Background: COMT is a key enzyme for inactivation and metabolism of catechols, including dopamine.
Results: MB-COMT is located in the cell body, axons and dendrites of cortical neurons, and oriented with its C-terminal catalytic domain in the extracellular space.
Conclusion: MB-COMT can inactivate synaptic and extrasynaptic dopamine on surface of cortical neurons.
Significance: MB-COMT specific inhibitors can be developed and may be less cytotoxic.
Keywords: Cell Surface Enzymes, Enzyme Inhibitors, Neural Metabolism, Neurons, Parkinson Disease, Schizophrenia, Catechol-o-methyltransferase, Cytotoxicity, Dopamine, Tolcapone
Abstract
Catechol-O-methyltransferase (COMT) is a key enzyme for inactivation and metabolism of catechols, including dopamine, norepinephrine, caffeine, and estrogens. It plays an important role in cognition, arousal, pain sensitivity, and stress reactivity in humans and in animal models. The human COMT gene is associated with a diverse spectrum of human behaviors and diseases from cognition and psychiatric disorders to chronic pain and cancer. There are two major forms of COMT proteins, membrane-bound (MB) COMT and soluble (S) COMT. MB-COMT is the main form in the brain. The cellular distribution of MB-COMT in cortical neurons remains unclear and the orientation of MB-COMT on the cellular membrane is controversial. In this study, we demonstrate that MB-COMT is located in the cell body and in axons and dendrites of rat cortical neurons. Analyses of MB-COMT orientation with computer simulation, flow cytometry and a cell surface enzyme assay reveal that the C-terminal catalytic domain of MB-COMT is in the extracellular space, which suggests that MB-COMT can inactivate synaptic and extrasynaptic dopamine on the surface of presynaptic and postsynaptic neurons. Finally, we show that the COMT inhibitor tolcapone induces cell death via the mechanism of apoptosis, and its cytotoxicity is dependent on dosage and correlated with COMT Val/Met genotypes in human lymphoblastoid cells. These results suggest that MB-COMT specific inhibitors can be developed and that tolcapone may be less hazardous at low doses and in specific genetic backgrounds.
Introduction
Genetic associations of the catechol-O-methyltransferase (COMT)2 gene with a diverse spectrum of human behaviors and diseases from cognition and psychiatric disorders to chronic pain and to cancer have revived interest in COMT research (1–4). COMT is a critical enzyme required for inactivation and metabolism of dopamine, norepinephrine, epinephrine, caffeine, estrogen, and other catechol compounds. The COMT gene encodes two major transcripts, the long and short transcripts (5). The long transcript can be translated into two major isoforms of COMT, the membrane-bound (MB) and the soluble (S) forms. MB-COMT is the main form of COMT in the brain and is postulated to play an important role in modulating cortical dopamine signaling (6–9). S-COMT is the main form of COMT in peripheral tissues, including liver and blood, and is postulated to play an important role in detoxification and metabolism of catechol compounds (10–12). MB-COMT has higher affinity for dopamine than S-COMT and is more important for inactivation of dopamine in the brain (13). Studies in mice (9, 14–16) and in humans (8, 17–22) indicate that COMT is particularly important in regulating cortical synaptic dopamine concentrations because of a relative absence of dopamine transporters, which regulate synaptic dopamine levels in the striatum. However, there is no MB-COMT specific inhibitor currently available for studying the roles of MB-COMT in dopamine-dependent brain functions. The only currently available COMT inhibitor that is permeable to the blood-brain barrier is tolcapone, an adjunct drug with l-DOPA (levodopa) for treatment of Parkinson disease (23). Tolcapone inhibits both MB-COMT and S-COMT. Inhibition of the S-COMT may be at least in part, a reason for its liver toxicity, which has led to the complete withdrawal of tolcapone from the European market. Thus, it may be important to develop MB-COMT specific inhibitors.
The COMT gene sequence varies dramatically among species and COMT enzyme activity diminishes substantially in the course of evolution (17), which correlates with dramatic evolutionary changes in cognitive function and other brain functions. The preponderance of MB-COMT in the primate brain relative to the rodent brain also suggests evolutionary refinement of COMT function in brain (17). A well characterized functional polymorphism, the Val-158–Met polymorphism, affects COMT enzyme activity in humans. The met allele, which is a human-specific mutation and possible even a modern human mutation (24), is associated with much lower enzyme activity and better cognitive function but increased pain sensitivity (25). Other polymorphisms in the human COMT gene have also been shown to affect enzyme activity, mRNA levels, mRNA structure and to be associated with clinical phenotypes (17, 25–27). Thus, it is reasonable to suspect that patients with different COMT genotypes might require individualized doses of catechol-based drugs including l-DOPA, consistent with a more personalized medicine strategy.
One of the most extensively characterized roles of COMT is in cortically mediated cognition. More rapid inactivation of dopamine in prefrontal cortex, a brain region with virtually undetectable levels of dopamine transporters, has been postulated to be the mechanism for the poorer cognitive functions for human subjects with higher COMT activity. Microdialysis analysis of dopamine levels in COMT knock-out mice showed significant increases of dopamine overflow in the frontal cortex but not in the striatum including nucleus accumbens, key regions involved in substance abuse (7). Similar results have been reported in PET studies of dopamine receptor occupancy in humans (28). Thus, inhibition of COMT might be expected to improve dopamine mediated cortical cognition without causing addiction related behaviors linked to increased dopamine in the ventral striatum. Indeed, clinical studies with tocalpone suggest improvement of cortical function without striatal effects (29).
Despite this wealth of information about the biochemistry and neurobiology of COMT, surprisingly little is known about the cell biology of MB-COMT. The cellular distribution and the orientation of MB-COMT on the cellular membrane of neurons including pyramidal neurons are controversial (11, 30–32). Indeed, a recent study in mice proposed that MB-COMT is not localized to the cell membrane, suggesting that dopamine would have to be transported from outside the cell for it to be metabolized by COMT (30). In this study, we demonstrate that MB-COMT is located in both the cell body and in axons and dendrites of rat cortical neurons. It co-localizes with secretegranin II, the lipid raft marker cholera toxin B, synaptotagmin, TGN 38, MAP2, Tau and PSD-95. The catalytic domain of MB-COMT is in the extracellular space of cortical neurons. Our functional analysis experiments reveal that higher levels of COMT activity will confer relative resistance to the cellular toxicity of tolcapone, particularly at moderate doses, suggesting that patients with COMT-Met genotypes might be especially susceptible to tolcapone toxicity.
EXPERIMENTAL PROCEDURES
Construction of MB-COMT-GFP Fusion Genes and Transfection
Human membrane-bound COMT (MB-COMT)-Val cDNA (GenbankTM accession no. BC011935) was subcloned into pEGFP-N1 and -C1 vectors (Clontech) to generate C-terminal and N-terminal tagged MB-COMT-GFP fusion genes, respectively. The orientation and open reading frames of the fusion constructs were confirmed by restriction enzyme site analyses and DNA sequencing. The fusion genes were introduced into primary neuronal or cell line cultures by transfection using Lipofectamine (Invitrogen).
Cortical Neuronal Culture
Whole cerebral cortex was dissected from embryonic day 18 Sprague-Dawley rats, dissociated in calcium- and magnesium-free Hanks' buffered salt solution containing 0.125% trypsin for 15 min, triturated in DMEM (Invitrogen)/10% FBS, and plated at 0.4 million cells per well in 6-well plates or 0.1 million cells per well per 24-well plates for immunostaining. Cells were grown at 37 °C, 5% CO2, and 95% humidity, first in 10% FBS/DMEM, followed 1 day later by serum-free medium and Neurobasal plus B27 (Invitrogen). Cultures were grown in serum-free medium for 10–12 days before the start of the experiments, and the medium was changed every 3 days. Fresh medium was applied 24 h before each experiment. These cultures yielded virtually all neurons.
Human Lymphoblastoid Cell Line
Blood was collected from normal subjects, and lymphocytes were transformed into lymphoblast cultures by standard Epstein-Barr virus transformation methods. Lymphocytes were selected from 20 individuals who were homozygous for each allele at the V158M locus (n = 10 per group, and the samples were matched by sex). The methods for recruitment and screening of volunteers are described in detail elsewhere (19). The DNA collection protocol was approved by the NIMH institutional review board, and all donors gave informed consent. Low speed centrifugation was used to collect 100,000,000 cells. The cell pellets were lysed in 300 μl of 1× lysis buffer (5 mm Tris-HCl (pH 7.4), 0.2% Triton X-100, and 1 mm DTT) with a sonicator. The total protein concentrations of the lysates were measured by the Bradford method.
Mouse Neuroblastoma 2A Cell Line
The mouse neuronblastoma 2A cell line was purchased from ATCC. The cell line is cultured in DMEM medium with 10% fetal bovine serum at 37 °C, 5% CO2.
Immunofluorescence Staining and Imaging
Primary cortical neurons were fixed in 4% paraformaldehyde in PBS (pH 7.4) and permeabilized in 0.2% Triton X-100. Cells were incubated with the following primary antibodies at 4 °C overnight: anti-TGN38 antibody (monoclonal, 1:500; BD Biosciences), anti-synaptophysin antibody (Roche Applied Science), anti-Tau antibody (Sigma), anti-MAP2 antibody (Sigma), anti-PSD-95 antibody (Sigma), and anti-secretegranin II antibody (SecII, polyclonal, 1:250; kindly provided by T. Watanabe) and then incubated with the appropriate TRITC-conjugated secondary antibodies (1:300; Jackson ImmunoResearch Laboratories) for 1 h at room temperature. Lipid raft was stained with fluorescein-labeled cholera toxin B (Invitrogen). The fluorescence images were captured by either a Cool SNAP CCD camera (Photometrics) mounted on an Olympus RX60 microscope or a Bio-Rad RTS2000 laser confocal microscope.
COMT Activity Assay
The COMT enzyme activity assay uses the organic solvent extraction method that separates the radioactive product, the methylated catechol, and the free radioactive coenzyme, [3H]AdoMet (33). Cells cultured in a six-well plate were homogenized in 1× lysis buffer. The cell lysates were centrifuged in a microcentrifuge at 14,000 rpm for 3 min. The supernatants were collected, and protein concentrations were determined. From each sample, 100 μg of the cell lysates at a concentration of 5 μg/μl was transferred to a fresh microcentrifuge tube and equilibrated to room temperature shortly before the enzyme assay. To each tube, we added 500 μl of the substrate mixture, which contained 10 mm Tris (pH 7.4), 1 mm MgCl2, 1.5 μCi of [3H]AdoMet, 10 μm of catechol, and 1 μm DTT. The tubes were then incubated at 37 °C for 20 min. The reactions were immediately terminated by the addition of 500 μl of 1 m HCl. The radioisotope-labeled catechol products from the reactions were extracted by adding 10 ml of scintillation fluid (Flow I (Molecular Diagnosis)) to the reaction mixture and then were measured for the radioactivity of the mixture in a scintillation counter. Relative COMT enzyme activity is presented as disintegrations per minute (dpm) per mg total protein. To establish a baseline control for nonspecific reactions that do not depend on COMT, 5 μl of the specific COMT inhibitor tolcapone (10 mg/ml) was added to a tube containing 100 μg of the human dorsolateral prefrontal cortex (DLPFC) sample. The high concentration of potent inhibitor blocked the specific reaction catalyzed by COMT, and the radioactivity from this reaction served as a baseline. To determine COMT activity on the cell surface, live cells in a six-well plate were rinsed with PBS twice and 500 μl of the substrate mixture, which contained 10 mm Tris (pH 7.4), 1 mm MgCl2, 1.5 μCi of [3H]AdoMet, 10 μm of catechol, 1 μm of DTT, and 0.8% NaCl, were added to each well. The cells were incubated at 37 °C for 20 min, and the reaction solution in each well was transferred to microcentrifuge tubes. The radioisotope-labeled catechol products from the reactions were extracted by adding 500 μl of 1 m HCl and 10 ml of scintillation fluid to the reaction mixture and then were measured for the radioactivity of the mixture in a scintillation counter. Because AdoMet does not penetrate the cell membrane, substrate methylation takes place on the cell surface and not intracellularly. To confirm that there is no reaction in the cytoplasm, the cells were attached on the six-well plate examined under a light microscope, and intracellular radioactivity was determined by lyzing the cells with 500 μl 1× lysis buffer and mixing the cell lysates with 500 μl of 1 m HCl, extracting with 10 ml of scintillation fluid (Flow I (Molecular Diagnosis)) and counting in scintillation counter.
Flow Cytometry Assay
Cells were washed with ice-cold PBS containing 2% BSA and incubated with phycoerythrin-conjugated anti-GFP antibody (Invitrogen) for 30 min on ice. After washing twice with cold PBS, cells were fixed with 2% paraformaldehyde in PBS and analyzed using FACScan (BD Biosciences). CellQuest software (BD Biosciences) was used to acquire and quantify the fluorescence signal intensities.
Tolcapone-induced Cytotoxicity Assay
Neuoblastoma 2A cells transfected with COMT-GFP or GFP vector and human lymphoblastoid cells in 96-well plates were treated with different concentrations of tolcapone for 24 h. Control cells were treated with dimethyl sulfoxide and used as a baseline, 0% of cell death, or 100% cell survival, for calculations. Cell viability was measured using Cell Counting kit-8 (CCK-8) (Dojindo Molecular Technologies, Inc.). 10 μl of CCK-8 solution was added to each well and incubated for 1 h at 37 °C. The absorbance was measured at 450 nm with a DTX 880 Multimode detector (Beckman Coulter, Brea, CA).
Western Blotting Analysis
Human lymphoblastoid cells were lyzed with a protease inhibitor Tris-glycerol extraction buffer (0.024% (4-(2-aminoethyl) benzenesulfonyl fluoride), 0.005% aprotinin, 0.001% leupeptin, 0.001% pepstatin A, 50% glycerol, and 0.6% Tris). The protein samples were prepared for immunoblotting by diluting the cell lysates with the lysis buffer and adding LDS sample buffer (1.25 μl of 4× buffer per sample) (Invitrogen). Samples were then heated to 90 °C for 5 min to denature proteins. For each sample, 5 μl was loaded onto precast 10% Bis-Tris polyacrylamide gels (Invitrogen), and proteins were separated by electrophoresis at 175 V for 0.5 h by use of a single power source. Each gel contained a molecular weight marker ladder (SeeBlue Plus 2 (Invitrogen)) and pooled samples from controls, which were used for normalization in triplicate. Seven separate gels were used in one experiment. Gels were transferred onto nitrocellulose membranes for 2 h at room temperature at 85 V in NuPAGE transfer buffer with 20% methanol. Membranes were blocked for 1 h in 10% goat serum in TBS with 0.1% Tween-20 (TBS-T) and then were incubated with the polyclonal rabbit anti-human caspase-3 antibody (1:500 dilution (Santa Cruz Biotechnology (catalog no. SC-7148))) or anti-human COMT antibody (Chemicon). Blots were rinsed in TBS-T, incubated in a peroxidase-conjugated goat anti-rabbit (1:1,000 dilution (Santa Cruz Biotechnology)) for 2 h in 5% normal goat serum in TBS-T and rinsed in TBS-T. Blots were developed in ECL Plus (Amersham Biosciences) and were exposed to Kodak BioMax film. Films were digitized using a scanner, and the resulting images were analyzed using National Institutes of Health Image gel plotting macros on a Macintosh computer. The values for all samples were expressed as a percentage of a mean of controls on the same gel.
Statistical Analysis
Paired two-tailed t tests and analysis of variance tests were used to analyze the data of cell survival after tolcapone treatment and the data of COMT enzyme activities and protein levels in human lymphoblastoid cells
RESULTS
Cellular Distribution of MB-COMT in Rat Cortical Neurons
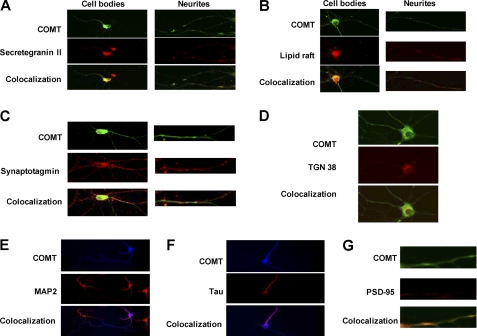
To identify whether MB-COMT is located in specific cell regions or compartments, we stained rat cortical neurons in primary neuronal cultures with anti-COMT antibody and anti-cellular markers antibodies (Fig. 1). Our results showed that MB-COMT is co-localized with secretegranin II (Fig. 1A), which suggests that COMT is packaged and sorted into secretory vesicles. The MB-COMT is also co-localized with a lipid raft stained with fluorescein-labeled cholera toxin B (Fig. 1B), implicating that MB-COMT is located in the more stable membrane region with a higher density of receptors. The MB-COMT is also co-localized with synaptotagmin (Fig. 1C), which suggests that it also localizes at the synapse of presynaptic neurons. MB-COMT is also co-localized with TGN38 (Fig. 1D), suggesting that it is processed and packaged at the Golgi apparatus. MB-COMT is also co-localized with MAP2, Tau, and PSD-95 (Fig. 1, E–G), which further suggests that it is located in dendrites and axons of neurons and synapses of postsynaptic neurons. Because MB-COMT is relatively evenly distributed across the neuron cell body and dendrites, it likely plays a role in elimination of not only synaptic but also diffused dopamine, norepinephrine, epinephrine, and potentially other catechols.
FIGURE 1.
Subcellular localization of MB-COMT in rat cortical neurons. Primary cortical neurons from rat brain were immunostained with anti-COMT and specific antibodies against selected cellular markers and imaged with a confocal microscope. A. co-localization of MB-COMT with secretegranin II, a marker for secretory vesicles. B, co-localization of MB-COMT with a lipid raft marker, cholera toxin B. C, co-localization of MB-COMT with synaptotagmin, a marker for synaptic vesicle. D, co-localization of MB-COMT with TGN38, a marker for Golgi apparatus. E, co-localization of MB-COMT with MAP2, a dendritic marker. F, co-localization of MB-COMT with Tau, an axonal marker. G, co-localization of MB-COMT with PSD-95, a marker for postsynaptic neurons.
Prediction of Membrane-bound Domain and Orientation of MB-COMT
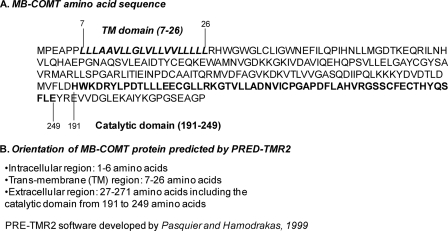
The orientation of MB-COMT in the cell membrane is critical for the mechanism of synaptic and diffused dopamine inactivation. If the C terminus, which contains the catalytic domain, is oriented toward the inside of the neuron, extracellular dopamine has to taken up by a transporter or internalized by endocytosis before it can be inactivated by MB-COMT. If the C terminus is oriented toward the extracellular space, then synaptic dopamine is likely inactivated directly by MB-COMT. To identify the orientation of MB-COMT, we first used the computer program PRE-TMR2 (34) to analyze MB-COMT amino acid sequences (Fig. 2A). The results showed that the hydrophobic sequence located between the seventh and the 26th amino acids is the transmembrane domain of the MB-COMT, and it is a type II transmembrane protein with the C terminus outside of the membrane (Fig. 2B). The accuracy of the prediction is ∼98%.
FIGURE 2.
Prediction of transmembrane domain and orientation of MB-COMT protein using PRED-TMR2. A, complete amino acid sequence of MB-COMT with the transmembrane domain and the catalytic domain indicated with bold letters. B, orientation of MB-COMT predicted by PRE-TMR2 showing that only six amino acids at the N terminus are intracellular and most amino acid sequences, including the catalytic domain, are extracellular.
Determination of Orientation of MB-COMT Using GFP Tag and Flow Cytometry
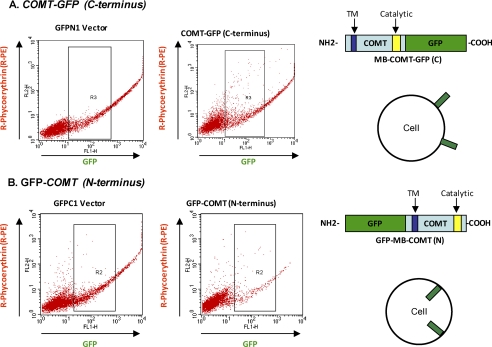
As mentioned above, the accuracy of the computer prediction is not 100%. To determine the orientation of the MB-COMT by experimentation, a GFP tag was added alternatively to the C terminus and the N terminus of MB-COMT to create the fusion constructs MB-COMT-GFP and GFP-MB-COMT, respectively (Fig. 3). Human lymphoblastoid cells transfected with the fusion constructs and their vector controls were stained with anti-GFP antibody conjugated with R-phycoerythrin (R-PE), sorted, and counted by flow cytometry. Our results showed that only the C-terminal GFP tag (Fig. 3A) but not N-terminal GFP (Fig. 3B) is detected by this antibody. This finding indicates that the C terminus of MB-COMT is outside of the cell and the catalytic domain in the C-terminal region is in the extracellular space.
FIGURE 3.
Catalytic domain at the C terminus of MB-COMT is in the extracellular space revealed by flow cytometry. A, lymphoblastoid cells transfected with COMT-GFP (C terminus) and its GFPN1 vector were immunostained with R-PE-conjugated anti-GFP antibody and analyzed with flow cytometry. The strong R-PE signal from the COMT-GFP transfected cells indicates that the GFP tag is located in the extracellular space as illustrated by the depiction on the right. B, lymphoblastoid cells transfected with GFP-COMT (N terminus) and its GFPC1 vector were immunostained with R-PE-conjugated anti-GFP antibody and analyzed with flow cytometry. The R-PE signal from the COMT-GFP transfected cells is the same as that from the GFPC1 vector-transfected cells, suggesting that the GFP tag is located in the cytoplasm as illustrated by the depiction on the right.
Detection of COMT Enzyme Activity on Surface of Neuroblasmtoma 2A Cell
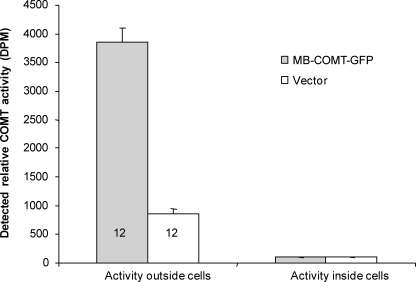
To further test whether the catalytic domain is in the extracellular space, we used a modified COMT enzyme assay to detect COMT enzyme activity on the surface of neuroblastoma 2A cells transfected with the C-terminal GFP MB-COMT or vector. The modified COMT assay utilizes the impermeability property of AdoMet to the cell membrane so that only extracellular COMT activity on the surface of intact live cells is measured. Our results showed that high levels of COMT enzyme activity on the cell surfaces were detected (Fig. 4), which further confirms that the catalytic domain of the MB-COMT is extracellular. The high level of enzyme activity in MB-COMT-GFP transfected cells suggests that the GFP tag at the C terminus of the MB-COMT did not disrupt COMT enzyme activity. The surface enzyme activity from the cells transfected with vector reveals that unmodified MB-COMT from the endogenous COMT gene has the same orientation as the MB-COMT-GFP protein, which excludes the possibility of an artifact from the fusion GFP tag. Moreover, we detected very low enzyme activity inside the cells with our modified COMT assay, suggesting that this assay can be used to screen specific COMT inhibitors that only inhibit MB-COMT.
FIGURE 4.
COMT activity on the surface of neuroblasmtoma 2A cells is detected by a modified COMT assay. COMT activities on the surface of 2A cells (n = 12) from six-well plates were analyzed using a modified COMT assay with 3H-labeled S-adenosylmethionine, impermeable to the cell membrane. COMT activities outside, but not inside, of the cells were detected with the modified assay. Activity outside the cells transfected by vector represents the COMT activity from the endogenous COMT gene.
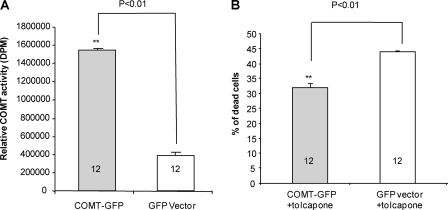
Lower Sensitivity to Tolcapone Toxicity in Neuroblastoma Cells Transfected with COMT-GFP and Human Lymphoblastoid Cells with Val/Val Genotype
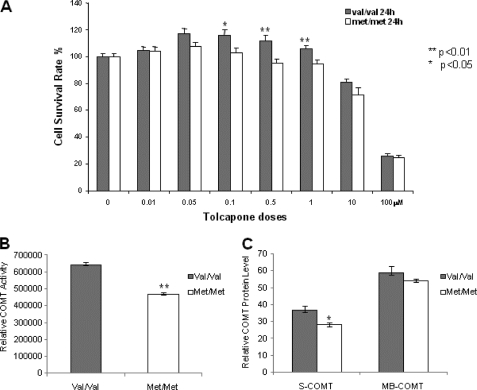
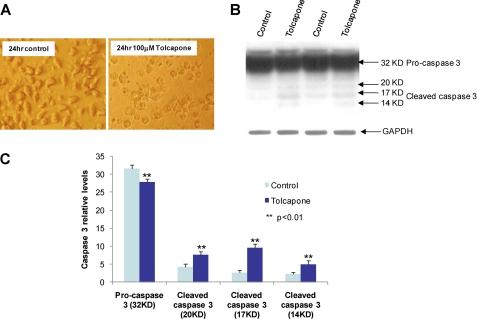
The toxicity of tolcapone is the key reason for its limited clinical use even though it causes liver necrosis in only a small minority of patients. Because human COMT-Val protein has higher enzyme activity than human COMT-Met protein, there may be differences in the sensitivity to tocalpone toxicity between patients carrying alternative genotypes. To test whether enzyme activity is correlated with sensitivity to tolcapone cytoxicity, we measured the enzyme activity of the neuroblastoma cells transfected with COMT-GFP and GFP vector as control and compared the percentage of dead cells induced by tolcapone in the transfected 2A cells. Our results show that there is significantly higher COMT activity (Fig. 5A) and lower percentage of cell death (Fig. 5B) in the 2A cells transfected with COMT-GFP than the controls transfected with GFP vector after tolcapone treatment. However, the small protective effect of the COMT-GFP is not proportional to the large increase of the COMT activity, which suggests that other factors might also underlie tolcapone toxicity. To further confirm the negative correlation between COMT enzyme activity and tolcapone toxicity, we measured the survival rate of human lymphoblastoid cells with Val/Val and Met/Met genotypes following tolcapone treatment (Fig. 6A). The results show that the lymphoblastoid cells with Val/Val genotype, a genotype associated with higher COMT activity, have statistically significant (p < 0.01) higher cell survival rates than those with the Met/Met genotype, a genotype associated with lower COMT activity, at tolcapone concentrations from 0.1 to 1.0 μm. The concentrations are within the range of the plasma tolcapone concentrations in human subjects administered 100 mg to 800 mg per day (35). The higher survival rate of the cells with Val/Val genotypes appears to correlate with higher COMT enzyme activity (Fig. 6B) and higher levels of S-COMT (Fig. 6C). At lower concentrations, however, from 0.01 to 0.1 μm, tolcapone is not cytotoxic with either Val/Val or Met/Met genotype, suggesting that low doses of tolcapone are relatively safe regardless of genotype. In fact, and quite surprisingly, at low concentrations from 0.01 to 1.0 μm, tolcapone appears to stimulate the growth of the cells with the Val/Val but not the Met/Met genotype. At high concentrations (10 and 100 μm), tolcapone is highly toxic to the cells, and there is no difference in the susceptibility to tolcapone cytotoxicity between the cells with Val/Val and Met/Met genotypes. Thus, tolcapone cytotoxicity is largely dependent on dosage and only partially on the COMT Val/Met genotype. To explore the potential mechanism of tolcapon-induced cytotoxicity, we analyzed the active or cleaved forms of caspase-3, a molecular marker for apoptosis, in the lymphoblastoid cells treated with high concentration (100 μm) of tolcapone. Our results showed that high concentration of tolcapone increases the level of the cleaved or active forms of caspase-3 (Fig. 7), which suggests that tolcapone induces cell death via apoptosis, at least at the high dose.
FIGURE 5.
Reduced tolcapone induced cell death in neuroblastoma 2A cells transfected with COMT-GFP. A, COMT activities of the 2A cells from six-well plates transfected with COMT-GFP (n = 12) and GFP vector (n = 12) were analyzed and presented in the bar graph. B, COMT activities of the 2A cells from six-well plates transfected with COMT-GFP (n = 12) and GFP vector (n = 12) were treated with 100 μm of tolcapone for 24 h. Tolcapone-induced cell death was analyzed and is presented in the bar graph using the controls treated with the vehicle, dimethyl sulfoxide, as baseline or 0% cell death.
FIGURE 6.
Effect of tolcapone on human lymphoblastoid cells with COMT Val/Val and Met/Met genotypes. Human lymphoblastoid cells were treated with different doses of tolcapone for 24 h in 2% FBS medium. A, the survival of human lymphoblastoid cells with the Val/Val and Met/Met genotypes were measured and presented as percentages of controls treated with vehicle dimethyl sulfoxide (DMSO) in the bar graph. B, COMT enzyme activity in human lymphoblastoid cells with the Val/Val and Met/Met genotypes. C, relative COMT protein levels of human lymphoblastoid cells with Val/Val and Met/Met genotypes from Western blotting analyses.
FIGURE 7.
Induction of cleaved forms of caspase-3 by tolcapone in human lymphoblastoid cells. A, human lymphoblastoid cells untreated and treated with 100 μm of tolcapone for 24 h. B, images of Western blots using anti-human caspase-3 antibody and anti-GAPDH antibody. C, relative levels of procaspase-3 and cleaved forms of caspase-3 from quantitative analysis of the Western blots using densitometry.
DISCUSSION
The major findings of this study are that MB-COMT is localized in axons, dendrites, and postsynaptic and presynaptic structures, as well as in lipid rafts and secretory vesicles, and that on the surface of cortical neurons or neuroblastoma cells, its catalytic domain is oriented toward the extracellular space. Because the catalytic domain is in the extracellular space, MB-COMT is capable of catalyzing the transfer of a methyl group from S-adenosylmethionine to catecholamines in synaptic and extracellular spaces, which is independent of endocytosis or other cross-membrane transport. Our results also suggest that COMT inhibitors impermeable to the cell membrane will be MB-COMT specific, at least in the brain. Finally, tolcapone at high doses induces cell death via the mechanism of apoptosis associated with activation of caspase-3. The cellular toxicity of tolcapone also appears to be associated with COMT enzyme activity, suggesting that patients with different COMT genotypes may be differentially sensitive to tolcapone toxicity.
Implications of Cellular Distribution of MB-COMT
MB-COMT is co-localized with presynaptic and postsynaptic markers, which suggests that MB-COMT is expressed in both presynaptic and postsynaptic neurons. As it is co-localized with the synaptic protein, synaptotagmin, it may play an important role in the inactivation of synaptic dopamine and affect dopaminergic transmission of presynaptic and postsynaptic neurons. However, MB-COMT is also evenly distributed on the cell body and in axons and dendrites; thus, it likely plays an important role in direct inactivation of extrasynaptic or diffused dopamine, which could also affect dopamine signaling. These results are consistent with evidence in COMT knock-out mice that dopamine levels are increased by 60% and that ∼50% of cortical dopamine synaptic flux in the mouse is determined by COMT (7). Another study showed that the levels of tyrosine hydroxylase, the rate-limiting enzyme for dopamine synthesis, are significantly different between human subjects carrying COMT-Val or COMT-Met (18). In a human PET imaging study of the availability of cortical dopamine D1 receptors as an indirect proxy of synaptic dopamine concentrations, significantly different occupancies were found between COMT-Val and COMT-Met carrying genotypes (28). All of these findings in the mouse and human support the hypothesis that COMT activity affects synaptic dopamine levels.
Orientation of Catalytic Domain of MB-COMT Is Critical for Design of COMT Inhibitors Specific for MB-COMT
The information on the orientation of MB-COMT is important for understanding the action site for MB-COMT and the design of MB-COMT-specific inhibitors. Prior to these experiments, the orientation of the catalytic domain of cell surface COMT has been unclear. An early study on the orientation of MB-COMT utilized microsome protection from proteinease digestion to show that MB-COMT is an integral membrane protein (36). However, the protection from proteinase K digestion by microsome is partial and membrane proteins in either orientation can be digested to some extent by proteinease K. In fact, a faint band of protected MB-COMT is seen in the 30-min treatment with proteinase K in that study, which suggests that MB-COMT is partially protected by microsome, and it is in the extracelluar orientation. A recent study using S-COMT-deficient mice also reported that MB-COMT is an intracellular protein, suggesting dopamine and other catechol compounds would need to be internalized before they are methylated (30). However, weak MB-COMT immunoreactivity on the cell membrane showed co-localization with Na,K-ATPase, the cellular membrane protein marker, in that study. Thus, different conclusions could be drawn from the results of the previous studies. Neither of the earlier studies performed any biochemical experiments aimed at localizing COMT enzyme activity.
Our computer analysis of MB-COMT orientation predicts extracellular localization of the catalytic domain of MB-COMT. The prediction suggests that MB-COMT is a type II trans-membrane protein with its amino terminus on the cytoplasmic side of the cell and the carboxyl terminus on the exterior. Using GFP-MB-COMT fusion proteins, we confirmed that MB-COMT is a type II trans-membrane protein in transfected lymphoblastoid cells. To exclude the potential effect of the GFP tag on the orientation of MB-COMT, we measured the surface COMT activity of intact neuroblastoma 2A cells. Detection of the COMT activity on the cell surface is direct evidence for the extracellular orientation of MB-COMT. Interestingly, this orientation offers the possibility to design MB-COMT-specific inhibitors, which cannot penetrate the cell membrane and will not inhibit S-COMT that is abundant in liver. However, it may be difficult to design a drug that can cross the blood brain barrier but not the cell membrane. Active transport across the blood brain barrier might be required for the MB-COMT-specific inhibitors.
Pharmacogenetics of COMT V158M Polymorphism
Untoward toxicity has been the reason for several effective drugs such as tolcapone, Vioxx, Avandia, and Baycol to be withdrawn from the market (37–40). Genetic risk factors might be associated with such side effects because the drugs do not show severe toxicity in the majority of patients. For tolcapone, it is unknown whether the toxicity is caused by direct inhibition of COMT or off-target effects other than those involving COMT. Our results show that tolcapone-induced cell death is dramatically impacted by dose and to a small extent also is associated with COMT enzyme level, which suggests that the toxicity is due at least in part to the blockade of functional COMT in human lymphoblastoid and mouse neuroblastoma cells. Toxicological studies of tolcapone in vivo using the rat and in vitro using human neuroblastoma SH-SY5Y cells have demonstrated that tolcapone could affect mitochondrial biochemistry and inhibit ATP production (41, 42). Inhibition of COMT may also affect the Akt cell survival pathway (43). It is also interesting to note that complete COMT knock-out in mice does not lead to liver failure (15), although tolcapone itself may also not be hepatotoxic in mice. Nevertheless, because COMT Val/Val carriers are more tolerant to the in vitro toxicity of tolcapone at moderate doses, the functional V158M genotype information may be useful in guiding therapy with COMT inhibitors. Interestingly, because polymorphisms in the 5′ domain and the 3′-untranslated region of the human COMT gene also have functional effects on COMT gene activity (17, 26, 27), the V158M polymorphism alone may not adequately characterize the spectrum of genetic variation related to COMT biology. It is also likely that other factors will contribute to the development of tolcapone hepatotoxicity. Indeed, a recent pharmacogenetic study showed that UDP-glucuronosyl transferase, an enzyme involved in the elimination of tolcapone, is associated with tolcapone-induced liver enzyme elevations (44). Thus, genes other than COMT should also be considered for personalized medicine using tolcapone. This earlier study, however, did not find a significant association between liver toxicity and COMT V158M or A72S polymorphisms. This negative result may be a reflection of the limited power of the study (n = 135 subjects) or that the relationship of liver enzyme elevations and hepatic necrosis may not be linear.
Acknowledgments
We thank Mitsuyuki Matsumoto for help with the original COMT enzyme assay and technical advice and Barbara Lipska for laboratory assistance.
This work was supported by NIMH, National Institutes of Health Intramural Research Program.
- COMT
- catechol-O-methyltransferase
- MB-COMT
- membrane-bound COMT
- S-COMT
- soluble COMT
- AdoMet
- S-adenosylmethionine
- R-PE
- R-phycoerythrin.
REFERENCES
- 1. Tan H. Y., Callicott J. H., Weinberger D. R. (2009) Cogn. Neuropsychiatry 14, 277–298 [DOI] [PubMed] [Google Scholar]
- 2. Weinberger D. R. (2005) Clin. Ther. 27, S8–15 [DOI] [PubMed] [Google Scholar]
- 3. Harrison P. J., Weinberger D. R. (2005) Mol. Psychiatry 10, 40–68 [DOI] [PubMed] [Google Scholar]
- 4. Parl F. F., Dawling S., Roodi N., Crooke P. S. (2009) Ann. N.Y. Acad. Sci. 1155, 68–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lundström K., Salminen M., Jalanko A., Savolainen R., Ulmanen I. (1991) DNA Cell Biol. 10, 181–189 [DOI] [PubMed] [Google Scholar]
- 6. Matsumoto M., Weickert C. S., Akil M., Lipska B. K., Hyde T. M., Herman M. M., Kleinman J. E., Weinberger D. R. (2003) Neuroscience 116, 127–137 [DOI] [PubMed] [Google Scholar]
- 7. Käenmäki M., Tammimäki A., Myöhänen T., Pakarinen K., Amberg C., Karayiorgou M., Gogos J. A., Männistö P. T. (2010) J. Neurochem. 114, 1745–1755 [DOI] [PubMed] [Google Scholar]
- 8. Meyer-Lindenberg A., Kohn P. D., Kolachana B., Kippenhan S., McInerney-Leo A., Nussbaum R., Weinberger D. R., Berman K. F. (2005) Nat. Neurosci. 8, 594–596 [DOI] [PubMed] [Google Scholar]
- 9. Papaleo F., Crawley J. N., Song J., Lipska B. K., Pickel J., Weinberger D. R., Chen J. (2008) J. Neurosci. 28, 8709–8723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nunes T., Machado R., Rocha J. F., Fernandes-Lopes C., Costa R., Torrão L., Loureiro A. I., Falcão A., Vaz-da-Silva M., Wright L., Almeida L., Soares-da-Silva P. (2009) Clin. Ther. 31, 2258–2271 [DOI] [PubMed] [Google Scholar]
- 11. Grossman M. H., Creveling C. R., Rybczynski R., Braverman M., Isersky C., Breakefield X. O. (1985) J. Neurochem. 44, 421–432 [DOI] [PubMed] [Google Scholar]
- 12. Soares-da-Silva P., Vieira-Coelho M. A., Parada A. (2003) Pharmacol. Toxicol. 92, 272–278 [DOI] [PubMed] [Google Scholar]
- 13. Lotta T., Vidgren J., Tilgmann C., Ulmanen I., Melén K., Julkunen I., Taskinen J. (1995) Biochemistry 34, 4202–4210 [DOI] [PubMed] [Google Scholar]
- 14. Huotari M., Gogos J. A., Karayiorgou M., Koponen O., Forsberg M., Raasmaja A., Hyttinen J., Männistö P. T. (2002) Eur. J. Neurosci. 15, 246–256 [DOI] [PubMed] [Google Scholar]
- 15. Gogos J. A., Morgan M., Luine V., Santha M., Ogawa S., Pfaff D., Karayiorgou M. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 9991–9996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tammimäki A., Männistö P. T. (2011) Basic Clin. Pharmacol. Toxicol. 108, 2–8 [DOI] [PubMed] [Google Scholar]
- 17. Chen J., Lipska B. K., Halim N., Ma Q. D., Matsumoto M., Melhem S., Kolachana B. S., Hyde T. M., Herman M. M., Apud J., Egan M. F., Kleinman J. E., Weinberger D. R. (2004) Am. J. Hum. Genet. 75, 807–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Akil M., Kolachana B. S., Rothmond D. A., Hyde T. M., Weinberger D. R., Kleinman J. E. (2003) J. Neurosci. 23, 2008–2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Egan M. F., Goldberg T. E., Kolachana B. S., Callicott J. H., Mazzanti C. M., Straub R. E., Goldman D., Weinberger D. R. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 6917–6922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tan H. Y., Chen Q., Goldberg T. E., Mattay V. S., Meyer-Lindenberg A., Weinberger D. R., Callicott J. H. (2007) J. Neurosci. 27, 13393–13401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tunbridge E. M., Weickert C. S., Kleinman J. E., Herman M. M., Chen J., Kolachana B. S., Harrison P. J., Weinberger D. R. (2007) Cereb. Cortex 17, 1206–1212 [DOI] [PubMed] [Google Scholar]
- 22. Frank M. J., Doll B. B., Oas-Terpstra J., Moreno F. (2009) Nat. Neurosci. 12, 1062–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Goetz C. G. (1998) Neurology 50, S26–30 [DOI] [PubMed] [Google Scholar]
- 24. Green R. E., Krause J., Briggs A. W., Maricic T., Stenzel U., Kircher M., Patterson N., Li H., Zhai W., Fritz M. H., Hansen N. F., Durand E. Y., Malaspinas A. S., Jensen J. D., Marques-Bonet T., Alkan C., Prüfer K., Meyer M., Burbano H. A., Good J. M., Schultz R., Aximu-Petri A., Butthof A., Höber B., Höffner B., Siegemund M., Weihmann A., Nusbaum C., Lander E. S., Russ C., Novod N., Affourtit J., Egholm M., Verna C., Rudan P., Brajkovic D., Kucan Z., Gusic I., Doronichev V. B., Golovanova L. V., Lalueza-Fox C., de la Rasilla M., Fortea J., Rosas A., Schmitz R. W., Johnson P. L., Eichler E. E., Falush D., Birney E., Mullikin J. C., Slatkin M., Nielsen R., Kelso J., Lachmann M., Reich D., Pääbo S. (2010) Science 328, 710–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nackley A. G., Shabalina S. A., Tchivileva I. E., Satterfield K., Korchynskyi O., Makarov S. S., Maixner W., Diatchenko L. (2006) Science 314, 1930–1933 [DOI] [PubMed] [Google Scholar]
- 26. Shifman S., Bronstein M., Sternfeld M., Pisanté-Shalom A., Lev-Lehman E., Weizman A., Reznik I., Spivak B., Grisaru N., Karp L., Schiffer R., Kotler M., Strous R. D., Swartz-Vanetik M., Knobler H. Y., Shinar E., Beckmann J. S., Yakir B., Risch N., Zak N. B., Darvasi A. (2002) Am. J. Hum. Genet. 71, 1296–1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Meyer-Lindenberg A., Nichols T., Callicott J. H., Ding J., Kolachana B., Buckholtz J., Mattay V. S., Egan M., Weinberger D. R. (2006) Mol. Psychiatry 11, 867–877 [DOI] [PubMed] [Google Scholar]
- 28. Slifstein M., Kolachana B., Simpson E. H., Tabares P., Cheng B., Duvall M., Frankle W. G., Weinberger D. R., Laruelle M., Abi-Dargham A. (2008) Mol. Psychiatry 13, 821–827 [DOI] [PubMed] [Google Scholar]
- 29. Apud J. A., Mattay V., Chen J., Kolachana B. S., Callicott J. H., Rasetti R., Alce G., Iudicello J. E., Akbar N., Egan M. F., Goldberg T. E., Weinberger D. R. (2007) Neuropsychopharmacology 32, 1011–1020 [DOI] [PubMed] [Google Scholar]
- 30. Myöhänen T. T., Schendzielorz N., Männistö P. T. (2010) J. Neurochem. 113, 1632–1643 [DOI] [PubMed] [Google Scholar]
- 31. Ulmanen I., Peränen J., Tenhunen J., Tilgmann C., Karhunen T., Panula P., Bernasconi L., Aubry J. P., Lundström K. (1997) Eur. J. Biochem. 243, 452–459 [DOI] [PubMed] [Google Scholar]
- 32. Karhunen T., Tilgmann C., Ulmanen I., Panula P. (1995) Neurosci. Lett 187, 57–60 [DOI] [PubMed] [Google Scholar]
- 33. Zürcher G., Da Prada M. (1982) J. Neurochem. 38, 191–195 [DOI] [PubMed] [Google Scholar]
- 34. Pasquier C., Hamodrakas S. J. (1999) Protein Eng. 12, 631–634 [DOI] [PubMed] [Google Scholar]
- 35. Dingemanse J., Jorga K. M., Schmitt M., Gieschke R., Fotteler B., Zürcher G., Da Prada M., van Brummelen P. (1995) Clin. Pharmacol. Ther. 57, 508–517 [DOI] [PubMed] [Google Scholar]
- 36. Ulmanen I., Lundström K. (1991) Eur. J. Biochem. 202, 1013–1020 [DOI] [PubMed] [Google Scholar]
- 37. Farmer J. A. (2003) Curr. Atheroscler Rep. 5, 96–100 [DOI] [PubMed] [Google Scholar]
- 38. Davies N. M., Jamali F. (2004) J. Pharm. Pharm. Sci. 7, 332–336 [PubMed] [Google Scholar]
- 39. Watkins P. (2000) Neurology 55, S51–52; discussion S53–56 [PubMed] [Google Scholar]
- 40. Scheen A. J. (2001) Diabetes Metab. 27, 305–313 [PubMed] [Google Scholar]
- 41. Haasio K., Lounatmaa K., Sukura A. (2002) Exp. Toxicol. Pathol. 54, 9–14 [DOI] [PubMed] [Google Scholar]
- 42. Korlipara L. V., Cooper J. M., Schapira A. H. (2004) Neuropharmacology 46, 562–569 [DOI] [PubMed] [Google Scholar]
- 43. Sei Y., Li Z., Song J., Ren-Patterson R., Tunbridge E. M., Iizuka Y., Inoue M., Alfonso B. T., Beltaifa S., Nakai Y., Kolachana B. S., Chen J., Weinberger D. R. (2010) PLoS One 5, e10789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Acuña G., Foernzler D., Leong D., Rabbia M., Smit R., Dorflinger E., Gasser R., Hoh J., Ott J., Borroni E., To Z., Thompson A., Li J., Hashimoto L., Lindpaintner K. (2002) Pharmacogenomics J. 2, 327–334 [DOI] [PubMed] [Google Scholar]