Abstract
The aggregation of α-synuclein (α-Syn), the primary component of Lewy bodies, into high molecular weight assemblies is strongly associated with Parkinson disease. This event is believed to result from a conformational change within native α-Syn. Molecular chaperones exert critical housekeeping functions in vivo including refolding, maintaining in a soluble state, and/or pacifying protein aggregates. The influence of the stress-induced heat shock protein 70 (Hsp70) on α-Syn aggregation has been notably investigated. The constitutively expressed chaperone Hsc70 acts as an antiaggregation barrier before cells are overwhelmed with α-Syn aggregates and Hsp70 expression induced. Here, we investigate the interaction between Hsc70 and α-Syn, the consequences of this interaction, and the role of nucleotides and co-chaperones Hdj1 and Hdj2 as modulators. We show that Hsc70 sequesters soluble α-Syn in an assembly incompetent complex in the absence of ATP. The affinity of Hsc70 for soluble α-Syn diminishes upon addition of ATP alone or together with its co-chaperones Hdj1 or Hdj2 allowing faster binding and release of client proteins thus abolishing α-Syn assembly inhibition by Hsc70. We show that Hsc70 binds α-Syn fibrils with a 5-fold tighter affinity compared with soluble α-Syn. This suggests that Hsc70 preferentially interacts with high molecular weight α-Syn assemblies in vivo. Hsc70 binding certainly has an impact on the physicochemical properties of α-Syn assemblies. We show a reduced cellular toxicity of α-Syn fibrils coated with Hsc70 compared with “naked” fibrils. Hsc70 may therefore significantly affect the cellular propagation of α-Syn aggregates and their spread throughout the central nervous system in Parkinson disease.
Keywords: Heat Shock Protein, Parkinson Disease, Protein Assembly, Protein Folding, Synuclein, Co-chaperones, Fibrils, Hsc70, Oligomers
Introduction
Fibrillar α-synuclein (α-Syn)2 is one of the principal components of intracellular Lewy bodies, whose presence in the central nervous system is a defining feature of Parkinson disease (PD) and other synucleinopathies (1). Symptoms of PD are apparent after more than 70% of dopaminergic terminals and/or neurons have been lost, with some of the remaining neurons containing filamentous α-Syn. The precise cellular function of soluble α-Syn is unknown. There is, however, evidence that it plays an important role at presynaptic termini (2–4) and it has recently been suggested that the protein is a cellular ferrireductase (5). Various factors, including genetic susceptibility and environmental influences, can induce the aggregation of the naturally unfolded, soluble α-Syn (6, 7), leading to PD pathogenesis.
Molecular chaperones assist newly synthesized proteins to reach their native-fold and are in charge of refolding misfolded or unfolded proteins (8). As the progression of PD is strongly associated with a change in α-Syn solubility, which is widely believed to be the consequence of a conformational change, there has been significant focus on the possible therapeutic role of molecular chaperones in the past 5 years (9). Previous investigations have focused on heat shock protein 70 (Hsp70) (10–13), a molecular chaperone whose expression is induced when a cellular environment is under stress such as heat shock, ischemia, or other stresses (14). The reports are, however, conflicting as, whereas Hsp70 has been shown to inhibit α-Syn fibril formation (11, 15), protect against α-Syn toxicity in vitro (13, 16, 17), and to reduce the amount of α-Syn aggregates in vivo (13), Hsp70 overexpression has also been shown neither to influence the formation of α-Syn oligomeric species nor to counteract the unbeneficial motor deficits associated to α-synucleinopathy in a mouse model (18).
The constitutively expressed heat shock protein Hsc70 is a major member of the 70-kDa HSP family (19). Human Hsc70 and Hsp70 share 85% primary structure identity, and whereas it has always been thought that the two chaperones play similar cellular roles, there is evidence to prove otherwise (20, 21). Hsc70 and its co-chaperones from the HSP40 family confront high molecular weight α-Syn assemblies prior to Hsp70, as the expression of the latter protein is stress dependent. Thus, Hsc70 action, if any, is critical in the early stages of α-Syn aggregation. It is only when this constitutive molecular chaperone and its co-chaperones are overwhelmed that Hsp70 expression is induced and comes into play.
Here we document the effect of Hsc70 on α-Syn assembly. We show that Hsc70 inhibits α-Syn assembly by sequestering it in an assembly incompetent state, and describe the nucleotide dependence of this interaction. An interaction between Hsc70 and preformed α-Syn fibrils is also described and we show that the fibrils can be saturated with Hsc70. The affinities of Hsc70 for soluble and fibrillar α-Syn were determined and the apparent dissociation constants measured show that Hsc70 binds to fibrillar α-Syn with a higher affinity. The effect of Hsc70 co-chaperones Hdj1 and Hdj2 on α-Syn assembly is also documented. Finally, our results reveal a major conformational change within Hsc70 upon binding to oligomeric α-Syn, and a reduced toxicity of α-Syn fibrils coated with Hsc70 compared with “naked” α-Syn fibrils.
EXPERIMENTAL PROCEDURES
Expression and Purification of α-Syn, Hsc70, and Its Co-chaperones Hdj1 and -2
Recombinant wild-type α-Syn was expressed in Escherichia coli strain BL21(DE3) (Stratagene) and purified as described (22). α-Syn concentration was determined spectrophotometrically using an extinction coefficient of 5960 m−1 cm−1 at 280 nm. Pure α-Syn (0.5–1 mm) in 50 mm Tris-HCl, pH 7.5, 50 mm KCl was filtered through sterile 0.22-μm filters and stored at −80 °C.
The vectors allowing the bacterial expression of His6, N-terminal-tagged Hsc70, Hdj1, and Hdj2 were obtained by subcloning the BamHI-NotI fragments from pcDNA5/FRT/TO-HSPA8 (Hsc70), pcDNA5/FRT/TO-DNAJB1 (Hdj1), and pcDNA5/FRT/TO-DNAJA1 (Hdj2) plasmids (23) into pPRO-EX-HTb (Invitrogen) at the same cloning sites. BL21(DE3) E. coli were transformed with the resulting expression vectors. The cells were then grown to an absorbance at 600 nm of 2 in a 16-liter fermentor at 37 °C. Protein expression was induced with 0.5 mm isopropyl 1-thio-β-d-galactopyranoside for 3 h at 20 °C. The cells were harvested and the pellets were resuspended in 25 mm HEPES, pH 7.5, 300 mm NaCl, 5% glycerol, and 5 mm β-mercaptoethanol, and lysed by freezing in liquid nitrogen followed by thawing, addition of 0.1 mg/ml of lysozyme, 10 mm imidazole, and sonication at 4 °C. After centrifugation at 5000 × g and 4 °C, the supernatant was loaded onto a nickel-charged chelating Sepharose column (GE Healthcare) equilibrated in 25 mm HEPES, pH 7.5, 300 mm NaCl, 40 mm imidazole, pH 8.0, and 5% glycerol, and eluted by a gradient of 40–500 mm imidazole. The fractions containing Hsc70, Hdj1, and Hdj2 were assembled and dialyzed against 50 mm Tris-HCl, pH 7.5, 150 mm KCl, filtered through a sterile 0.22-μm filter and stored at −80 °C. The activity of the purified Hsc70 alone or in the presence of its co-chaperones Hdj1 and Hdj2 was assessed using a luciferase refolding assay. Briefly, firefly luciferase (Sigma) at 1 mg/ml was denatured in 7 m guanidine hydrochloride for 2 h at room temperature. 1 μl of denatured luciferase was added to 139 μl of refolding buffer (25 mm HEPES, pH 7.5, 50 mm KCl, 5 mm MgCl2, 2 mm DTT) in the absence or presence of Hsc70 (2 μm) alone or Hsc70 and Hdj1 or Hdj2 (2 μm). The mixtures were incubated at 30 °C and the time course of refolding activity was measured, in the absence or presence of 2 mm ATP or ADP, by withdrawing 5-μl aliquots and mixing with 95 μl of luciferase assay reagent (Promega) at different time intervals. Luminescence was only observed in the presence of ATP, suggesting that Hsc70 is only fully functional (binding, refolding, and releasing client proteins) where ATP is present. Luminescence measurements were performed in a Cary Eclipse fluorescence spectrophotometer (Varian Inc., Palo Alto, CA) in bioluminescence mode at 550 nm. Native luciferase activity was taken as 100%. The ATPase activity of Hsc70 alone or in the presence of its co-chaperones and unfolded luciferase was also monitored as described (24).
Assembly of α-Syn into Fibrils
Soluble wild-type α-Syn was assembled in 50 mm Tris-HCl, pH 7.5, 150 mm KCl, 15 μm thioflavin T (ThioT), in the absence or presence of Hsc70, with or without ADP-MgCl2 or ATP-MgCl2 and co-chaperones Hdj1 or Hdj2 in 1-ml cuvettes (path length 1 cm). Assembly was induced by incubating the cuvette at 37 °C with magnetic stirring in the Cary Eclipse spectrofluorimeter with excitation and emission wavelengths set at 440 and 480 nm, respectively, and an averaging time of 10 s. The nature of the oligomeric species was assessed using a Jeol 1400 transmission electron microscope (Jeol Ltd.) following adsorption of the samples onto carbon-coated 200-mesh grids and negative staining with 1% uranyl acetate. The images were recorded with a Gatan Orius CCD camera (Gatan). The assembly of α-Syn into fibrils was also assessed using a sedimentation assay. Aliquots (90 μl) were withdrawn at different time intervals from the assembly reaction and spun at 40,000 × g at 20 °C in a TL100 tabletop ultracentrifuge (Beckman). The proteins within the supernatant and pellet fractions were analyzed by SDS-PAGE and quantified following staining/destaining using the ImageJ software.
Analytical Ultracentrifugation
Sedimentation velocity measurements were carried out using a Beckman Optima XL-A ultracentrifuge equipped with an AN60-Ti four-hole rotor and cells with two-channel 12-mm path length centerpieces. 400 μl of protein were spun at 90,000 × g at 15 °C. Sample displacement profiles were obtained by recording the absorbance at 280 nm every 5 min. Data were analyzed with the programs Sedfit (25) and Svedberg. The partial specific volume (0.7305 ml/g), buffer viscosity (1.1534 cP), and density (1.00765 g/ml) were calculated with the software Sednterp.
Circular Dichroism
For CD measurements, 5, 10, or 25 μm Hsc70 was incubated for 1 h at 37 °C in 50 mm Tris-HCl, pH 7.5, 50 mm KCl, 0.5 mm ATP, and 0.05 mm MgCl2 with 50 μm soluble α-Syn. Far-UV CD spectra were recorded at 20 °C using a JASCO J-810 dichrograph equipped with a thermostated cell holder using a 0.1-cm path length quartz cuvette. Each spectrum was the average of 10 acquisitions recorded in the 260–195 nm range with 0.5-nm steps, a bandwidth of 2 nm, and at a speed of 100 nm min−1. All spectra were buffer corrected.
Filter Trap Assay, SDS-PAGE, Semidenaturing Detergent-Agarose Gel Electrophoresis (SDD-AGE), and Western Blotting
Binding of Hsc70 to fibrillar α-Syn was followed by a filter retardation assay where fibrils and associated proteins are retained on a membrane. α-Syn fibrils (0.5, 1, or 2 μm) were incubated for 1 h at 37 °C with increasing concentrations of Hsc70 (0.1 to 1 μm), in 650 μl of assembly buffer or 0.5% SDS. 200 μl of each sample were filtered in triplicate through cellulose acetate membranes (0.2 μm pore size, Millipore Corp., Bedford, MA) using a 48-slot slot-blot filtration apparatus (GE Healthcare). After filtration of the sample, 500 μl of buffer or 0.5% SDS were filtered twice in each slot. The cellulose acetate membranes were incubated with 3% skim milk, probed with an antibody against Hsc70 (Assay Designs, MI), and developed with the enzyme-coupled luminescence technique (ECL, Thermo Scientific) according to the recommendation of the manufacturer. SDS-PAGE was performed in 7.5–13% Tricine-SDS-polyacrylamide gels (26).
SDD-AGE was carried out as previously described (27). Briefly, samples of α-Syn (100 μm) in the absence or presence of Hsc70 (1–10 μm), at the beginning (0 min) or at the steady state of the assembly reaction (1000 min) were mixed with 4× sample buffer (2× Tris acetate-EDTA (TAE), 20% glycerol, 8% SDS, 0.05% bromphenol blue). After 10 min of incubation at room temperature, samples were loaded onto a 1.6% agarose gel in 1× TAE buffer containing 0.1% SDS. Following electrophoresis, proteins were blotted onto nitrocellulose membranes by capillary transfer in Tris-buffered saline buffer. The membrane was analyzed by Western blotting using an anti-α-Syn antibody (BD Biosciences) and chemiluminescence reagents (Pierce) before stripping the antibody by heating for 1 h at 50 °C in 62.5 mm Tris-HCl, pH 6.8, 2% SDS, 100 mm β-mercaptoethanol, and reanalyzing with an anti-Hsc70 antibody (Assay Designs) and chemiluminescence reagents.
Cell Viability Assays
Murine endothelioma H-END cells were kindly provided by Prof. F. Bussolino (University of Turin, Italy) and cultured in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum (FBS), 2.0 mm glutamine, 100 units/ml of penicillin, and 100 μg/ml of streptomycin in a 5% CO2 humidified atmosphere at 37 °C. All materials used for cell culture were from PAA Laboratoires GmbH (Pasching, Austria). The toxicity of soluble and fibrillar α-Syn was assessed by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide reduction inhibition assay, as previously described (28). Formazan absorbance was measured at 570 nm in a FlexStation3 microplate reader (Molecular Devices).
RESULTS
Effect of Hsc70 on α-Syn Assembly
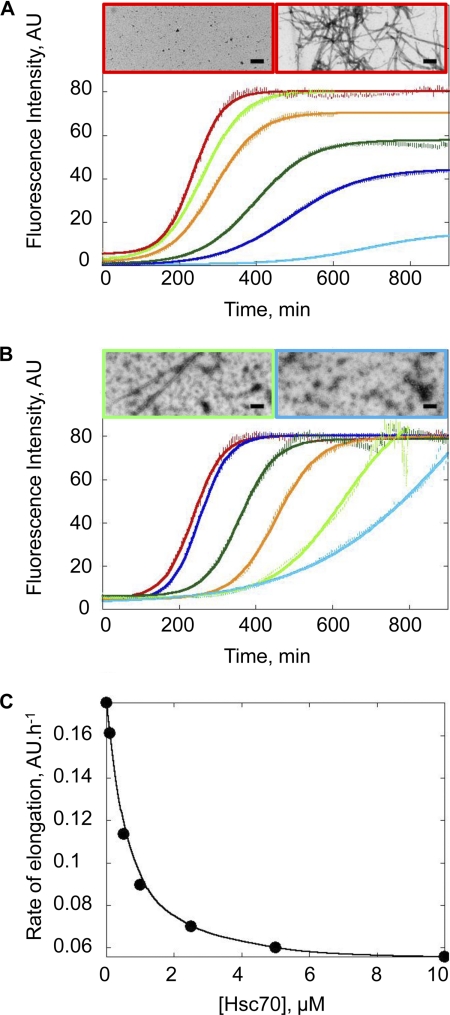
The effect of Hsc70 on α-Syn assembly was monitored using ThioT binding, a widely used dye that fluoresces upon binding to fibrillar species (29), and electron microscopy (EM). In the absence of Hsc70, α-Syn readily assembles into fibrils in vitro, with a lag phase of ∼160 min at 100 μm. The lack of fibrils at the onset of the assembly reaction, and their presence after 1000 min was confirmed by EM after negative staining (Fig. 1A). The length of the lag phase preceding α-Syn assembly increased upon addition of increasing concentrations of Hsc70, indicating that the chaperone slows down α-Syn assembly. EM images of sample aliquots removed at the steady state show that as the concentration of Hsc70 increases, there are less and less fibrils present with a concomitant increase in the amount of oligomeric species (Fig. 1B). Where 10 μm Hsc70 is present (molar ratio 1:10, Hsc70:α-Syn), no fibrils are observed in the electron microscope, even though a high ThioT signal was measured. This shows that ThioT not only binds to fibrillar α-Syn but also to the high molecular weight α-Syn-Hsc70 assemblies. The affinity of Hsc70 for soluble α-Syn (Fig. 1C) was derived from measurements of the elongation rates of a constant α-Syn concentration in the presence of increasing concentrations of Hsc70. The observed dissociation constant (Kd) was 0.5 μm−1.
FIGURE 1.
Assembly of α-Syn in the presence of Hsc70. A, time courses of α-Syn assembly at 37 °C and increasing concentrations (10 μm, light blue; 20 μm, dark blue; 30 μm, dark green; 60 μm, orange; 80 μm, light green; and 100 μm, red) in 50 mm Tris-HCl, pH 7.5, 150 mm KCl. The assembly reactions were monitored by ThioT binding. AU, arbitrary units. Negative stained electron micrographs of α-Syn (100 μm) at 0 min (left) and 1000 min (right) after the onset of the assembly reaction. Bar, 0.2 μm. B, time courses of α-Syn (100 μm) assembly in the absence (red) and presence of increasing amounts of Hsc70 (0.1 μm, dark blue; 0.5 μm, dark green; 1 μm, orange; 5 μm, light green; 10 μm, light blue). Negative stained electron micrographs of α-Syn (100 μm) assemblies obtained in the presence of 5 (left) or 10 μm Hsc70 (right) at 1000 min. Bar, 0.2 μm. C, rate of α-Syn fibril elongation at a constant α-Syn concentration (100 μm) and increasing Hsc70 concentrations (0.1–10 μm).
The Interaction between Soluble α-Syn and Hsc70
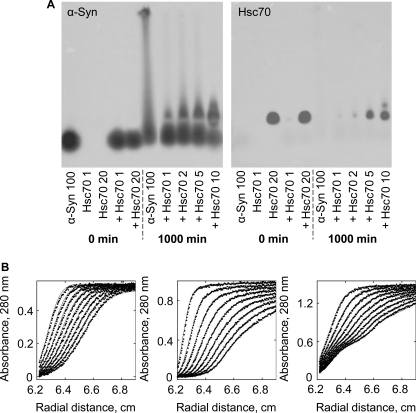
To assess the consequences of Hsc70 addition to assembly competent α-Syn, and better characterize the oligomeric species that form in the presence of Hsc70, we adapted a SDD-AGE assay, first designed to assess the aggregation of yeast prions (30). Comparison of the SDD-AGE profiles of α-Syn in the absence and presence of increasing amounts of Hsc70 at the onset of assembly and at steady state reveal the formation, with time, of α-Syn-Hsc70 assembly incompetent complexes. Although a smear characteristic of fibrillar material was observed upon incubation of α-Syn in the absence of Hsc70, it is no longer apparent in the presence of increasing concentrations of Hsc70. Instead, a slower migrating band was observed, the intensity of which increases with increasing concentrations of Hsc70. This α-Syn band co-localizes with Hsc70 on the gels. This strongly suggests the formation of an α-Syn·Hsc70 assembly incompetent complex.
Analytical ultracentrifugation is a versatile tool used to characterize protein-protein interactions in solution, under equilibrium conditions. We therefore used analytical ultracentrifugation to further characterize the interaction between soluble α-Syn and Hsc70. Fig. 2B shows the sedimentation boundaries of α-Syn (80 μm, left panel), Hsc70 (20 μm, middle panel), and the α-Syn/Hsc70 mixture (80 and 20 μm, respectively, right panel) at regular intervals. Raw data (symbols) were modeled to a 1-, 3-, or 4-component system and the best fits (continuous lines) yielded a single species with sedimentation coefficient (s20,w) of 1 S for α-Syn; three species with sedimentation coefficients 3.8, 5.7, and 8.2 S for Hsc70, and four species with sedimentation coefficients 1, 3.5, 4.8, and 5.9 S for the α-Syn/Hsc70 mixture. No rapidly sedimenting material was detected while the rotor was accelerating to reach the operating speed (180,000 × g), ruling out the presence of large aggregates. The sedimentation coefficient measured for α-Syn (1 S) is compatible with the molecular mass of a natively unfolded 14-kDa polypeptide. The species we observe for Hsc70 have been previously described and correspond to monomeric, dimeric, and oligomeric Hsc70 (31). Determination of sedimentation coefficients gives valuable information on the hydrodynamic shape of protein complexes and conformational changes within these complexes. Instead of an increase in the sedimentation coefficient of Hsc70 in complex with α-Syn, we observed a marked decrease of the sedimentation coefficient of the complex. This is indicative of a major conformational change that takes place within the complex or Hsc70 upon its interaction with α-Syn. This change is further assessed.
FIGURE 2.
Soluble α-Syn-Hsc70 interaction. A, anti-α-Syn (left) and anti-Hsc70 (right) antibody staining after SDD-AGE analysis and Southern blotting onto nitrocellulose membranes of sample aliquots at the onset of assembly (0 min) and at the steady state (1000 min). The concentration of α-Syn (100 μm) is held constant, although Hsc70 varies from 1 to 10 μm as indicated. B, sedimentation velocity of α-Syn (80 μm, left), Hsc70 (20 μm, middle), and α-Syn/Hsc70 mixture (80 and 20 μm, respectively, right) in 50 mm Tris-HCl, pH 7.5, 150 mm KCl. The positions of the moving boundaries shown were recorded at 5-min intervals by spectrophotometric scanning at 280 nm. The continuous lines are best fits of the experimental data (●). The rotor speed was 180,000 × g and the temperature 15 °C.
The Interaction between Fibrillar α-Syn and Hsc70
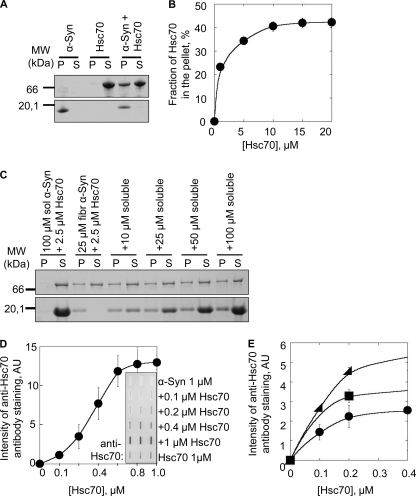
To determine whether Hsc70 interacts with not only soluble but also fibrillar α-Syn, preformed α-Syn fibrils were incubated with Hsc70 for 1 h at 37 °C. The fibrils were pelleted, and the pellet and supernatant fractions were analyzed by SDS-PAGE. As shown in Fig. 3A, controls show that α-Syn is found solely within the pellet, whereas Hsc70 is in the supernatant. After coincubation, Hsc70 was found in the pellet. This indicates that Hsc70 does indeed bind to fibrillar α-Syn. To further characterize the interaction between fibrillar α-Syn and Hsc70, α-Syn fibrils (60 μm) preincubated for 1 h in the presence of increasing concentrations of Hsc70 (0–20 μm) were spun and the amount of Hsc70 in the supernatants and pellets was determined. This yielded a saturation curve (Fig. 3B) showing saturation at a molar ratio of 1:4 for Hsc70:α-Syn. This assay also shows that the binding of Hsc70 to fibrillar α-Syn does not lead to fibrils disassembly, as seen by the sustained absence of soluble α-Syn in the supernatant fractions following incubation with Hsc70.
FIGURE 3.
Fibrillar α-Syn-Hsc70 interaction. A, SDS-PAGE analysis of the pellet (P) and supernatant (S) fractions of fibrillar α-Syn (80 μm), Hsc70 (8 μm), and fibrillar α-Syn (80 μm) incubated with Hsc70 (8 μm) for 1 h at 37 °C. B, fraction of Hsc70 in the pellet after incubation at 37 °C for 1 h at a constant amount of fibrillar α-Syn (80 μm) with increasing concentrations of Hsc70 (0–20 μm), followed by ultracentrifugation at 90,000 × g for 20 min. C, competition between soluble and fibrillar α-Syn for the binding of Hsc70. The binding of Hsc70 to fibrillar α-Syn was assessed by SDS-PAGE analysis of the pellet and supernatant fractions after incubating fibrillar α-Syn (25 μm) with Hsc70 (2.5 μm) and increasing concentrations of soluble α-Syn (0–100 μm), for 1 h at room temperature. D, quantification of the amount of Hsc70 bound to a constant concentration of fibrillar α-Syn (1 μm) using a filter trap assay followed by anti-Hsc70 antibody staining (inset) at increasing Hsc70 concentrations (0–1 μm). E, quantification of the amount of Hsc70 trapped on nitrocellulose filters in the presence of fibrillar α-Syn (circle, 0.5 μm; square, 1 μm; triangle, 2 μm) at increasing concentrations of Hsc70 (0–0.4 μm). The molecular mass markers (in kilodaltons) are shown to the left in A and C. AU, arbitrary units.
Having established that Hsc70 binds to both soluble and fibrillar α-Syn, we designed an assay to determine which form Hsc70 binds preferentially. The assay consisted of challenging Hsc70 bound to α-Syn fibrils with increasing amounts of soluble α-Syn. After the challenge (1 h), the fibrils were sedimented and the amount of Hsc70 in the supernatant and pellet fractions was assessed by SDS-PAGE. The data presented in Fig. 3C show clearly that Hsc70 remains bound to fibrillar α-Syn even when 4-fold soluble α-Syn is added to the reaction mixture. This strongly suggests that Hsc70 binds to fibrillar α-Syn with a higher affinity.
To determine the affinity of Hsc70 for fibrillar α-Syn, a filter assay where fibrils are retained on an acetate membrane, along with anything that binds to them was used. After washing to remove unbound protein, the membranes were stained with an anti-Hsc70 antibody. There is minimal Hsc70 retained on the membrane in the absence of α-Syn fibrils. In contrast, Hsc70 is quantitatively retained where fibrils are present (Fig. 3D). Using this assay and increasing the concentration of fibrillar α-Syn and Hsc70 we determined the affinity of Hsc70 for α-Syn fibrils. The dissociation constant we measured between α-Syn fibrils and Hsc70 is 0.1 μm−1 (Fig. 3E), consistent with a tighter binding of the chaperone to fibrillar α-Syn.
Nucleotide Dependence of Hsc70-α-Syn Interaction and Consequences on Assembly
The affinity of Hsc70 for its client polypeptides is modulated by nucleotides (ATP or ADP) (32, 33). We therefore documented the effect of ATP and ADP on the inhibition of α-Syn assembly by Hsc70, knowing that the assembly of α-Syn is unaffected by the presence of ATP or ADP (supplemental Fig. S1). In the absence of added nucleotides, the client protein binding site of Hsc70 is in an open conformation. Hsc70 binds α-Syn and an assembly inhibitory effect is observed (Fig. 4A). In the presence of ADP, the Hsc70 client protein binding site is in a closed conformation. Hsc70 does not bind α-Syn and no effect on assembly is observed (Fig. 4A). In the presence of ATP, and upon subsequent ATP hydrolysis, the Hsc70 client binding site cycles between an open and a closed conformation with simultaneous binding and release of client proteins within the medium (33, 34). Consistent with this, the assembly of α-Syn into protein fibrils in the presence of Hsc70 and ATP is slightly inhibited but to a lesser extent than observed for nucleotide-free Hsc70 (Fig. 4A). In all cases fibrillar α-Syn is observed at steady state (800 min, electron micrographs Fig. 4B) under the experimental conditions we used. Limited proteolysis of Hsc70-ATP and Hsc70-ADP shows the ADP state to be more resistant to digestion by proteinase K than the ATP state (supplemental Fig. S2). This is coherent with Hsc70 being in a closed conformation with ADP, as the ATPase domain is less susceptible to protease cleavage, and in an open conformation with ATP. The ability of Hsc70 to correctly refold and release chemically denatured luciferase solely in the presence of ATP (supplemental Fig. S3) and its ATPase activity (supplemental Fig. S4) also demonstrates it is fully functional as its activity is modulated by the nature of added nucleotides. We conclude from our observations that the nucleotide-dependent effect of Hsc70 on α-Syn assembly is due to changes in the proportion of open and closed forms of Hsc70.
FIGURE 4.
Effect of nucleotides on the assembly of α-Syn in the presence of Hsc70. A, time courses of α-Syn (100 μm) assembly (○) in the presence of Hsc70 (1 μm) in the absence of nucleotide (▴) and the presence of ATP (0.5 mm, ■) or ADP (0.5 mm, ●) in 50 mm Tris-HCl, pH 7.5, 150 mm KCl, 0.05 mm MgCl2. B, negative stained electron micrographs of α-Syn assemblies at 1000 min, labeled with symbols corresponding to those used for the assembly reactions shown in A. Bar, 0.2 μm. AU, arbitrary units.
Hsc70 Sequesters α-Syn in an Assembly Incompetent State
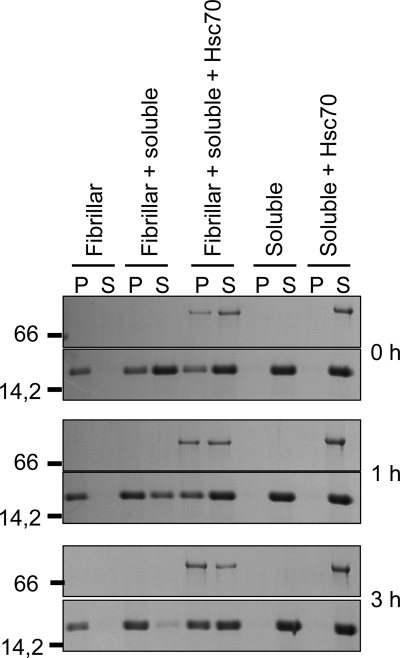
Preformed α-Syn fibrils elongate by efficient incorporation of the soluble form of the protein, which can be followed using the ThioT binding assay. The aggregation of α-Syn into nonfibrillar oligomeric species in the presence of Hsc70 is also accompanied by an increase in ThioT fluorescence (Fig. 1B). We therefore followed the time course of elongation by assessing the decrease of soluble α-Syn in the supernatant and concomitant increase of fibrillar α-Syn in the pellet fractions, after incubation of soluble α-Syn with preformed fibrils and ultracentrifugation using SDS-PAGE. This experiment was performed at room temperature without agitation so that the soluble α-Syn would not assemble. Fig. 5 shows that the vast majority of the soluble α-Syn has been incorporated into the fibrils within 3 h. In contrast, soluble α-Syn remained in the supernatant fraction upon incubation with fibrillar α-Syn when it was preincubated for 30 min with Hsc70 (Fig. 5). This indicates that soluble α-Syn in complex with Hsc70 is not incorporated into preformed fibrils, in other words that Hsc70 sequesters α-Syn in an assembly incompetent state. Interestingly, and in agreement with the higher affinity of Hsc70 for fibrillar α-Syn we measured, a progressive binding of Hsc70 to the fibrils was observed (Fig. 5).
FIGURE 5.
Inhibition of soluble α-Syn incorporation within preformed α-Syn fibrils by Hsc70. Time course of fibrillar α-Syn elongation assessed by SDS-PAGE analysis of the pellet (P) and supernatant (S) fractions after incubating fibrillar α-Syn (25 μm) with soluble α-Syn (25 μm) in the absence or presence of Hsc70 (2.5 μm), as indicated. The molecular mass markers (in kilodaltons) are shown to the left of the panel.
Hsc70 Undergoes a Major Conformational Change upon Binding α-Syn
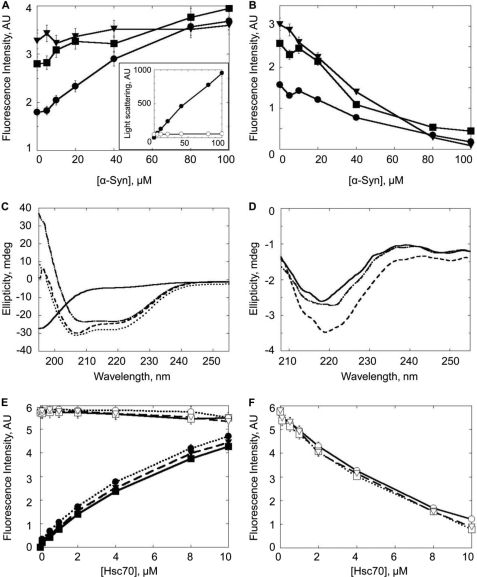
Hsc70 undergoes a remarkable conformational change upon interacting with α-Syn as revealed by the analytical ultracentrifugation measurements we performed (Fig. 2). We further assessed the conformational change accompanying α-Syn binding to Hsc70 using tryptophan fluorescence measurements. Upon titration of different concentrations of Hsc70 (5, 10, or 25 μm) by increasing concentrations of α-Syn (0–100 μm) (Fig. 6A), there is an overall decrease in tryptophan fluorescence (excitation and emission wavelengths 285 and 305 nm, respectively) (Fig. 6B). It is important to note that the changes in fluorescence seen are not the result of Hsc70 aggregation or α-Syn assembly, which we have controlled by measuring the light scattering of the samples (Fig. 6A, inset). We then used circular dichroism to further confirm a conformational change. Fig. 6C shows the CD spectrum of α-Syn, Hsc70, and α-Syn incubated with Hsc70 for 1 h. The theoretical spectrum of the α-Syn·Hsc70 complex is also shown. A significant difference at 222 nm was observed upon subtracting the experimental spectrum from the theoretical spectrum (Fig. 6D). This change corresponds to a decrease in Hsc70 α-helical content. This change varies from 6 to 9% depending on the Hsc70:α-Syn ratio. Finally, whereas a linear increase in tryptophan fluorescence is recorded for increasing concentrations of Hsc70 alone, no such increase was observed in the presence of a constant, saturating concentration of α-Syn (Fig. 6E). When the contribution of increasing concentrations of Hsc70 was subtracted from the overall fluorescence signal, a very significant decrease in tryptophan fluorescence was observed (Fig. 6F). We used this change to assess whether Hsc70 co-chaperones Hdj1 or Hdj2 further affect this conformational change. Neither Hdj1 nor Hdj2 affected the Hsc70 conformational change mediated by its interaction with soluble α-Syn.
FIGURE 6.
Conformational changes within Hsc70 upon interaction with soluble α-Syn. Top panels, changes in tryptophan fluorescence (excitation 285 nm, emission 305 nm) of Hsc70 incubated for 1 h at 37 °C, in 50 mm Tris-HCl, pH 7.5, 150 mm KCl, 0.5 mm ATP, and 0.05 mm MgCl2 with increasing concentrations of soluble α-Syn. A, fluorescence intensity recorded for 5 μm Hsc70 (●), 10 μm Hsc70 (■), or 25 μm Hsc70 (▾) with increasing concentrations of α-Syn. Inset, light scattering (at 350 nm) of Hsc70 (25 μm) with increasing concentrations of α-Syn (0.1 to 100 μm) (○) and fibrillar α-Syn as a control (0 to 100 μm, ●). B, change in fluorescence intensity after subtracting the contribution of α-Syn from the overall signal in α-Syn/Hsc70 mixtures. Middle panel, far-UV circular dichroism. C, spectra of Hsc70 (25 μm, dot-dashed line), α-Syn (50 μm, solid line), and α-Syn·Hsc70 complex (dashed line). The latter spectrum clearly differs from the theoretical one (dotted line) obtained by summing the spectra of Hsc70 and α-Syn. D, difference spectra obtained upon subtracting experimental spectra for 5 (solid line), 10 (dot-dashed line), or 25 μm (dotted line) Hsc70 with 50 μm α-Syn from the calculated theoretical respective spectra. The spectra were obtained at 20 °C using a JASCO J-810 dichrograph equipped with a thermostated cell holder using a 0.1-cm path length quartz cuvette. Each spectrum was the average of 10 acquisitions recorded in the 260–195 nm range with 0.5-nm steps, a bandwidth of 2 nm, and at a speed of 100 nm min−1. Lower panel, changes in tryptophan fluorescence (excitation 285 nm, emission 305 nm) of α-Syn (100 μm) incubated for 1 h at 37 °C, in 50 mm Tris-HCl, pH 7.5, 150 mm KCl, 0.5 mm ATP, and 0.05 mm MgCl2 with increasing concentrations of Hsc70 (0.1 to 10 μm, solid line), Hsc70 and Hdj1 (dotted line), or Hsc70 and Hdj2 (dashed line). A, fluorescence intensity recorded for increasing concentrations of Hsc70, Hsc70 and Hdj1, or Hsc70 and Hdj2 alone (filled symbols) or in the presence of α-Syn (open symbols). B, change in fluorescence intensity after subtracting the contribution of Hsc70, Hsc70 and Hdj1, or Hsc70 and Hdj2 from the overall signal in α-Syn-Hsc70, Hsc70 and Hdj1, or Hsc70 and Hdj2 mixtures.
Hsc70 Co-chaperones Hdj1 and Hdj2 and the Assembly of α-Syn into Fibrils
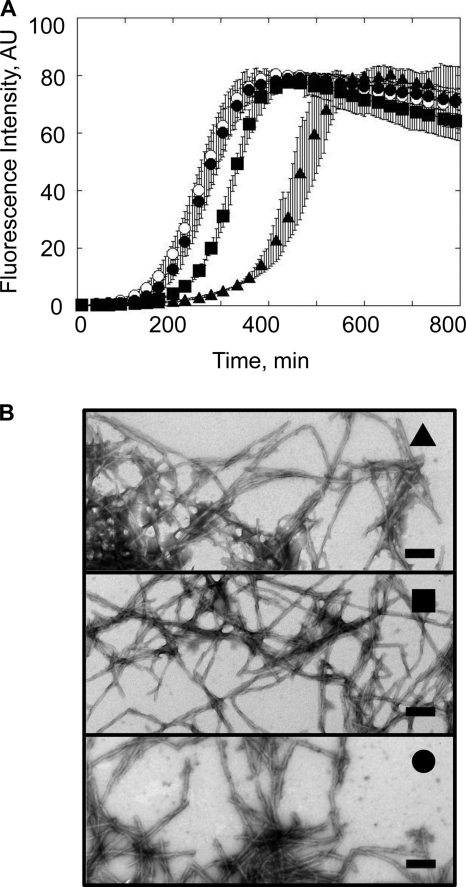
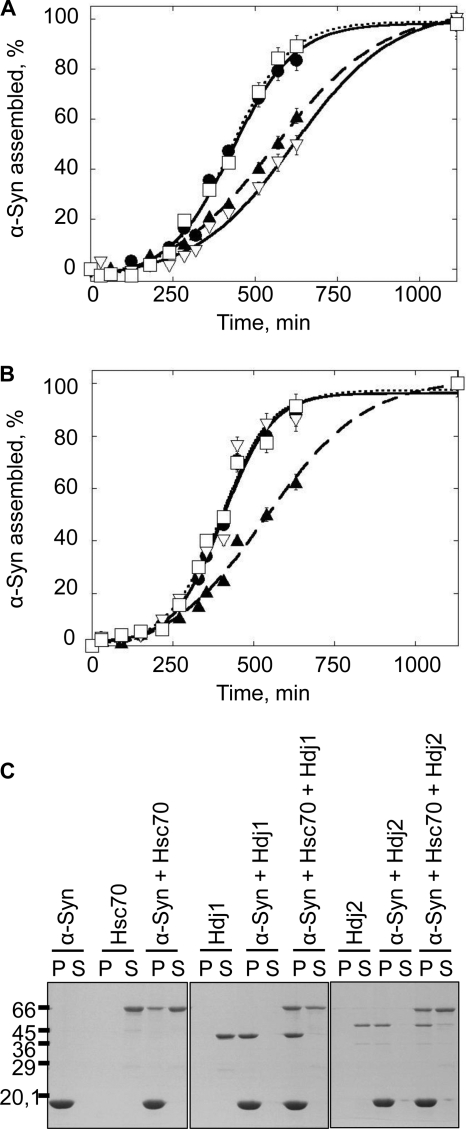
We first assessed the effect of Hdj1 and Hdj2 on α-Syn assembly into fibrils in the absence or presence of Hsc70. As the ThioT signal we recorded upon assembling α-Syn in the presence of Hsc70 and Hdj1 or Hdj2 were noisy, the reactions were monitored by removing aliquots at different time intervals from the assembling samples, spinning the aliquot at 40,000 × g, and quantifying the disappearance of α-Syn from the supernatant and appearance of fibrillar α-Syn by SDS-PAGE. The assembly kinetics of α-Syn (100 μm) in the presence Hsc70 or Hdj1 alone (1 μm) and Hsc70 and Hdj1 (1 μm each) are presented in Fig. 7A. Hdj1 on its own slows down the assembly of α-Syn into fibrils. The Hsc70 concentration we used throughout these experiments (1 μm) significantly delays α-Syn assembly (Fig. 1B). Thus, α-Syn assembly should be further slowed down if the effects of Hsc70 and Hdj1 were additive when both proteins are present within the assembly reaction. Surprisingly, the assembly kinetics of α-Syn in the presence of Hsc70 and Hdj1 and α-Syn in the absence of added chaperones and co-chaperones overlay perfectly well, suggesting that Hdj1 abolishes the assembly inhibitory effect of Hsc70. Similar observations were made with Hdj2 (Fig. 7B). Unlike Hdj1, however, Hdj2 alone has no effect on the assembly of α-Syn. This is consistent with previous work describing the independent effects of DNAJB (Hdj1) and DNAJA (Hdj2) co-chaperone subfamilies on the aggregation of polyglutamine-containing proteins (35). Hsc70 sequesters α-Syn in an assembly incompetent state (Fig. 1B). Hsc70 affinity for α-Syn decreases in the presence of ATP and its assembly inhibitory effects diminishes (Fig. 4A). In the presence of ATP, Hdj1 or Hdj2 speed up the binding and release of client proteins from Hsc70. Thus, the amount of assembly competent α-Syn is higher in the presence of Hsc70 and Hdj1 or Hdj2 than in the presence of Hsc70 or its co-chaperones alone. In addition, the amounts of free Hsc70 and Hdj1 or Hdj2 decrease when the chaperone and co-chaperones are present together because of their interaction. Our observations suggest therefore that Hsc70 co-chaperones most probably counteract the Hsc70 inhibitory activity by increasing the amounts of free assembly competent α-Syn.
FIGURE 7.
Hdj1 and Hdj2 modulate the interaction between soluble or fibrillar α-Syn and Hsc70. A, time courses of α-Syn (100 μm) assembly in the absence (●) or presence of Hsc70 (1 μm) alone (▴), Hdj1 (1 μm) alone (▿), and Hsc70 and Hdj1 (1 μm each, □) in 50 mm Tris-HCl, pH 7.5, 150 mm KCl, 0.5 mm ATP, and 0.05 mm MgCl2. B, time courses of α-Syn (100 μm) assembly in the absence (●) or presence of Hsc70 (1 μm) alone (▴), Hdj2 (1 μm) alone (▿), and Hsc70 and Hdj2 (1 μm each, □) in 50 mm Tris-HCl, pH 7.5, 150 mm KCl, 0.5 mm ATP, and 0.05 mm MgCl2. The assembly reactions in A and B were monitored by quantifying α-Syn within the supernatant and pellet fractions by SDS-PAGE, as described under “Experimental Procedures.” C, SDS-PAGE analysis of the pellet (P) and supernatant (S) fractions of preformed α-Syn fibrils (60 μm) in the absence and presence of Hsc70 (6 μm), Hdj1 (6 μm), Hdj2 (6 μm), or Hsc70 and its co-chaperone (6 μm each), as indicated. Samples were incubated for 1 h at 37 °C prior to 20 min centrifugation at 90,000 × g, 20 °C. The molecular mass markers (in kilodaltons) are shown to the left of B.
We then examined the effect of Hdj1 and Hdj2 on Hsc70 interaction with fibrillar α-Syn. Control reactions where α-Syn fibrils (60 μm) were incubated in the presence of Hdj1 or Hdj2 alone revealed that both co-chaperones bind to α-Syn fibrils with a high affinity as witnessed by their co-sedimentation with fibrillar α-Syn (Fig. 7C). In the presence of Hdj1, increased amounts of Hsc70 bind to and sediment with α-Syn fibrils. A similar increased Hsc70 binding to the fibrils, although to a lesser extent, was observed in the presence of Hdj2. The yeast orthologs of Hdj1 and Hdj2, Sis1p and Ydj1p, are widely believed to bind client proteins and facilitate their interaction with Ssa1p, the Hsc70 yeast ortholog (36). Our observations are consistent with this view and suggest that Hdj1 and Hdj2 mediate the binding of Hsc70 to α-Syn fibrils. Alternatively, the increased binding of Hsc70 to α-Syn fibrils could simply be due to the binding of Hsc70 to fibril-bound Hdj1 or Hdj2.
Physiological Consequences of Hsc70 and Its Co-chaperones Interacting with Fibrillar α-Syn
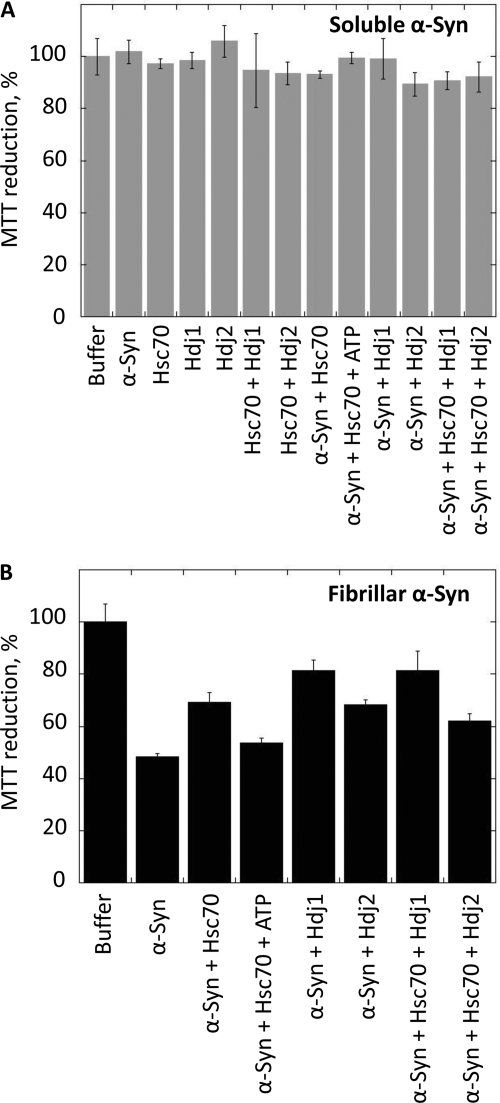
We then assessed the physiological consequences of the interaction of Hsc70 with α-Syn fibrils in the absence or presence of ATP and co-chaperones. α-Syn fibril toxicity was assessed using murine endothelioma H-END cells, previously shown to be highly affected by different protein assemblies (28, 37) using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. Fig. 8A shows that neither soluble α-Syn nor Hsc70 and its co-chaperones affect cell viability. In contrast, α-Syn fibrils are highly toxic to the cells as witnessed by the 52 ± 1% reduction in cell viability observed upon treatment of the cells for 24 h with 1 μm α-Syn fibrils (Fig. 8B). We then determined whether α-Syn fibril toxicity is affected upon their incubation with Hsc70 and/or its co-chaperones. Preformed α-Syn fibrils were therefore incubated for 1 h at 37 °C in the presence of Hsc70, Hdj1, Hdj2 or Hsc70, and Hdj1 or Hdj2. As unbound Hsc70, Hdj1, or Hdj2 could potentially counteract the toxicity of α-Syn fibrils, the fibrils were spun 20 min at 16,100 × g. The pelleted fibrils, free of unbound Hsc70, Hdj1, and Hdj2 were resuspended and diluted in the cell culture medium. The proportion of viable cells increases from 48 ± 1% upon addition of α-Syn fibril to 69 ± 3% in the presence of fibrillar α-Syn incubated with Hsc70. The addition of ATP (0.5 mm) to the latter fibrils prior to their dilution in the cell culture medium restores toxicity as the proportion of viable cells decreases to 54 ± 2%. The proportion of viable cells exposed to fibrillar α-Syn incubated with Hdj1 alone is 81 ± 4%, whereas that of fibrillar α-Syn incubated with Hdj2 alone is 68 ± 2%, consistent with the tighter binding of Hdj1 to fibrillar α-Syn (Fig. 7C). Similar survival rates (81 ± 7 and 66 ± 3%) were measured for cells exposed to fibrillar α-Syn incubated with Hdj1 and Hsc70 or Hdj2 and Hsc70 suggesting that Hdj1 or Hdj2 and Hsc70 do not counteract toxicity in an additive manner. We conclude from these observations that Hsc70 and its co-chaperones binding to fibrillar α-Syn has physiological consequences in that it reduces the toxicity of α-Syn fibrils. We further conclude, upon comparison of the data presented in Figs. 7C and 8B, that the toxicity reduction we observe is proportional to the amount of Hsc70 bound to the fibrils as the latter is significantly higher in the presence of Hdj1 than in the presence of Hdj2 and α-Syn fibrils incubated with Hdj1 and Hsc70 are less toxic than those incubated with Hdj2 and Hsc70.
FIGURE 8.
Viability of H-END cells upon exposure to soluble (A, gray) or fibrillar (B, black) α-Syn in the absence or presence of Hsc70 and/or Hdj1 or Hdj2, as indicated. Before exposure to cells, soluble or fibrillar α-Syn (100 μm) was incubated for 1 h at 37 °C with Hsc70 (25 μm), Hdj1 (25 μm), Hdj2 (25 μm), Hsc70 and Hdj1 (25 μm each protein), or Hsc70 and Hdj2 (25 μm each protein). Fibrillar α-Syn was centrifuged for 20 min at 16,100 × g and the pellet was resuspended in the culture medium to remove unbound Hsc70, Hdj1, or Hdj2. The final protein concentration within the culture medium was 1 μm. The cells were incubated with the different proteins for 24 h. Cell viability is expressed as the percentage of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction using cells treated with the same volume of buffer as a reference (100% 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide reduction). The values are averages ± S.D. obtained from three independent experiments.
DISCUSSION
The pathogenesis of PD is strongly associated with the aggregation of α-Syn into high molecular weight assemblies. This is widely believed to result from a conformational change within soluble α-Syn. Molecular chaperones exert critical housekeeping functions in vivo including refolding, maintaining proteins in a soluble state, and/or pacifying protein aggregates (8). Hsp90, for example, modulates α-Syn assembly (38). The inducible molecular chaperone, Hsp70, has been previously shown to inhibit α-Syn assembly into fibrils (11, 15). This is also the case in our hands, as shown in supplemental Fig. S5. Hsp70 has also been previously shown to reduce the size of α-Syn aggregates in vivo, and protect against α-Syn toxicity (13, 16, 17). However, and in contradiction with the latter, Hsp70 induction has also been shown to be unbeneficial in a mouse model of α-synucleinopathy (18). The constitutive molecular chaperone Hsc70 is among the very first anti-aggregation barriers any aggregation-prone protein must overcome. It is only when Hsc70 activity is overwhelmed that the cellular stress response is induced and the isoform Hsp70 is expressed. Thus, Hsp70 appears late in the cellular response to protein aggregation. Considering that Hsc70 encounters soluble and aggregating α-Syn before its inducible counterpart, it is of importance to investigate the interaction of Hsc70 with soluble and/or fibrillar α-Syn to determine what role, if any, Hsc70 may have in the onset of PD.
We therefore designed experiments to document the interaction between Hsc70 and α-Syn, the consequences of α-Syn-Hsc70 interaction, and the role of modulators of α-Syn-Hsc70 interaction, e.g. nucleotides and Hsc70 co-chaperones. We show here that Hsc70, in vitro and in the absence of ATP, inhibits α-Syn fibril formation (Fig. 1). Hsc70 binds soluble α-Syn with a high affinity (0.5 μm−1). We show using a technique (SDD-AGE) adapted for α-Syn assembly into fibrils, by electron microscopy analysis and fibril elongation assays that α-Syn is maintained within the α-Syn·Hsc70 complex in an assembly incompetent state (Figs. 1, 2, and 5). The interaction between Hsc70 and α-Syn is, as expected, dependent on the nature of the nucleotide bound to Hsc70 and Hsc70 co-chaperones Hdj1 and Hdj2 (Figs. 4 and 7). We demonstrate that the affinity of Hsc70 for α-Syn decreases in the presence of ATP (Fig. 4) and that the sequestering activity of Hsc70 is abolished in the presence of ATP and Hdj1 or Hdj2 (Fig. 7). Analytical ultracentrifugation, intrinsic fluorescence measurements, and circular dichroism reveal a major conformational change occurring within Hsc70 upon its interaction with α-Syn (Figs. 2 and 6). Finally, we also show that Hsc70 binds fibrillar α-Syn (Fig. 3), the consequence of which is a significant reduction in fibril-associated toxicity (Fig. 8).
The major conformational change within Hsc70 we report unequivocally demonstrates that Hsc70 binds, holds, and releases α-Syn in a nucleotide-dependent manner. By facilitating nucleotide exchange, Hsc70 co-chaperones accelerate the binding, holding, and release cycle. In its Hsc70-bound form, α-Syn is assembly incompetent. Consistent with this is the observation that Hsp70 in the absence of added nucleotides and co-chaperones inhibits α-Syn assembly (see Refs. 11 and 15 and supplemental Fig. S5). In the absence of ATP, the chaperone binds client proteins, but as no nucleotide cycling takes place, the client proteins are not released (33, 34). The inhibition effect seen is therefore simply the consequence of α-Syn sequestration. The functional cycle of Hsp70 comprises ATP binding and hydrolysis, an exchange mediated by co-chaperones. As ATP and co-chaperones Hdj1 or Hdj2 are added to the Hsc70, the system closer resembles that of a cellular environment. The proportion of α-Syn bound at any time to Hsc70 decreases and consequently, the pool of free, assembly competent α-Syn increases leading to a reduction in the molecular chaperone assembly inhibitory effect. Therefore, our study reconciles conflicting results. Indeed, we show that ATP and Hsc70 co-chaperones, which are in abundance in vivo, counteract the Hsp70-mediated α-Syn assembly inhibition reported in the absence of added nucleotide. This accounts for why no beneficial effect of Hsp70 overexpression was seen in a mouse model of α-synucleinopathy (18).
The finding that Hsc70 binds to preformed α-Syn fibrils with a higher affinity than soluble α-Syn (Fig. 3) suggests that the main role of Hsc70 within the cell is preferentially binding the high molecular weight α-Syn assemblies as opposed to a minor role in inhibiting α-Syn assembly. We show that besides interacting individually with soluble α-Syn and affecting assembly, Hsc70 co-chaperones Hdj1 and Hdj2 further increase Hsc70 binding to α-Syn assemblies (Fig. 7). Hsc70, Hdj1, and/or Hdj2 binding to high molecular weight α-Syn assemblies undoubtedly affects their physicochemical properties. The first possible consequence is a reduction of α-Syn aggregate size as observed upon overexpression of Hsp70 in vivo (18) that could be due to the binding of Hsc70 and its co-chaperones to preformed fibrils, leading to changes in their bundling propensity. The second possible consequence could be a change in the physiological properties of the fibrils. We therefore compared cell viability in the presence of soluble and fibrillar α-Syn in the absence or presence of Hsc70, Hdj1, Hdj2, and Hsc70 and Hdj1 or Hdj2 (Fig. 8). No toxicity was associated to soluble α-Syn in contrast to what is observed for α-Syn fibrils. The binding of Hsc70 and its co-chaperones to fibrillar α-Syn has an important physiological consequence in that it reduces fibril toxicity.
We and others previously contributed evidence for intercellular propagation of α-Syn aggregates in vivo (39, 40). α-Syn assemblies were in particular shown to be taken up by cells through endocytosis, interact with intracellular α-Syn and seed the assembly of endogenous α-Syn. The cell-to-cell propagation of α-Syn aggregates was also proposed to contribute to the progressive spreading of α-synucleinopathies throughout the nervous system (41, 42). The changes in the physicochemical properties of the high molecular weight α-Syn assemblies upon binding of Hsc70, Hdj1 and/or Hdj2 could certainly affect their capacity to bind the cell membrane and/or be taken up. Thus, intercellular propagation of α-Syn assemblies that are, for example, actively exported or passively released upon neuronal death in the brain may strongly depend on their Hsc70, Hdj1 and/or Hdj2 surface saturation levels. In other words, the changes proffered by tightly bound chaperones may be sufficient to interfere with and/or halt cell to cell transmission, and consequently delay the systematic spread of misfolded α-Syn assemblies strongly associated to Parkinson disease. Further characterization of the potential pacifying role of molecular chaperones and co-chaperones will lead to a better understanding of their role in modulating the propagation of misfolded protein assemblies in Parkinson diseases. Such studies together with a thorough description of the changes affecting the cellular proteostasis machinery upon the aggregation of α-Syn will provide additional insights into the molecular events underlying PD pathogenesis and the cellular defenses selected through evolution to suppress the formation of α-Syn assemblies and/or their intercellular propagation.
Supplementary Material
Acknowledgment
We thank Dr. Harm Kampinga for molecular chaperone plasmids.
This work was supported by the French Ministry of Education, Research and Technology, the Centre National de la Recherche Scientifique (CNRS), the Era-Net Neuron, Agence Nationale pour la Recherche Grant ANR-08-NEUR-001-01, and the Human Frontier Science Program.
This work is dedicated to Dr. Tamas Erdos.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S5.
- α-Syn
- alpha-synuclein
- Hsc
- constitutive heat shock protein
- PD
- Parkinson disease
- SDD-AGE
- semi-denaturing detergent-agarose gel electrophoresis
- ThioT
- thioflavin T
- Tricine
- N-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]glycine.
REFERENCES
- 1. Spillantini M. G., Schmidt M. L., Lee V. M., Trojanowski J. Q., Jakes R., Goedert M. (1997) Nature 388, 839–840 [DOI] [PubMed] [Google Scholar]
- 2. Norris E. H., Giasson B. I., Lee V. M. (2004) Curr. Top. Dev. Biol. 60, 17–54 [DOI] [PubMed] [Google Scholar]
- 3. Jakes R., Spillantini M. G., Goedert M. (1994) FEBS Lett. 345, 27–32 [DOI] [PubMed] [Google Scholar]
- 4. Burré J., Sharma M., Tsetsenis T., Buchman V., Etherton M. R., Südhof T. C. (2010) Science 329, 1663–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Davies P., Moualla D., Brown D. R.(2011) PLoS One 6, e15814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Uversky V. N., Li J., Bower K., Fink A. L. (2002) Neurotoxicology 23, 527–536 [DOI] [PubMed] [Google Scholar]
- 7. Ross C. A., Smith W. W. (2007) Parkinsonism Relat. Disord. 13, Suppl. 3, S309–315 [DOI] [PubMed] [Google Scholar]
- 8. Vabulas R. M., Raychaudhuri S., Hayer-Hartl M., Hartl F. U. (2010) Cold Spring Harbor Perspect. Biol. 2, a004390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Witt S. N. (2010) Biopolymers 93, 218–228 [DOI] [PubMed] [Google Scholar]
- 10. Danzer K. M., Ruf W. P., Putcha P., Joyner D., Hashimoto T., Glabe C., Hyman B. T., McLean P. J. (2011) FASEB J. 25, 326–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dedmon M. M., Christodoulou J., Wilson M. R., Dobson C. M. (2005) J. Biol. Chem. 280, 14733–14740 [DOI] [PubMed] [Google Scholar]
- 12. Huang C., Cheng H., Hao S., Zhou H., Zhang X., Gao J., Sun Q. H., Hu H., Wang C. C. (2006) J. Mol. Biol. 364, 323–336 [DOI] [PubMed] [Google Scholar]
- 13. Klucken J., Shin Y., Masliah E., Hyman B. T., McLean P. J. (2004) J. Biol. Chem. 279, 25497–25502 [DOI] [PubMed] [Google Scholar]
- 14. Richter K., Haslbeck M., Buchner J. (2010) Mol. Cell 40, 253–266 [DOI] [PubMed] [Google Scholar]
- 15. Luk K. C., Mills I. P., Trojanowski J. Q., Lee V. M. (2008) Biochemistry 47, 12614–12625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Opazo F., Krenz A., Heermann S., Schulz J. B., Falkenburger B. H. (2008) J. Neurochem. 106, 529–540 [DOI] [PubMed] [Google Scholar]
- 17. Outeiro T. F., Klucken J., Strathearn K. E., Liu F., Nguyen P., Rochet J. C., Hyman B. T., McLean P. J. (2006) Biochem. Biophys. Res. Commun. 351, 631–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shimshek D. R., Mueller M., Wiessner C., Schweizer T., van der Putten P. H. (2010) PLoS One 5, e10014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ohtsuka K., Suzuki T. (2000) Brain Res. Bull. 53, 141–146 [DOI] [PubMed] [Google Scholar]
- 20. Goldfarb S. B., Kashlan O. B., Watkins J. N., Suaud L., Yan W., Kleyman T. R., Rubenstein R. C. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 5817–5822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hageman J., van Waarde M. A., Zylicz A., Walerych D., Kampinga H. H. (2011) Biochem. J. 435, 127–142 [DOI] [PubMed] [Google Scholar]
- 22. Ghee M., Melki R., Michot N., Mallet J. (2005) FEBS J. 272, 4023–4033 [DOI] [PubMed] [Google Scholar]
- 23. Hageman J., Kampinga H. H. (2009) Cell Stress Chaperones 14, 1–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Melki R., Carlier M. F., Pantaloni D. (1990) Biochemistry 29, 8921–8932 [DOI] [PubMed] [Google Scholar]
- 25. Schuck P. (2000) Biophys. J. 78, 1606–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Laemmli U. K. (1970) Nature 227, 680–685 [DOI] [PubMed] [Google Scholar]
- 27. Halfmann R., Lindquist S. (2008) J. Vis. Exp. 17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pieri L., Bucciantini M., Guasti P., Savistchenko J., Melki R., Stefani M. (2009) Biophys. J. 96, 3319–3330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wolfe L. S., Calabrese M. F., Nath A., Blaho D. V., Miranker A. D., Xiong Y. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 16863–16868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kryndushkin D. S., Alexandrov I. M., Ter-Avanesyan M. D., Kushnirov V. V. (2003) J. Biol. Chem. 278, 49636–49643 [DOI] [PubMed] [Google Scholar]
- 31. Benaroudj N., Batelier G., Triniolles F., Ladjimi M. M. (1995) Biochemistry 34, 15282–15290 [DOI] [PubMed] [Google Scholar]
- 32. Chirico W. J., Markey M. L., Fink A. L. (1998) Biochemistry 37, 13862–13870 [DOI] [PubMed] [Google Scholar]
- 33. Borges J. C., Ramos C. H. (2006) Arch. Biochem. Biophys. 452, 46–54 [DOI] [PubMed] [Google Scholar]
- 34. Freeman B. C., Myers M. P., Schumacher R., Morimoto R. I. (1995) EMBO J. 14, 2281–2292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hageman J., Rujano M. A., van Waarde M. A., Kakkar V., Dirks R. P., Govorukhina N., Oosterveld-Hut H. M., Lubsen N. H., Kampinga H. H. (2010) Mol. Cell 37, 355–369 [DOI] [PubMed] [Google Scholar]
- 36. Kampinga H. H., Craig E. A. (2010) Nat. Rev. Mol. Cell Biol. 11, 579–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pieri L., Bucciantini M., Nosi D., Formigli L., Savistchenko J., Melki R., Stefani M. (2006) J. Biol. Chem. 281, 15337–15344 [DOI] [PubMed] [Google Scholar]
- 38. Falsone S. F., Kungl A. J., Rek A., Cappai R., Zangger K. (2009) J. Biol. Chem. 284, 31190–31199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hansen C., Angot E., Bergström A. L., Steiner J. A., Pieri L., Paul G., Outeiro T. F., Melki R., Kallunki P., Fog K., Li J. Y., Brundin P. (2011) J. Clin. Invest. 121, 715–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Desplats P., Lee H. J., Bae E. J., Patrick C., Rockenstein E., Crews L., Spencer B., Masliah E., Lee S. J. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 13010–13015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Braak H., Del Tredici K., Rüb U., de Vos R. A., Jansen Steur E. N., Braak E. (2003) Neurobiol. Aging 24, 197–211 [DOI] [PubMed] [Google Scholar]
- 42. Brundin P., Melki R., Kopito R. (2010) Nat. Rev. Mol. Cell Biol. 11, 301–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.