ATPase domain and C-terminal region of CSB can now interact with either chromatin or the C-terminal 30 amino acids of p53 (light blue). When p53 levels are low, the interaction of CSB with the C-terminal domain of p53 promotes the sequence-independent association of the p53 core domain with chromatin. b, when p53 levels are elevated upon stress, p53-chromatin association is independent of CSB. Increased levels of p53 sequester CSB, preventing CSB-chromatin association (11). The bracketed trimeric complex in a is an intermediate that is transient or unstable in the gel shift assays.
Under conditions of normal cell growth or low stress, when p53 levels are relatively low, both CSB-chromatin and CSB-p53 interactions would occur (Fig. 6a). However, under conditions of high stress, when p53 levels are drastically elevated, CSB-p53 interactions would increase, promoting a decrease in CSB-chromatin associations by occluding the chromatin interaction surface of the CSB protein (Fig. 6b).
It has been suggested that the N-terminal region of CSB participates in substrate recognition (5). Therefore, p53 binding to the central ATPase domain and C-terminal region of CSB may free up the N-terminal substrate recognition domain and lead to a more efficient search by CSB for its preferred substrate, such as DNA lesion-stalled transcription (Fig. 6a). A prediction from this model would be that CSB-mediated DNA repair would be less efficient in cells defective for the p53 protein. Indeed, evidence suggests that cells from patients with Li-Fraumeni syndrome, which have p53 mutations, can be less efficient in transcription-coupled repair as well as global genome nucleotide excision repair (15, 24, 25).
In addition to DNA repair, CSB can also function as a transcription elongation factor (38–40). It has been suggested that activated p53 can sequester CSB and inhibit its function in transcription elongation, resulting in stalled transcription (11). Such stalling can lead to locally decondensed chromatin at certain loci during mitosis and, consequently, metaphase chromosome fragility (11). The results presented here are consistent with the hypothesis that p53 up-regulation can result in CSB sequestration (Fig. 6b and Table 1).
What could be the biological significance of the CSB-mediated enhancement of p53-chromatin association (34, 41)? As has been suggested for sequence-specific transcription factors, p53 may bind to chromatin in a sequence-independent manner to reduce the search for its targets from three dimensions to one, for instance by sliding along DNA (42, 43). Our in vitro studies revealed that CSB facilitated p53 binding to nucleosomes only in a sequence-independent manner (Fig. 3). However, our in vivo observations revealed that CSB could also enhance the association of p53 with its response elements inside cells (Fig. 5). Thus, the increased sequence-independent chromatin association, mediated by CSB, may lead to enhanced scanning by p53 for its response elements, and this may account for our in vivo observations (Fig. 5, b and c). Accordingly, by promoting p53-chromatin association, CSB may assist p53 in monitoring and maintaining genome integrity, such as in scanning for damaged DNA or in helping p53 find its target genes.
In addition to CSB, p53 has been found to interact with enzymes important for nucleotide excision repair, such as XPB and XPD (15). Of interest, the C-terminal region of p53 also mediates interaction with XPB and XPD. It has been suggested that p53 can directly modulate the enzymatic activities of DNA repair proteins (15). The mechanism we propose here may represent another aspect of p53 function in DNA repair, i.e. enhancing chromatin scanning by DNA repair proteins and thus facilitating their targeting to their sites of action (42).
Given that the biological response to p53 activity is intimately tied to p53 levels, it is tempting to speculate that the dynamic relationship between the chromatin association of p53 and CSB we have described here could be part of a sensing mechanism that a cell uses to monitor genome integrity. For instance, in the absence of CSB, cells will readily undergo apoptosis when challenged with agents that stall transcription, such as exposure to UV irradiation. Therefore, CSB may participate in tailoring the response of a cell to p53 activation, impacting the decision between cell survival and programmed cell death as well as the aging process.
Supplementary Material
Acknowledgments
We are especially grateful to Geeta Narlikar for critical discussions of the model. We thank Girish Hemashettar for technical assistance. We are grateful to Geeta Narlikar, Julia Leu, and Donna George for critical reading of this manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grant GM 084983 (to H.-Y. F.). This work was also supported by the Academic Development Fund of the University of Pennsylvania.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2 and Table S1.
- CSB
- Cockayne syndrome complementation group B.
REFERENCES
- 1. Hogan C., Varga-Weisz P. (2007) Mutat. Res. 618, 41–51 [DOI] [PubMed] [Google Scholar]
- 2. Peterson C. L. (2002) EMBO Rep. 3, 319–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Flaus A., Martin D. M., Barton G. J., Owen-Hughes T. (2006) Nucleic Acids Res. 34, 2887–2905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Narlikar G. J., Fan H. Y., Kingston R. E. (2002) Cell 108, 475–487 [DOI] [PubMed] [Google Scholar]
- 5. Lake R. J., Geyko A., Hemashettar G., Zhao Y., Fan H. Y. (2010) Mol. Cell 37, 235–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Citterio E., Van Den Boom V., Schnitzler G., Kanaar R., Bonte E., Kingston R. E., Hoeijmakers J. H., Vermeulen W. (2000) Mol. Cell. Biol. 20, 7643–7653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Licht C. L., Stevnsner T., Bohr V. A. (2003) Am. J. Hum. Genet. 73, 1217–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Newman J. C., Bailey A. D., Weiner A. M. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 9613–9618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stevnsner T., Muftuoglu M., Aamann M. D., Bohr V. A. (2008) Mech. Ageing Dev. 129, 441–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Troelstra C., van Gool A., de Wit J., Vermeulen W., Bootsma D., Hoeijmakers J. H. (1992) Cell 71, 939–953 [DOI] [PubMed] [Google Scholar]
- 11. Yu A., Fan H. Y., Liao D., Bailey A. D., Weiner A. M. (2000) Mol. Cell 5, 801–810 [DOI] [PubMed] [Google Scholar]
- 12. Yuan X., Feng W., Imhof A., Grummt I., Zhou Y. (2007) Mol. Cell 27, 585–595 [DOI] [PubMed] [Google Scholar]
- 13. Hanawalt P. C., Spivak G. (2008) Nat. Rev. Mol. Cell Biol. 9, 958–970 [DOI] [PubMed] [Google Scholar]
- 14. Fousteri M., Vermeulen W., van Zeeland A. A., Mullenders L. H. (2006) Mol. Cell 23, 471–482 [DOI] [PubMed] [Google Scholar]
- 15. Wang X. W., Yeh H., Schaeffer L., Roy R., Moncollin V., Egly J. M., Wang Z., Freidberg E. C., Evans M. K., Taffe B. G., Bohr V. A., Weeda G., Hoeijmakers J. H., Forrester K., Harris C. C. (1995) Nat. Genet. 10, 188–195 [DOI] [PubMed] [Google Scholar]
- 16. Ko L. J., Prives C. (1996) Genes Dev. 10, 1054–1072 [DOI] [PubMed] [Google Scholar]
- 17. Levine A. J. (1997) Cell 88, 323–331 [DOI] [PubMed] [Google Scholar]
- 18. Vigneron A., Vousden K. H. (2010) Aging 2, 471–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vousden K. H., Prives C. (2009) Cell 137, 413–431 [DOI] [PubMed] [Google Scholar]
- 20. Beckerman R., Prives C. (2010) Cold Spring Harb. Perspect. Biol. 2, a000935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sahin E., Colla S., Liesa M., Moslehi J., Müller F. L., Guo M., Cooper M., Kotton D., Fabian A. J., Walkey C., Maser R. S., Tonon G., Foerster F., Xiong R., Wang Y. A., Shukla S. A., Jaskelioff M., Martin E. S., Heffernan T. P., Protopopov A., Ivanova E., Mahoney J. E., Kost-Alimova M., Perry S. R., Bronson R., Liao R., Mulligan R., Shirihai O. S., Chin L., DePinho R. A. (2011) Nature 470, 359–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Filippi S., Latini P., Frontini M., Palitti F., Egly J. M., Proietti-De-Santis L. (2008) EMBO J. 27, 2545–2556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee S., Elenbaas B., Levine A., Griffith J. (1995) Cell 81, 1013–1020 [DOI] [PubMed] [Google Scholar]
- 24. Ford J. M., Hanawalt P. C. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 8876–8880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Therrien J. P., Drouin R., Baril C., Drobetsky E. A. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 15038–15043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fan H. Y., Trotter K. W., Archer T. K., Kingston R. E. (2005) Mol. Cell 17, 805–815 [DOI] [PubMed] [Google Scholar]
- 27. Shrader T. E., Crothers D. M. (1989) Proc. Natl. Acad. Sci. U.S.A. 86, 7418–7422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fan H. Y., He X., Kingston R. E., Narlikar G. J. (2003) Mol. Cell 11, 1311–1322 [DOI] [PubMed] [Google Scholar]
- 29. Szak S. T., Mays D., Pietenpol J. A. (2001) Mol. Cell. Biol. 21, 3375–3386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Narlikar G. J., Phelan M. L., Kingston R. E. (2001) Mol. Cell 8, 1219–1230 [DOI] [PubMed] [Google Scholar]
- 31. Campeau E., Ruhl V. E., Rodier F., Smith C. L., Rahmberg B. L., Fuss J. O., Campisi J., Yaswen P., Cooper P. K., Kaufman P. D. (2009) PLoS ONE 4, e6529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mattia M., Gottifredi V., McKinney K., Prives C. (2007) Mol. Cell. Biol. 27, 1309–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hupp T. R., Meek D. W., Midgley C. A., Lane D. P. (1992) Cell 71, 875–886 [DOI] [PubMed] [Google Scholar]
- 34. Selivanova G., Iotsova V., Kiseleva E., Ström M., Bakalkin G., Grafström R. C., Wiman K. G. (1996) Nucleic Acids Res. 24, 3560–3567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fresht A. (1998) Structure and Mechanism in Protein Science, 1st Ed., W. H. Freeman & Co., New York [Google Scholar]
- 36. Liu Y., Kulesz-Martin M. (2001) Carcinogenesis 22, 851–860 [DOI] [PubMed] [Google Scholar]
- 37. van den Boom V., Citterio E., Hoogstraten D., Zotter A., Egly J. M., van Cappellen W. A., Hoeijmakers J. H., Houtsmuller A. B., Vermeulen W. (2004) J. Cell Biol. 166, 27–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tantin D., Kansal A., Carey M. (1997) Mol. Cell. Biol. 17, 6803–6814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee S. K., Yu S. L., Prakash L., Prakash S. (2001) Mol. Cell. Biol. 21, 8651–8656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Selby C. P., Sancar A. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 11205–11209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kern S. E., Kinzler K. W., Baker S. J., Nigro J. M., Rotter V., Levine A. J., Friedman P., Prives C., Vogelstein B. (1991) Oncogene 6, 131–136 [PubMed] [Google Scholar]
- 42. McKinney K., Mattia M., Gottifredi V., Prives C. (2004) Mol. Cell 16, 413–424 [DOI] [PubMed] [Google Scholar]
- 43. Tafvizi A., Huang F., Leith J. S., Fersht A. R., Mirny L. A., van Oijen A. M. (2008) Biophys. J. 95, L01–L03 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.