Abstract
Infectious prion diseases 1 – scrapie of sheep 2 and chronic wasting disease (CWD) of several species in the deer family 3,4 – are transmitted naturally within affected host populations. Although several possible sources of contagion have been identified in excretions and secretions from symptomatic animals 5–8, the biological importance of these sources in sustaining epidemics remains unclear. Here we show that asymptomatic CWD-infected mule deer (Odocoileus hemionus) excrete CWD prions in their feces long before they develop clinical signs of prion disease. Intracerebral (i.c.) inoculation of irradiated deer feces into transgenic (Tg) mice overexpressing cervid PrP revealed infectivity in 14 of 15 fecal samples collected from 5 deer at 7–11 months before the onset of neurological disease. Although prion concentrations in deer feces were considerably lower than in brain tissue from the same deer collected at the disease terminus, the estimated total infectious dose excreted in feces by an infected deer over the disease course may approximate the total contained in brain tissue. Prolonged fecal prion excretion by infected deer provides a plausible natural mechanism that might explain the high incidence and efficient horizontal transmission of CWD within deer herds 3,4,9, as well as prion transmission between susceptible deer species.
Prions are transmissible, proteinaceous agents that cause fatal neurodegenerative diseases 1. In cervids, including deer (Odocoileus spp.), elk (Cervus elaphus nelsoni), and moose (Alces alces shirasi), prions cause CWD 4,10. The incidence of CWD can be remarkably high in both captive and wild herds 3,4,11 and epidemiologic data argue that efficient horizontal transmission drives epidemic dynamics 9,11,12. Although cervids can be infected orally 13,14 and seem to be able to contract CWD from contaminated environments 15, precisely how and when CWD prions are shed into the environment have not been described. Previous studies have identified CWD prions in saliva, blood, urine, antler velvet, and muscle, lymphoid, and other tissues of symptomatic cervids with late-stage disease 5–7,14,16. These sources of CWD prions may contribute to the spread of CWD, but none parsimoniously explains natural CWD transmission both within and between species in the deer family. In order to fit observed patterns, a natural CWD transmission mechanism must be effected within biologically realistic limits of the carrier medium, cannot require “cannibalism” 9,15, and should be indirect to explain both environmental persistence and spread among multiple host species 4,10,12,15. Because empirical data 13–15 and modeling 12 suggested fecal excretion of prions throughout much of the disease course as potentially important to CWD transmission, we investigated whether prions are shed in feces from mule deer during the course of CWD infection.
To obtain a standard based on which we could determine the infectivity of tissues and excrement containing CWD prions, we first performed endpoint titrations of the Elk1 CWD isolate 17 by i.c. inoculation into transgenic mice overexpressing elk prion protein [Tg(ElkPrP) mice] using 10 ten-fold dilutions of a 10% brain homogenate ranging from 10−1 to 10−10 (Supplementary Fig. 1A). Based on the method of Spearman-Kärber 18, we determined the titer of this homogenate to be 7.4 ± 0.24 log infectious (ID50 ± SE) U/ml; using Cox regression analysis 19, which estimates the ID50 based on Kaplan-Meier survival times and accounts for censored events, the titer was 7.5 log ID50 U/ml with a 95% confidence interval (CI) between 7.0 and 7.9 logID50 U/ml. Next, we correlated the ID50 values with the respective incubation times for dilutions 10−1 to 10−6 (<50% of the mice developed disease for dilutions ≥10−7) (Supplementary Fig. 1B) as a basis for determining unknown titers using the incubation time assay 20. The dynamic range of the incubation time assay strongly depends on the combination of animal model and prion strain used. In our system, dilutions of the Elk1 inoculum ≤10−3 showed similar incubation times, whereas dilutions >10−7 did not cause disease, suggesting the dynamic range of this assay to be between 10−3–10−7 dilutions with median incubation times between 140–400 days (Supplementary Fig. 1A).
We assessed prion excretion in feces collected from 5 mule deer before and every 3–6 months after oral infection with CWD prions until the animals died or developed signs of CWD and were euthanized at 16–20 months (Supplementary Table 1). The presence of CWD prions in these mule deer was confirmed at ~250 dpi by positive tonsil and rectal mucosa biopsies (Supplementary Table 1) 21. Tg(ElkPrP) mice are highly susceptible to CWD prions of deer and seem to pose no species barrier despite one amino-acid difference at codon 226: elk express glutamate and deer PrP harbors glutamine 17,22. Therefore, we used bioassays to estimate CWD titers in brain tissue from these 5 deer (Fig. 1) as well as other orally infected deer with longer incubation times (Supplementary Fig. 2A), by i.c. inoculation into Tg(ElkPrP) mice. We observed median incubation times of 131–207 days and estimated prion doses between 2.9–5.7 logID50 units (Supplementary Table 2); titer estimates based on Cox regression analysis were similar (Supplementary Table 2). We found a linear correlation between the incubation period in deer and prion titers accumulated in their brains (Supplementary Fig. 2B).
Fig. 1.
Kaplan-Meier plots indicating incubation times in Tg(ElkPrP) mice after i.c. inoculation with 1% (wt/vol) brain homogenate from mule deer (ID numbers indicated) that developed CWD in 16–20 months after oral infection with CWD prions.
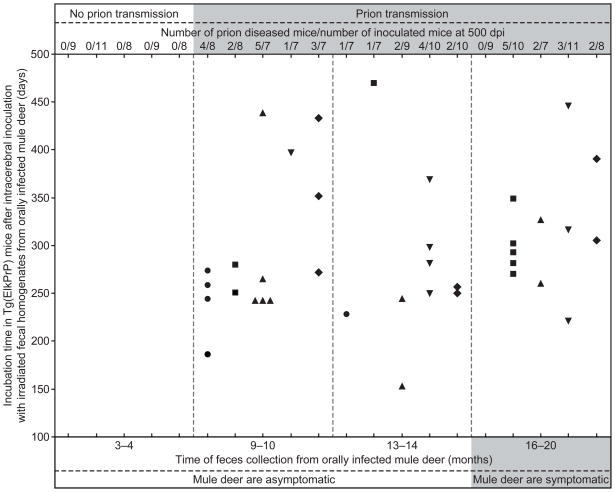
In addition to brain homogenates, we performed bioassays using irradiated fecal homogenates collected from infected mule deer by i.c. inoculation into Tg(ElkPrP) mice. Irradiation was used in order to damage nucleic acids and inactivate bacteria and viruses with minimal effects on prion titers 23; irradiation of the Elk1 CWD isolate did not diminish its titer when assayed in Tg(ElkPrP) mice (data not shown). Fecal samples collected from deer before oral infection and 3–4 months after infection did not transmit prion disease to Tg(ElkPrP) mice, whereas 14 of 15 fecal samples collected >4 months after inoculation transmitted disease to 37 of 126 Tg(ElkPrP) mice (29%; Fig. 2). Prion disease in mice with neurologic symptoms was confirmed by detecting proteinase K (PK)-resistant PrPSc in brain homogenates and in some cases by neuropathology (Fig. 3, Supplementary Fig. 3). Neuropathologic changes seen in symptomatic Tg(ElkPrP) mice inoculated with fecal material collected from mule deer were similar to those in Tg(ElkPrP) mice inoculated with brain homogenates from the same CWD-positive mule deer (Fig. 4).
Fig. 2.
Feces from 5 mule deer (WA04, circle; W1504, square; FA04, triangle; 25B04, inverted triangle; G804, diamond) were sampled before and at 4 time points following oral infection; the last collection was taken when the deer developed signs of CWD. Irradiated fecal homogenates were i.c. inoculated in Tg(ElkPrP) mice, some of which developed prion disease between 153–470 dpi when inoculated with fecal preparations collected from deer >4 months after their oral infection. Feces collected from deer before (data not shown) or at 3–4 months after oral infection did not transmit disease to Tg(ElkPrP) mice.
Fig. 3.
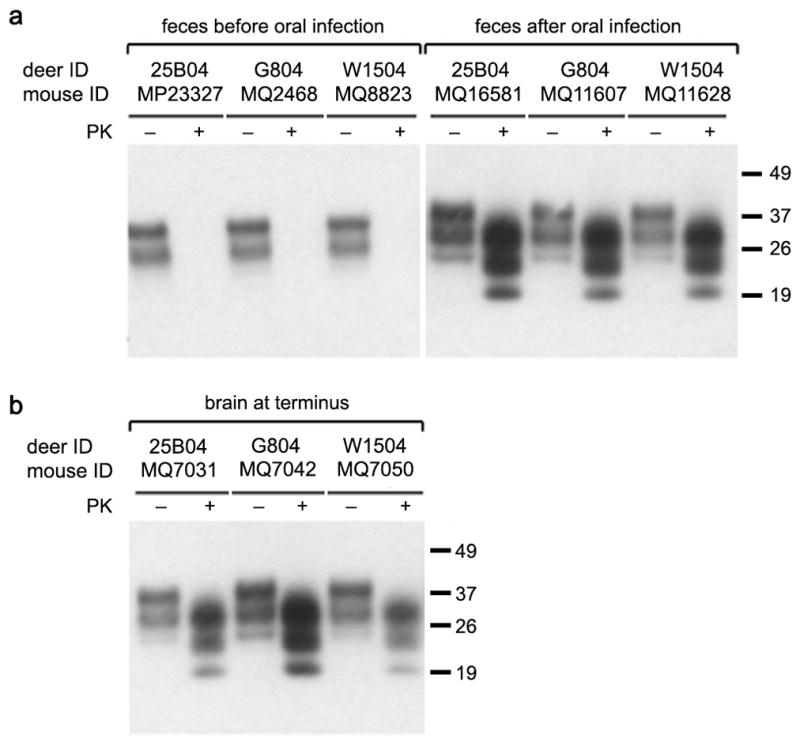
Western blots of brain homogenates of Tg(ElkPrP) mice inoculated i.c. with feces (A) or brain homogenates (B) from mule deer. (A) Mice inoculated with mule deer feces collected before the deer were orally infected with CWD prions were sacrificed at 481 dpi and showed no PK-resistant PrPSc in their brains. In contrast, some mice inoculated with feces from CWD-infected mule deer collected at >4 months after oral infection had PrPSc in their brains. (B) Inoculation with brain homogenates from CWD- infected deer resulted in prion disease and PrPSc in the brains of ill Tg(ElkPrP) mice. Samples were undigested (−) or digested with proteinase K (+). Molecular masses of protein standards are shown in kilodaltons (kDa).
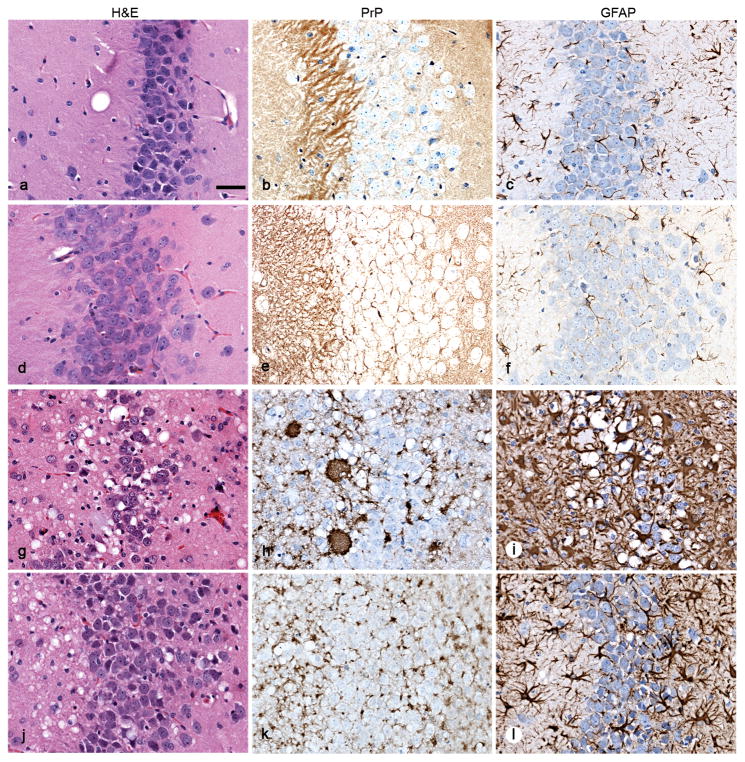
Fig. 4.
Neuropathology of brain sections from Tg(ElkPrP) mice inoculated with fecal (d–i) or brain (j–l) homogenates of mule deer. Sections show vacuolation (left), PrPSc deposition (middle), and astrocytic gliosis (right). Uninoculated, 658-day-old, control mice showed no vacuolation (a), no PrPSc deposits (b), and mild, age-related gliosis (c). Mice inoculated with feces from uninfected deer remained healthy without vacuolation (d) or PrPSc deposits (e), and showed mild, age-related gliosis at 503 dpi (f). Mice inoculated with feces from infected deer developed neurologic symptoms in 153–438 days, showed vacuolation (g), PrPSc deposits (h), and severe gliosis (i) similar to mice inoculated with brain homogenates of deer with CWD (j–l). Bar, 25 μm.
Transmission rates of CWD prions from some irradiated fecal samples to Tg(ElkPrP) mice were as high as 62% but most fecal homogenates caused disease in less than 50% of the inoculated mice. Compared to 30 μl of a 10% brain homogenate from elk harboring −5.9 log ID50 units, we estimated that 30 μl of 10% fecal homogenates contained between −1.6 and 0 log ID50 units (i.e., between 0.03 and 1 ID50 units) (Supplementary Table 3 and Supplementary Note). Prion titers in the feces from infected deer were variable for individual deer, but excretion of infectious prions was generally continuous until the deer became symptomatic, 16–20 months after oral infection. No trend was evident in infectivity levels in feces over time beginning at 9 months after oral infection (p = 0.99). This observed prion shedding pattern is consistent with the relatively early and rapid accumulation of prions in lymphoid tissue associated with the alimentary tract 13,14.
Fecal contamination of vegetation and soil provides a mechanism for transmitting a wide variety of parasitic bacteria, protozoa, and helminths among herbivores 24. Fecal prion shedding also appears to be a plausible explanation for the efficient transmission of CWD. Based on our titration results, an infected deer may shed nearly as many prions in feces over the disease course as accumulate in its brain in terminal disease. Assuming a constant infectious dose of 0 log ID50 units in 3 μg of feces (equivalent to 5.5 log ID50 units/g of wet feces), the cumulative total dose of prions shed during a 10-month period based on 780 g wet weight of feces produced per day 25 is 10.9 log ID50 units, which is similar to 10.8–12.3 log ID50 units found in brains of terminally sick mule deer, assuming an average brain weight of 200 g. Although prion titers in feces were relatively low, exposing deer to multiple oral doses in feces-contaminated environments could increase their overall probability of infection 26. Moreover, both the persistence and infectivity of prions shed in feces may be enhanced by interaction with clay soil microparticles 27, and mule deer may consume 8–30 g of soil daily depending on season 28, thereby facilitating infection by fecal prions. The foregoing mechanism is consistent with observed conditions under which captive mule deer have shown remarkably high rates of prion infection 3,4,9,12, and explains how CWD could effectively transmit among mule deer in natural systems.
Our findings show that CWD prions can be shed into the environment in feces from symptomatic and, perhaps more importantly, from asymptomatic deer. These data support the fecal-oral route as a likely natural mechanism for the transmission of CWD prions among deer and other susceptible cervid species, and possibly for scrapie prions among sheep and goats 29. Prion shedding through much of the disease course would facilitate exposure of both conspecifics and susceptible sympatric species, as well as geographic spread as deer move between seasonal ranges. Prion contamination of forest, shrub-steppe, and grassland habitats may be largely responsible for horizontal transmission of CWD among mule deer and perhaps other species.
Methods Summary
Oral infection of mule deer
At weaning, mule deer fawns were orally infected with approximately 1 g of nonspecific, pooled, infectious brain material placed at the base of the tongue; based on previous analyses, the inoculum pool contained approximately 3 μg PrPSc per g of brain tissue 30 and showed prion conversion in vitro and infectivity in vivo 13,14,30. All infected deer surviving >250 dpi showed evidence of PrPSc accumulation in tonsil and rectal mucosa biopsies 21, indicating successful infection. All infected deer that survived >490 dpi showed clinical signs of CWD prior to death and evidence of prion infection on postmortem examination.
Mice and inoculations
Production of Tg(ElkPrP+/+)12584, or Tg(ElkPrP) for simplicity, has been described previously. Tg(ElkPrP) mice do not express endogenous mouse PrP and homozygously express the PRNP allele encoding ElkPrP(M132) from the cosSHa.Tet cosmid vector. For i.c. inoculation, weanling mice were injected into the right parietal lobe with 30 μl of diluted sample using a 27-gauge, disposable hypodermic syringe. Inoculated animals were examined daily for their clinical status and three times weekly for neurologic dysfunction and scored for prion disease based on standard diagnostic criteria. Diseased animals were euthanized and their brains removed. One half brain was frozen and the other half immersion-fixed in 10% neutrally buffered formalin for biochemical and neuropathologic analyses, respectively. All mouse studies were reviewed and approved by the UCSF Institutional Animal Care and Use Committee.
Additional methods for care of mule deer, feces sampling and treatment, statistical analyses, Western immunoblotting, and histopathology are described in the Supplementary Information. Full Methods accompany this paper.
Supplementary Material
Acknowledgments
G.T. was supported by a fellowship from the Larry L. Hillblom Foundation. This work was supported by the Colorado Division of Wildlife and grants from the US Department of Defense National Prion Research Program (NP020152) and the National Institutes of Health (AG02132). The authors thank Pierre Lessard and the staff of the Hunters Point animal facility for support with the transgenic animal experiments, Kurt Giles for screening of transgenic animals, Ana Serban for antibodies, Kristen Pomeroy for technical assistance, Jiri Safar for discussions, Hang Nguyen for editorial assistance, and Beth Williams for her early insights on CWD transmission.
Footnotes
Reprints and permissions information is available at npg.nature.com/reprintsandpermissions.
The authors declare no competing financial interests.
Author contributions
G.T., M.W.M., and S.B.P. designed the transgenic mouse studies; G.T., M.W.M., L.L.W., T.M.S., C.P., A.L., and S.J.D. performed various aspects of the research on mule deer or transgenic mice; G.T., M.W.M, D.V.G., S.J.D., and S.B.P. analyzed the data; G.T., M.W.M., S.J.D., and S.B.P. wrote the paper. All authors discussed the results and commented on the manuscript.
Supplementary information accompanies this paper. Supplementary information is linked to the online version of the paper at www.nature.com/nature.
References
- 1.Prusiner SB. Prions. In: Knipe DM, et al., editors. Fields Virology. Lippincott Williams & Wilkins; Philadelphia: 2007. pp. 3059–3092. [Google Scholar]
- 2.Detwiler LA, Baylis M. The epidemiology of scrapie. Rev Sci Tech. 2003;22:121–143. doi: 10.20506/rst.22.1.1386. [DOI] [PubMed] [Google Scholar]
- 3.Williams ES, Young S. Chronic wasting disease of captive mule deer: a spongiform encephalopathy. J Wildl Dis. 1980;16:89–98. doi: 10.7589/0090-3558-16.1.89. [DOI] [PubMed] [Google Scholar]
- 4.Williams ES. Chronic wasting disease. Vet Pathol. 2005;42:530–549. doi: 10.1354/vp.42-5-530. [DOI] [PubMed] [Google Scholar]
- 5.Mathiason CK, et al. Infectious prions in the saliva and blood of deer with chronic wasting disease. Science. 2006;314:133–136. doi: 10.1126/science.1132661. [DOI] [PubMed] [Google Scholar]
- 6.Angers RC, et al. Chronic wasting disease prions in elk antler velvet. Emerg Infect Dis. 2009;15:696–703. doi: 10.3201/eid1505.081458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haley NJ, Seelig DM, Zabel MD, Telling GC, Hoover EA. Detection of CWD prions in urine and saliva of deer by transgenic mouse bioassay. PLoS ONE. 2009;4:e4848. doi: 10.1371/journal.pone.0004848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Konold T, Moore SJ, Bellworthy SJ, Simmons HA. Evidence of scrapie transmission via milk. BMC Vet Res. 2008;4:14. doi: 10.1186/1746-6148-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller MW, Williams ES. Prion disease: horizontal prion transmission in mule deer. Nature. 2003;425:35–36. doi: 10.1038/425035a. [DOI] [PubMed] [Google Scholar]
- 10.Baeten LA, Powers BE, Jewell JE, Spraker TR, Miller MW. A natural case of chronic wasting disease in a free-ranging moose (Alces alces shirasi) J Wildl Dis. 2007;43:309–314. doi: 10.7589/0090-3558-43.2.309. [DOI] [PubMed] [Google Scholar]
- 11.Miller MW, et al. Lions and prions and deer demise. PLoS ONE. 2008;3:e4019. doi: 10.1371/journal.pone.0004019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller MW, Hobbs NT, Tavener SJ. Dynamics of prion disease transmission in mule deer. Ecol Appl. 2006;16:2208–2214. doi: 10.1890/1051-0761(2006)016[2208:dopdti]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 13.Sigurdson CJ, et al. Oral transmission and early lymphoid tropism of chronic wasting disease PrPres in mule deer fawns (Odocoileus hemionus) J Gen Virol. 1999;80:2757–2764. doi: 10.1099/0022-1317-80-10-2757. [DOI] [PubMed] [Google Scholar]
- 14.Fox KA, Jewell JE, Williams ES, Miller MW. Patterns of PrPCWD accumulation during the course of chronic wasting disease infection in orally inoculated mule deer (Odocoileus hemionus) J Gen Virol. 2006;87:3451–3461. doi: 10.1099/vir.0.81999-0. [DOI] [PubMed] [Google Scholar]
- 15.Miller MW, Williams ES, Hobbs NT, Wolfe LL. Environmental sources of prion transmission in mule deer. Emerg Infect Dis. 2004;10:1003–1006. doi: 10.3201/eid1006.040010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Angers RC, et al. Prions in skeletal muscles of deer with chronic wasting disease. Science. 2006;311:1117. doi: 10.1126/science.1122864. [DOI] [PubMed] [Google Scholar]
- 17.Tamgüney G, et al. Transmission of elk and deer prions to transgenic mice. J Virol. 2006;80:9104–9114. doi: 10.1128/JVI.00098-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dougherty R. Animal virus titration techniques. In: Harris RJC, editor. Techniques in Experimental Virology. Academic Press; New York: 1964. pp. 169–224. [Google Scholar]
- 19.Cox DR. Regression models and life-tables. J R Stat Soc Ser A Method. 1972;34:187–220. [Google Scholar]
- 20.Prusiner SB, et al. Measurement of the scrapie agent using an incubation time interval assay. Ann Neurol. 1982;11:353–358. doi: 10.1002/ana.410110406. [DOI] [PubMed] [Google Scholar]
- 21.Wolfe LL, et al. PrPCWD in rectal lymphoid tissue of deer (Odocoileus spp. ) J Gen Virol. 2007;88:2078–2082. doi: 10.1099/vir.0.82342-0. [DOI] [PubMed] [Google Scholar]
- 22.Tamgüney G, et al. Transmission of scrapie and sheep-passaged bovine spongiform encephalopathy prions to transgenic mice expressing elk prion protein. J Gen Virol. 2009;90:1035–1047. doi: 10.1099/vir.0.007500-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miekka SI, et al. Inactivation of viral and prion pathogens by gamma-irradiation under conditions that maintain the integrity of human albumin. Vox Sang. 2003;84:36–44. doi: 10.1046/j.1423-0410.2003.00256.x. [DOI] [PubMed] [Google Scholar]
- 24.Judge J, Greig A, Kyriazakis I, Hutchings MR. Ingestion of faeces by grazing herbivores—risk of inter-species disease transmission. Agric Ecosyst Environ. 2005;107:267–274. [Google Scholar]
- 25.Arthur WJ, Jr, Alldredge AW. Seasonal estimates of masses of mule deer fecal pellets and pellet groups. J Wildl Manage. 1980;44:750–752. [Google Scholar]
- 26.Diringer H, Roehmel J, Beekes M. Effect of repeated oral infection of hamsters with scrapie. J Gen Virol. 1998;79:609–612. doi: 10.1099/0022-1317-79-3-609. [DOI] [PubMed] [Google Scholar]
- 27.Johnson CJ, Pedersen JA, Chappell RJ, McKenzie D, Aiken JM. Oral transmissibility of prion disease is enhanced by binding to soil particles. PLoS Pathog. 2007;3:e93. doi: 10.1371/journal.ppat.0030093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arthur WJ, III, Alldredge AW. Soil ingestion by mule deer in Northcentral Colorado. J Range Manage. 1979;32:67–71. [Google Scholar]
- 29.Hadlow WJ, Kennedy RC, Race RE. Natural infection of Suffolk sheep with scrapie virus. J Infect Dis. 1982;146:657–664. doi: 10.1093/infdis/146.5.657. [DOI] [PubMed] [Google Scholar]
- 30.Raymond GJ, et al. Evidence of a molecular barrier limiting susceptibility of humans, cattle and sheep to chronic wasting disease. EMBO J. 2000;19:4425–4430. doi: 10.1093/emboj/19.17.4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.