Abstract
Paclitaxel (Taxol) is one of the most effective treatment options for patients suffering from a variety of cancers. A major side effect seen in a high percentage of patients treated with paclitaxel is irreversible peripheral neuropathy. We previously reported that prolonged treatment with paclitaxel activates a calcium-dependent enzyme, calpain, which degrades neuronal calcium sensor 1 (NCS-1) and subsequent loss of intracellular calcium signaling. Because it appears that activation of calpain is an early step in this destructive cascade, we proposed that inhibition of calpain will protect against the unwanted side effects of paclitaxel treatment. First, NCS-1 levels and intracellular calcium signaling were found to be protected by the presence of lactacystin, a protesome inhibitor. To reinforce the role of calpain in this process, we showed that increased concentrations of calpastatin, a naturally occurring calpain inhibitor, were protective. Next, we tested two mutated versions of NCS-1 developed with point mutations at the P2 position of the calpain cleavage site of NCS-1 to decrease the likelihood of NCS-1 degradation. One mutant was cleaved more favorably by calpain compared with NCS-1 WT, whereas the other mutant was less favorably cleaved. Expression of either mutated version of NCS-1 in neuroblastoma cells protected intracellular calcium signals from paclitaxel-induced changes. These results support our hypothesis that it is possible to protect cells from paclitaxel-induced degradation of NCS-1 by inhibiting calpain activity.
Keywords: Calcium Channels; Calcium Signaling; Calpain; Inositol 1,4,5-Trisphosphate; Protein Degradation; Calpastatin; Intracellular Calcium Channels; Live Cell Imaging; NCS-1; Paclitaxel
Introduction
Paclitaxel (Taxol) is one of the most effective and commonly used treatment options for patients suffering from a variety of cancers, including breast and ovarian cancer (1, 2). Unfortunately, a major side effect seen in a high percentage of patients treated with this and several other chemotherapeutic drugs is an irreversible peripheral neuropathy (3, 4). Although the paclitaxel mechanism of action, polymerization of tubulin and the formation of stable microtubule polymers, was first discovered in 1979, the cellular mechanisms responsible for the development of peripheral pain are still unclear (5). Strategies are needed to prevent this unwanted effect of paclitaxel without altering its chemotherapeutic action.
Recently, neuronal calcium sensor 1 (NCS-1)2 was identified as a novel paclitaxel-binding partner (6). NCS-1 is a high affinity, low capacity calcium-binding protein containing four EF hand binding domains, three of which functionally bind calcium (7). NCS-1 was initially thought to be found solely in neuronal cells; however, more recently, it has been found in a variety of cell types where it enhances intracellular calcium signaling (8–11). NCS-1 has a number of binding partners (12). Relevant to this study, NCS-1 binds to the inositol 1,4,5-trisphosphate receptor (InsP3R) and modulates its function in a calcium-dependent manner (11). Paclitaxel increases the interaction between NCS-1 and the InsP3R (11, 13). Prolonged treatment with paclitaxel activates calpain, a calcium-dependent enzyme with numerous targets, including NCS-1 (13, 14). The loss in functional NCS-1 results in a decrease in the activity of the InsP3R, which in turn decreases ability of the cell to produce intracellular calcium signals (6, 11, 13, 15). It is likely that the interaction among paclitaxel, NCS-1, and the InsP3R is an early step in the mechanism leading to the production of peripheral neuropathy by paclitaxel.
Inhibition of calpain proteases can induce protective effects against sensory neuropathy caused by prolonged treatment with paclitaxel (13, 16). In this study, we show several ways of preventing NCS-1 degradation. First, NCS-1 levels and intracellular calcium signaling were found to be protected by the addition of lactacystin, a proteasome inhibitor (26). To reinforce the role of calpain in this process, we showed that increased concentrations of calpastatin (CAST), a naturally occurring calpain inhibitor, were protective. Next, two mutant versions of NCS-1 were developed with point mutations at the P2 position of the calpain cleavage site of NCS-1. Using the consensus calpain cleavage map, this putative P2 site of NCS-1 was substituted with amino acids believed to be unfavorable for calpain cleavage (17). One mutant was cleaved more efficiently by calpain compared with the NCS-1 WT, and the other mutant was less efficiently cleaved. The expression of either NCS-1 mutant in a neuroblastoma cell line protected intracellular calcium signaling in paclitaxel-treated cells.
EXPERIMENTAL PROCEDURES
NCS-1 Expression and Purification
NCS-1 wild-type (WT) and mutants, I35H and I35A, were expressed as previously described (14). Mutations were made at amino acid 35 of NCS-1 to either a histidine or an alanine using the QuikChange multiple site-directed mutagenesis kit (Stratagene). The WT and mutated versions of NCS-1 were also made with a HA tag at the C terminus denoted NCS-1-HA.
Isothermal Titration Calorimetry (ITC)
Calcium titrations were performed as described previously (14). Briefly, purified NCS-1 WT and mutants were stripped of calcium using a modification of a previously published protocol (22). A Bio-Rad Econo-Pac 10DG column was used to exchange the protein buffer to 50 mm Hepes, 100 mm KCl, pH 7.5. NCS-1 was then dialyzed against 10 mm EDTA, pH 2.0, for 1 h to strip all calcium. This was followed by a second dialysis in deionized water for 1 h, followed by 10 mm Tris, pH 7.4, for 1 h. The final dialysis was against 50 mm Tris, 100 mm KCl, and 0.5 mm DTT, pH 7.2, overnight. ITC measurements were performed on a VP ITC instrument with a protein concentration of 100 μm in the sample cell and 5 mm calcium concentration. The experiments were run at 20 °C with a total of 60–80 injections of 1.5-μl injection volume (3-s duration) and 240-s intervals. Data were processed using Origin 7.0 (Microcal) and fit using a two-site model, and dissociation constants (Kd), stoichiometry (n), enthalpy (ΔH), and entropy (ΔS) of binding determined.
Digestion of NCS-1 by μ-Calpain
Digestion of NCS-1 WT and mutants was performed using a modification of a previously published protocol (14). Briefly, μ-calpain was purchased from Sigma (calpain-1, active from human plasma; Sigma C-6108). Control reactions contained NCS-1 without μ-calpain and μ-calpain without NCS-1. The reaction volume was 15 μl with a mass ratio of 1:4 μ-calpain:NCS-1. Reactions proceeded in digestion buffer containing 10 mm Hepes, 1 mm DTT, 100 mm KCl, 5.0 mm calcium for 30 min at 30 °C. The reaction was quenched on ice by the addition of 1 μl of 50 mm EDTA and 15 μl of SDS-PAGE buffer.
Western Blot Analysis
The human neuroblastoma cell line, SHSY-5Y, was cultured and transfected with NCS-1 WT or mutants with and without the HA tag and CAST as previously described (23). Cells were treated for 6 h with 800 ng/ml paclitaxel. Lysate preparation was performed as described previously (11), and immunoblotting was performed using the Invitrogen NuPage system and IBlot according to the manufacturer's protocol. Antibodies used were anti-NCS-1 (Santa Cruz Biotechnology, Santa Cruz, CA), anti-β-actin (Abcam, Cambridge, MA), anti-calpain 1 (Calbiochem, Darmstadt, Germany), anti-calpain 2 (Abcam), anti-CAST (Santa Cruz Biotechnology), and anti-HA (Santa Cruz Biotechnology). Protein expression was quantified by scanning densitometry by UN-SCAN-IT (Silk Scientific, Orem, UT) or Odyssey 2.0 (LI-COR Biotechnology, Lincoln NE) and normalized to β-actin loading controls. All Western blotting experiments were performed with three independent cultures.
Live Cell Imaging
SHSY-5Y live cell imaging was performed as described previously (6). Briefly, SHSY-5Y cells overexpressing NCS-1 WT, I35H, I35A, and/or CAST were incubated for 30 min at 37 °C in 5% CO2 in Hepes buffer containing 0.1% Pluronic F-127 and 5 μm Fluo-4 (Molecular Probes). Hepes buffer contained 20 mm Hepes, 130 mm NaCl, 4.7 mm KCl, 1 mm MgSO4, 1.2 mm KH2PO4, 5 mm glucose, pH 7.4. Calcium-containing experiments also included 1.3 mm CaCl2 in the buffer, whereas calcium-free experiments contained 10 mm EGTA in the buffer. A Zeiss LSM 510 META scanning laser confocal microscope equipped with a C-Apochromat X40/1.2 water immersion objective (Zeiss, Thornwood, NY) was used. For ATP and carbachol stimulation experiments, cells were excited with either 500 nm-1 μm ATP or 200 nm carbachol in calcium-containing or calcium-free Hepes buffer to induce a transient release of calcium from intracellular stores. 3 μm thapsigargin, an inhibitor of the intracellular calcium pump (SERCA), was added to indicate that the intracellular stores were filled and the cells were viable. Calcium-induced fluorescence intensity ratio F/F0 was plotted as a function of time in seconds with F0 calculated as the average of the first 10 points of the base line. Response duration was defined as the time the calcium signal remains consecutively over 50% of the peak value.
NCS-1 Binding to Paclitaxel
Changes in NCS-1 intrinsic fluorescence after addition of paclitaxel was measured by changes in the peak of NCS-1 fluorescence spectra at 334 nm acquired using a FluoroMax-3 fluorometer with Datamax software and temperature control (Jobin Yvon Horiba, NJ). Samples were excited at 270 nm, and spectra were collected between 280 and 400 nm with 2-nm increments at 20 °C. Experiments were performed in 10 mm Hepes, 100 mm KCl, 1 mm CaCl2, pH 7.2, with 0.5 μg of NCS-1 held constant and increasing concentrations from 0–800 ng/ml paclitaxel. Control experiments with buffer alone and increasing concentrations of paclitaxel alone were subtracted from the experiments containing NCS-1. Changes in the peak height at 334 nm were plotted as a function of drug concentration to determine the binding constant (Kd).
Calpain Activity Assay
SHSY-5Y cells were grown in 6-well plates to between 70 and 80% confluence and then transfected with CAST or treated with Lipofectamine alone (control) and allowed to grow for 48 h. Cells were then treated with paclitaxel for 6 h, followed by two rinses with 1 × PBS. Cells were then lysed with CytoBuster protein extraction reagent (Novagen, Calbiochem), and the lysate was cleared by centrifugation for 10 min at 13,000× g and used for the Calpain-Glo protease assay (Promega) according to the manufacturer's protocol. In this assay, calpain activity was measured by luminescence. The relative luminescence was averaged over 10 s and background subtracted, then normalized to the amount of total protein in the lysates.
Immunoprecipitation Assay
The cell line SHSY-5Y was cultured and transfected with NCS-1 WT or mutants with HA tag. Cells were treated for 6 h with 800 ng/ml paclitaxel. Cells were harvested by incubation with 100 μl of lysis buffer M-PER mammalian protein extraction reagent and protease inhibitors (protease inhibitor mixture, lyophilized powder; Sigma) on ice for 2.5 min. After harvesting and centrifuging the lysate, the supernatant was saved and immediately used for immunoprecipitation. The amount of protein in each sample was assayed (BCA protein assay kit; Pierce). For immunoprecipitation, 100 μg of protein of the whole cell lysate was precleared with 5 μl of protein A/G-Sepharose beads (Santa Cruz Biotechnology). In a second microcentrifuge tube 5 μl of beads/sample were washed and then incubated with 5 μl of T1NH antibody/25 μl of beads in 500 μl M-PER buffer in the cold room rotating for 1 h. The supernatant was removed, washed twice M-PER with 50 μm calcium and protease inhibitors, and then resuspended in 100 μl of A/G-Sepharose bead slurry/tube.100 μg of lysate was added up to a final volume of 500 μl with M-PER plus 50 μm calcium and protease inhibitor rotating for 2 h in the cold room. The beads were then washed three times with M-PER plus 50 μm calcium. The final pellet of beads was resuspended in 20 μl of NuPage sample buffer (Invitrogen), and the whole sample was analyzed by gel electrophoresis and Western blotting as described previously. Antibodies used were 1:1000 anti-NCS-1 (Santa Cruz Biotechnology) and 1:2000 anti-InsP3R1 T1NH from Gregory Mignery's laboratory (24).
RESULTS
Inhibition of Calpain Protects NCS-1 Levels and Calcium Imaging
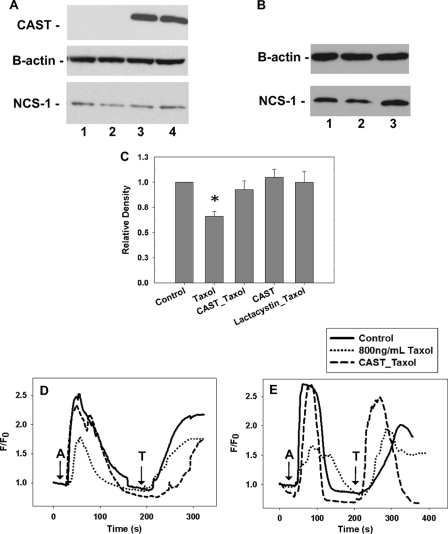
Known inhibitors of calpain were tested on both NCS-1 levels and intracellular calcium signaling in a neuroblastoma cell line, SHSY-5Y. Western blot analysis revealed that treatment of SHSY-5Y cells with paclitaxel for 6 h led to a reduction in NCS-1 levels by 34 ± 2.3% (Fig. 1, A and B). A marked reduction (45 ± 2.0%) in intracellular calcium signaling was also found in paclitaxel-treated cells, both in the presence and absence of extracellular calcium (Fig. 1, D and E). To prevent this reduction in protein and signaling, two classes of inhibitors were considered. First, lactacystin, an irreversible inhibitor of proteasomes was investigated as the majority of proteins in cells are degraded by the proteasome system (26, 27). We found that lactacystin rescued both NCS-1 levels and the intracellular calcium signal in SHSY-5Y cells to near that of control cells (Fig. 1, B and C). To avoid off-target effects when using an agent not naturally found in cells, SHSY-5Y cells were transfected with CAST, the predominant calpain inhibitor found naturally in cells, to determine whether increased expression of CAST would have protective effects. After cells were transfected with CAST alone there was a slight increase in NCS-1 levels compared with control cells (Fig. 1, A and C). SHSY-5Y cells transfected with CAST 24–48 h prior to paclitaxel treatment had NCS-1 levels near that of control cells (Fig. 1, A and C). As expected from the biochemical assessment showing protection of NCS-1 levels by CAST, calcium signaling was also rescued when examined in cells transfected with CAST. Both the magnitude and duration of the calcium signals in paclitaxel-treated cells were similar to those of control cells (Fig. 1D).
FIGURE 1.
Lactacystin and overexpression of CAST, a calpain inhibitor, rescues NCS-1 levels and the calcium signal in SHSY-5Y cells. A, representative Western blot showing that NCS-1 levels are significantly decreased in SHSY-5Y cells treated with paclitaxel and expression of CAST rescued NCS-1 levels. Lane 1, control; lane 2, paclitaxel-treated cells; lane 3, CAST + paclitaxel; lane 4, CAST. B, Representative Western blot showing lactacystin-rescued NCS-1 levels. Lane 1, control; lane 2, paclitaxel-treated cells; lane 3, lactacystin + paclitaxel. C, Western blot analysis shows that either the presence of lactacystin or expression of CAST rescued NCS-1 levels close to that of control cells (n = 5). *, p ≤ 0.05. Error bars, S.E. All treatments were normalized to control cells. D and E, intracellular calcium signal measured in the presence (D) and absence (E) of external calcium. With a 6-h treatment of paclitaxel, calcium transients were reduced 45%. CAST rescued the calcium signal in cells treated for 6 h with paclitaxel to that of control cells (60 cells, n = 3). (A, 1 μm ATP; T, 10 μm thapsigargin).
To verify that the primary pathway affected was due to intracellular calcium signals, calcium imaging experiments were performed using calcium-free extracellular media. Once again, the intracellular calcium signals were reduced in paclitaxel-treated cells, whereas cells overexpressing CAST had intracellular calcium signals near those of control cells (Fig. 1E). These results clearly support our hypothesis that inhibition of calpain has protective effects on both NCS-1 levels and intracellular calcium signaling in cells with prolonged exposure to paclitaxel.
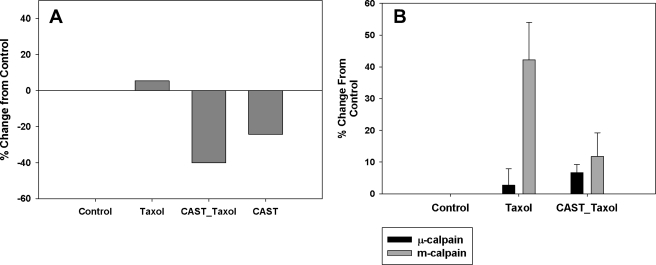
Effects of CAST on Calpain
Calpain activity in control SHSY-5Y cells was compared with cells overexpressing CAST by the use of the standard activity assay: calpain-Glo protease assay (Promega). Previously, we showed that there is an increase in calpain activity in cells treated with paclitaxel for 6 h (13). Cells transfected with CAST were treated with paclitaxel for 6 h and were found to have lower calpain activity compared with both control and paclitaxel-treated cells (Fig. 2A). These results show that overexpression of CAST resulted in a reduction of calpain activity even in the presence of paclitaxel. Next, calpain 1 (μ-calpain) and calpain 2 (m-calpain) levels were assessed in cells treated with paclitaxel. Western blot analysis was performed on the lysates of cells treated as described above. Interestingly, μ-calpain levels did not change; however, m-calpain levels increased in the presence of paclitaxel and returned back to control levels in cells transfected with CAST prior to treatment with paclitaxel for 6 h (Fig. 2B).
FIGURE 2.
Calpain activity is significantly decreased in the presence of CAST. A, calpain activity was significantly increased in cells treated with paclitaxel. Cells transfected with CAST 24 h prior to 6-h paclitaxel treatment had significantly reduced calpain activity. B, Western blot analysis shows that m-calpain, and not μ-calpain, levels were affected by 6-h paclitaxel treatment. Error bars, S.E.
Mutant Versions of NCS-1 Still Bind Calcium, InsP3R, and Paclitaxel
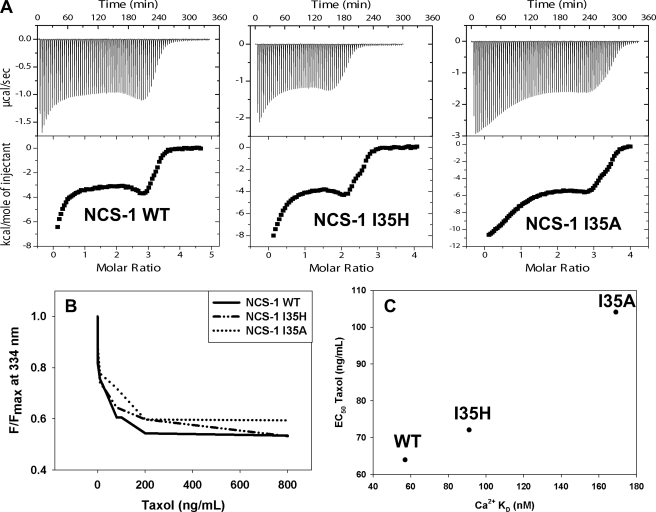
To verify that NCS-1 is a target of calpain in cells, two mutant versions of NCS-1 were developed with point mutations at the P2 position of the calpain cleavage site of NCS-1. The P2 position is amino acid I35 in NCS-1. From the analysis of the calpain cleavage site published previously (17), we selected histidine (I35H) and alanine (I35A) for substitution at position 35 of NCS-1 because these amino acids are the least favored for calpain cleavage. The P2 site of NCS-1 was selected because examination of the three-dimensional structure of NCS-1 (18, 19) suggested that mutations at Ile-35 would not alter protein folding. ITC was performed to ensure that neither of these mutations altered the calcium binding ability of NCS-1. The calcium binding affinity of NCS-1 I35H was similar to that of NCS-1 WT, whereas the calcium binding affinity of NCS-1 I35A was slightly decreased (Fig. 3A and Table 1). Immunoprecipitation of InsP3R was performed to ensure that these mutations did not interfere with the ability of NCS-1 to bind with the InsP3R because this interaction is important for modulation of intracellular calcium signaling. Neither mutation was found to interfere with the binding of NCS-1 to the InsP3R (supplemental Fig. S2).
FIGURE 3.
Binding of NCS-1 WT, I35H, I35A to calcium, InsP3R, and paclitaxel. A, ITC of NCS-1 WT, I35H, and I35A. NCS-1 and the mutants I35H and I35A were titrated with calcium and the resulting isotherm fitted with a two-site binding model using the program ORIGIN. B, paclitaxel binding to NCS-1 WT, I35H, and I35A. An assay utilizing the intrinsic tryptophan fluorescence of NCS-1 was used to determine the binding affinity with paclitaxel for NCS-1 WT and both mutants. C, correlation between paclitaxel and calcium binding of NCS-1 WT, I35H, and I35A. The EC50 of paclitaxel was plotted as a function of calcium binding affinity of NCS-1 WT, I35H, and I35A.
TABLE 1.
ITC thermodynamic parameters of NCS-1 WT, 135H, and 135A
Results of fitting ITC data to a two-site binding model are shown. The program ORIGIN was used to obtain the thermodynamic parameters for NCS-1 WT and both mutants.
| ITC Ca2+titration of NCS-1 WT, I35H, and I35A | |||||
|---|---|---|---|---|---|
| NCS-1 | Site | KA | Kda | ΔHb | ΔSc |
| m−1 | nm | kcal/mol | kcal/mol | ||
| WT | 1 | 6.6 × 107 ± 2.5 × 107 | 57 | −2.37 ± 0.13 | 7.72 |
| 2 | 4.7 × 106 ± 2.2 × 106 | −3.19 ± 0.50 | 19.67 | ||
| I35H | 1 | 7.2 × 107 ± 2.0 × 107 | 91 nM | −8.28 ± 0.34 | 7.70 |
| 2 | 1.7 × 106 ± 3.4 × 105 | −4.03 ± 0.58 | 14.75 | ||
| I35A | 1 | 1.1 × 106 ± 1.8 × 105 | 169 nM | −5.50 ± 0.84 | 8.87 |
| Site 2 | 3.2 × 107 ± 6.4 × 106 | −1.07 ± 0.25 | 2.44 | ||
a ΔH, enthalpy.
b ΔS, entropy of binding.
c Overall Kd = 1/√K1K2.
Similarly, it was important to determine whether the mutations in NCS-1 altered the ability of NCS-1 to bind paclitaxel. Binding between paclitaxel and NCS-1 was assessed using tryptophan fluorescence. With increasing concentrations of paclitaxel, the binding curves were similar for all three variants of NCS-1 (Fig. 3B). NCS-1 WT bound paclitaxel with an affinity of 64.0 ± 2.4 ng/ml, NCS-1 I35H with an affinity of 72.1 ± 8.4 ng/ml, and NCS-1 I35A with an affinity of 104.1 ± 9.7 ng/ml. Interestingly, as the calcium binding affinity of NCS-1 decreased, so did the affinity for paclitaxel (Fig. 3C). Nonetheless, both mutants were still able to bind calcium and paclitaxel with high affinity.
Calpain Cleaves NCS-1 WT, I35H, and I35A
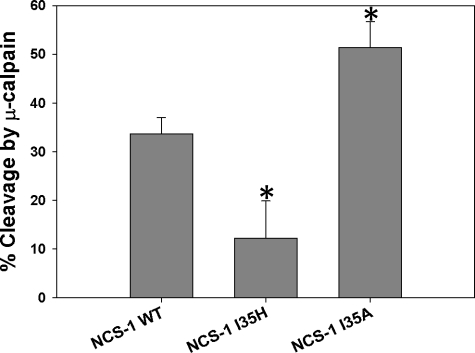
To confirm the assumption that mutations at position 35 of NCS-1 did affect the ability of calpain to recognize the cleavage site of NCS-1, purified μ-calpain was incubated with NCS-1 WT, I35H, or I35A in the presence of calcium. Interestingly, NCS-1 I35A was found to be a better substrate for calpain than the WT, whereas NCS-1 I35H was cleaved by calpain less than NCS-1 WT (Fig. 4 and supplemental Fig. S3).
FIGURE 4.
Cleavage reactions of NCS-1 WT, I35H, and I35A by μ-calpain. Digestion reactions were performed using 1:4 molar ratios of μ-calpain to NCS-1 WT, I35H, and I35A (n = 3). Changes in NCS-1 levels after calpain cleavage were determined using UnSCAN-It. Error bars, S.E. *, p ≤ 0.05.
Expression of NCS-1 WT, I35H, and I35A Protect Intracellular Calcium Signaling
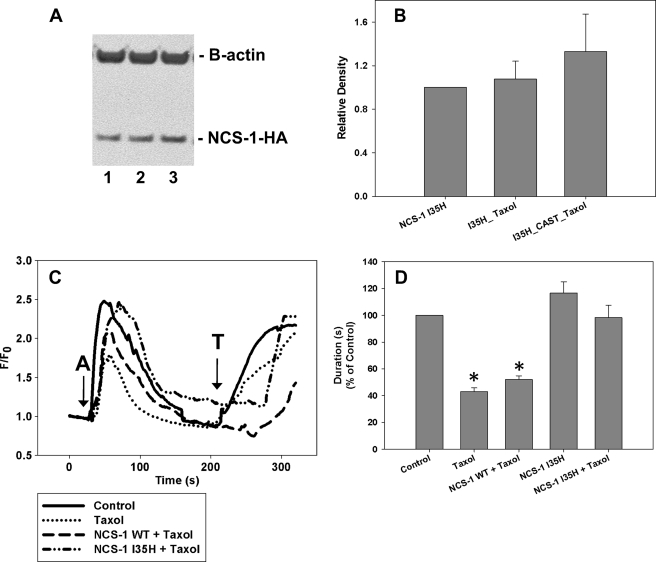
The two NCS-1 mutants provide potentially different modes of action for protecting NCS-1 levels in cells. Western blot analysis revealed that SHSY-5Y cells transfected with NCS-1 I35H-HA prior to the 6-h paclitaxel treatment showed no reduction in the levels of this mutant (Fig. 5, A and B). The addition of CAST to NCS-1 I35H-HA and paclitaxel had no additional effect. However, cells transfected with NCS-1 I35A-HA prior to paclitaxel treatment showed significant reduction in the levels of this mutant (supplemental Fig. S1, A and B) as predicted from in vitro experiments (Fig. 3B). Calcium imaging experiments showed a marked reduction in intracellular calcium signaling both in amplitude (45%) and duration (57%) in cells treated with paclitaxel for 6 h in both the presence and absence of extracellular calcium (Fig. 5, C and D). To test the effects of the mutated NCS-1 on intracellular calcium signals, SHSY-5Y cells were transfected with NCS-1 WT, I35H, or I35A. Overexpression of NCS-1 WT alone provided only partial protection of the amplitude of the intracellular calcium signal, whereas the duration was still markedly reduced compared with control cells (Fig. 5, C and D). Expression of NCS-1 I35H or I35A was able to protect fully against paclitaxel-induced reductions in both the amplitude and duration of calcium signaling; with either mutant version of NCS-1 the intracellular calcium signals were virtually identical to those of control cells (Fig. 5, C and D, and supplemental Fig. S1, C and D).
FIGURE 5.
Overexpression of NCS-1 I35H rescues NCS-1 levels and the calcium signal in SHSY-5Y cells. A, representative Western blot shows that NCS-1 I35H-HA levels are not significantly changed with paclitaxel treatment. Lane 1, NCS-1 I35H-HA; lane 2, NCS-1 I35H-HA + paclitaxel; lane 3, NCS-1 I35H-HA + CAST + paclitaxel. B, NCS-1 I35H-HA was not decreased by paclitaxel treatment, nor was it enhanced by the addition of CAST prior to paclitaxel treatment (n = 3). *, p ≤ 0.05. All treatments were normalized to cells transfected with HA-tagged NCS-1 I35H. C, the intracellular calcium signal was reduced after a 6-h treatment with paclitaxel. The overexpression of NCS-1 WT prior to paclitaxel treatment was unable to rescue the calcium signal fully to that of control cells. However, cells expressing NCS-1 I35H prior to treatment with paclitaxel had calcium signals similar to that of control cells (15–20 cells, n = 3). (A, 1 μm ATP; T, 10 μm thapsigargin). D, comparison of the normalized response durations to 1 μm ATP in control cells, cells treated with paclitaxel, and cells overexpressing NCS-1 WT or I35H is shown. All treatments are normalized to control cells. All values are mean ± S.E. (error bars).
The presence of calpain inhibitors or mutant NCS-1 was able to protect cells from paclitaxel-induced decreases in calcium signaling. To determine whether additive or synergetic effects were possible if both treatments were present simultaneously, SHSY-5Y cells were transfected with both CAST and either NCS-1 I35H or I35A 24–48 h prior to the 6-h paclitaxel treatment. No additional protection was found in cells transfected with CAST and NCS-1 I35H or I35A compared with either protein alone. Calcium imaging experiments were performed in both calcium-containing and calcium-free media, and similar results were obtained (data not shown). These results show there are no synergistic effects when both proteins, CAST and NCS-1, are present at elevated levels.
DISCUSSION
The aim of this study was to determine whether calpain activation is an important step in the mechanism leading to paclitaxel-induced NCS-1 degradation. Prolonged treatment of paclitaxel induces activation of calpain, NCS-1 degradation, and reductions in intracellular calcium signaling (6, 13). Previous literature suggests that the inhibition of calpain proteases has protective effects against sensory neuropathy caused by treatment with chemotherapeutic drugs (13, 16). Confirmation of the importance of calpain in the mechanism was an essential test of the hypothesis. Inhibitors of both the proteosome and calpain, lactacystin, and CAST, the naturally occurring calpain inhibitor, were tested in paclitaxel-treated cells, and in both cases endogenous levels of NCS-1 were protected against paclitaxel-induced degradation. Interestingly, cells transfected with CAST alone have a slightly elevated NCS-1 level compared with control cells, suggesting that CAST is protecting against the normally occurring NCS-1 degradation. The presence of either lactacystin or CAST in paclitaxel-treated cells also restored intracellular calcium signals to those seen in control cells. These results clearly show that calpain is a critical component in the degradation of NCS-1 and that by inhibiting calpain, the intracellular calcium signal was protected.
Previously, cells treated with paclitaxel were found to have elevated levels of calpain activity (13). The presence of CAST reduced calpain activity compared with that seen in both control and paclitaxel-treated cells. It was also discovered that the levels of m-calpain appear to be more affected by paclitaxel than μ-calpain. These two calpain isoforms use the same cleavage site and are inhibited by the same pharmacological reagents (20, 25). However, it was not possible to separate the activity of the two isoforms of calpain. This is because effective reagents to knock down the isoforms selectively are lacking. The expression of CAST decreases the overall calpain activity, but this natural inhibitor is an effective inhibitor of both isoforms of calpain (20).
To test the suggestion that NCS-1 is an important component of the effects of paclitaxel, two mutant versions of NCS-1 were developed with point mutations at the P2 position of the calpain cleavage site of NCS-1 (Ile-35), with amino acids thought to be poorly recognized by calpain. Isoleucine, naturally found at this position in NCS-1, is one of the top three amino acids favored by calpain at this position in the cleavage site (17). Alanine and histidine are among the least favored amino acids (17) and appear to have the least effect on proper protein folding (18, 19). Although these two mutations have slightly different effects on NCS-1 binding of calcium and paclitaxel, both protected calcium signaling in intact cells. Previously, we showed that mutating NCS-1 to inhibit calcium binding decreased its ability to interact with the InsP3R and this alteration led to a decrease in the open probability of the InsP3R (11). Therefore, we suggest that the ability of NCS-1 to bind calcium is crucial for this protein to function properly, and interaction with the InsP3R is essential for proper calcium signaling in neuronal cells. Neither mutation was found to interfere with the ability of NCS-1 to bind and interact with the InsP3R and thus will not affect calcium signaling in cells.
We ascertained that NCS-1 I35H was favored less than both NCS-1 WT and I35A by μ-calpain. Interestingly, NCS-1 I35A was a better substrate for μ-calpain, a finding that was opposite the result initially expected when designing this mutant. It is possible that the mutation from isoleucine to alanine led to a structural change in NCS-1 that resulted in a cleavage site for calpain that is more exposed, thus enhancing the likelihood of cleavage by calpain. Although the alanine mutation had an unexpected effect on cleavage by calpain, it still provided protection of overall NCS-1 levels. Overexpression of NCS-1 WT alone provided only a partial protection of the amplitude of the intracellular calcium signal but not the duration, which remained reduced compared with control cells. However, expression of NCS-1 I35H or I35A in SHSY-5Y cells treated with paclitaxel rescued both the amplitude and duration of the intracellular calcium signals back to that of control cells. Therefore, it appears that the presence of either mutant provides better protection against the negative effects of paclitaxel treatment on cells compared with NCS-1 WT alone. A possible explanation for the protective properties of the alanine mutant could be that the availability of a version of NCS-1 that is a more favorable target for calpain cleavage can divert paclitaxel-induced degradation of NCS-1 WT. In contrast, the histidine mutant was less likely to be cleaved by calpain, and it also functions in a manner similar to endogenous NCS-1, therefore allowing NCS-1-dependent processes in the cell to continue unaltered.
Previous findings suggested that calpain activation is an important step in the mechanism of paclitaxel-induced peripheral neuropathy (6, 13, 14, 16). Many chemotherapeutic drugs cause an enhanced calcium signal which leads to hyperactivation of neurons which in turn, activates enzymes that induce pathological changes in the neurons (21). The conclusions of this study support our hypothesis that the mechanism for paclitaxel-induced peripheral neuropathy begins with paclitaxel binding to NCS-1, thereby enhancing NCS-1 binding to the InsP3R, resulting in an increase in intracellular calcium. The increased level of calcium activates calpain which then cleaves NCS-1. With degraded levels of NCS-1 now present in the cell, it is unable to interact fully with the InsP3R, thereby decreasing calcium signaling in the cell. This reduction in overall calcium signaling results in the changes to neurons that we suggest induces peripheral neuropathy.
Supplementary Material
Acknowledgments
We thank Andjelka Celic, Edward Petri, and Michelle Mo for invaluable advice regarding the design of the experiments and thoughtful discussions and comments on the manuscript. We also thank Dr. Ben Turk for invaluable advice regarding the design of the mutant versions of NCS-1.
This work was supported, in whole or in part, by National Institutes of Health Grants DK57751 and DK61747. This work was also supported by National Institutes of Health Training Grant 5T32DK007356-32 administered by the Department of Digestive Diseases at Yale University and by Department of Defense for Breast Cancer Research Grant DOD J00181.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S3.
- NCS-1
- neuronal calcium sensor 1
- CAST
- calpastatin
- InsP3R
- inositol 1,4,5-trisphosphate receptor
- ITC
- isothermal titration calorimetry
- μ-calpain
- calpain 1
- m-calpain
- calpain 2.
REFERENCES
- 1. Spencer C. M., Faulds D. (1994) Drugs 48, 794–847 [DOI] [PubMed] [Google Scholar]
- 2. Preston N. J. (1996) Eur. J. Cancer Care 5, 147–152 [DOI] [PubMed] [Google Scholar]
- 3. Hilkens P. H., ven den Bent M. J. (1997) J. Peripher. Nerv. Syst. 2, 350–361 [PubMed] [Google Scholar]
- 4. Mielke S., Sparreboom A., Mross K. (2006) Eur. J. Cancer 42, 24–30 [DOI] [PubMed] [Google Scholar]
- 5. Schiff P. B., Fant J., Horwitz S. B. (1979) Nature 277, 665–667 [DOI] [PubMed] [Google Scholar]
- 6. Boehmerle W., Splittgerber U., Lazarus M. B., McKenzie K. M., Johnston D. G., Austin D. J., Ehrlich B. E. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 18356–18361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Burgoyne R. D., Weiss J. L. (2001) Biochem. J. 353, 1–12 [PMC free article] [PubMed] [Google Scholar]
- 8. McFerran B. W., Graham M. E., Burgoyne R. D. (1998) J. Biol. Chem. 273, 22768–22772 [DOI] [PubMed] [Google Scholar]
- 9. Nakamura T. Y., Pountney D. J., Ozaita A., Nandi S., Ueda S., Rudy B., Coetzee W. A. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 12808–12813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hilfiker S. (2003) Biochem. Soc. Trans. 31, 828–832 [DOI] [PubMed] [Google Scholar]
- 11. Schlecker C., Boehmerle W., Jeromin A., DeGray B., Varshney A., Sharma Y., Szigeti-Buck K., Ehrlich B. E. (2006) J. Clin. Invest. 116, 1668–1674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Petko J. A., Kabbani N., Frey C., Woll M., Hickey K., Craig M., Canfield V. A., Levenson R. (2009) BMC Neurosci. 10, 27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boehmerle W., Zhang K., Sivula M., Heidrich F. M., Lee Y., Jordt S. E., Ehrlich B. E. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 11103–11108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Blachford C., Celiæ A., Petri E. T., Ehrlich B. E. (2009) Cell Calcium 46, 257–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aravind P., Chandra K., Reddy P. P., Jeromin A., Chary K. V., Sharma Y. (2008) J. Mol. Biol. 376, 1100–1115 [DOI] [PubMed] [Google Scholar]
- 16. Wang M. S., Davis A. A., Culver D. G., Wang Q., Powers J. C., Glass J. D. (2004) Brain 127, 671–679 [DOI] [PubMed] [Google Scholar]
- 17. Cuerrier D., Moldoveanu T., Davies P. L. (2005) J. Biol. Chem. 280, 40632–40641 [DOI] [PubMed] [Google Scholar]
- 18. Bourne Y., Dannenberg J., Pollmann V., Marchot P., Pongs O. (2001) J. Biol. Chem. 276, 11949–11955 [DOI] [PubMed] [Google Scholar]
- 19. Sussman J. L., Lin D., Jiang J., Manning N. O., Prilusky J., Ritter O., Abola E. E. (1998) Acta Crystallogr. D Biol. Crystallogr. 54, 1078–1084 [DOI] [PubMed] [Google Scholar]
- 20. Goll D. E., Thompson V. F., Li H., Wei W., Cong J. (2003) Physiol. Rev. 83, 731–801 [DOI] [PubMed] [Google Scholar]
- 21. Florea A. M., Büsselberg D. (2009) Neurotoxicology 30, 803–810 [DOI] [PubMed] [Google Scholar]
- 22. Fisher J. R., Sharma Y., Iuliano S., Piccioti R. A., Krylov D., Hurley J., Roder J., Jeromin A. (2000) Protein Expr. Purif. 20, 66–72 [DOI] [PubMed] [Google Scholar]
- 23. Estrada M., Uhlen P., Ehrlich B. E. (2006) J. Cell Sci. 119, 733–743 [DOI] [PubMed] [Google Scholar]
- 24. Ramos-Franco J., Caenepeel S., Fill M., Mignery G. (1998) Biophys. J. 75, 2783–2793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huang Y., Wang K. K. (2001) Trends Mol. Med. 7, 355–362 [DOI] [PubMed] [Google Scholar]
- 26. Fenteany G., Schreiber S. L. (1998) J. Biol. Chem. 273, 8545–8548 [DOI] [PubMed] [Google Scholar]
- 27. Bingol B., Schuman E. M. (2005) Curr. Opin. Neurobiol. 15, 536–541 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.