Abstract
Background
Studies have shown that women with vulvodynia are more psychologically distressed than women without vulvodynia. These studies, however, have not effectively established temporal associations between diagnosed psychiatric disorders and vulvodynia.
Methods
The Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) was administered to 240 case-control pairs of women with and without vulvodynia. Interviews established age at first onset of diagnosed mood and anxiety disorder. Age information was used to determine whether the first episode of mood and/or anxiety was antecedent or subsequent to the first onset of vulvodynia symptoms. Conditional logistic regressions tested whether antecedent depression or anxiety was more likely among women with or without vulvodynia. Cox proportional hazards modeling was then used to estimate risk of subsequent new or recurrent onset of mood or anxiety disorder.
Results
After adjusting for education, race, age at menarche, age at first tampon use, and age at first sexual intercourse, odds of vulvodynia were four-times more likely among women with antecedent mood or anxiety compared to women without (95% confidence interval [CI] 2.1-7.5). Vulvodynia was associated with new or recurrent onset of mood or anxiety disorder after adjustment (hazard ratio [HR] 1.7, 95% CI 1.1-2.6) and did not significantly change after including history of mood or anxiety disorder before the onset of vulvodynia or reference age of controls in the models.
Conclusions
This is the first community-based epidemiologic study demonstrating that DSM-IV-diagnosed antecedent depression and anxiety disorders influence the risk of vulvodynia and that vulvodynia increases the risk of both new and recurrent onset of psychopathology.
Introduction
Although vulvodynia, or chronic vulvar pain, has long been documented, the medical and psychologic underpinnings remain elusive.1 With unexplained vulvar pain now known to be common (lifetime risk and prevalence of 16% and 7%, respectively),2 the continued lack of information available about antecedent risk factors and treatment has become a source of frustration for many women and their healthcare providers.3 Diagnosable psychologic disorders, such as depression and anxiety, have not been thoroughly examined in the pathogenesis of vulvodynia, as they have for many medical conditions (e.g., urinary tract or yeast infections).1,7 One reason for this disparity lies in the complexity of determining if psychologic disorders precede the onset of vulvodynia or occur primarily as a consequence of chronic vulvar pain.
It is well documented that women with vulvodynia are more psychologically distressed than women without vulvodynia.5–10 However, there is less certainty about the influence of psychiatric morbidity as an antecedent risk factor. This is largely due to several limitations in earlier studies that relied on self-reported diagnoses, lacked the capacity to establish age at first onset of depression or anxiety, and as a result, were unable to establish the temporal relationship between psychiatric disorders and vulvar pain onset.4–9 Moreover, the lack of structured clinical interviews in these prior studies meant documenting only symptoms of depression and anxiety in women; these measures preclude establishing a psychiatric diagnosis.4–9 Lastly, the selection of only women actively seeking treatment for vulvar pain in these earlier studies excludes the nearly 40% of women choosing not to seek treatment for their unexplained vulvar pain.2,5–7,9,10 Thus, the lack of studies that use a community-based population source has limited the generalizability of prior research findings.5,6,8–10
The study described in this report is based on women with and without vulvodynia who were identified from both the community and within clinical settings. In addition, we used Structured Clinical Interviews for DSM-IV Axis I Disorders (SCID),11 considered to be the gold standard for assessment of psychiatric morbidity. By pinpointing the age at first onset of vulvar pain and psychiatric morbidity, we sought to determine if diagnosed antecedent depression and anxiety are associated with risk of vulvodynia and if vulvodynia increases the risk of new or recurrent onset of depression or anxiety.
Materials and Methods
This study was approved by the Human Subjects Research Committee at Brigham and Women's Hospital in Boston and the University of Minnesota. Written informed consent was obtained from all participating women.
Target population
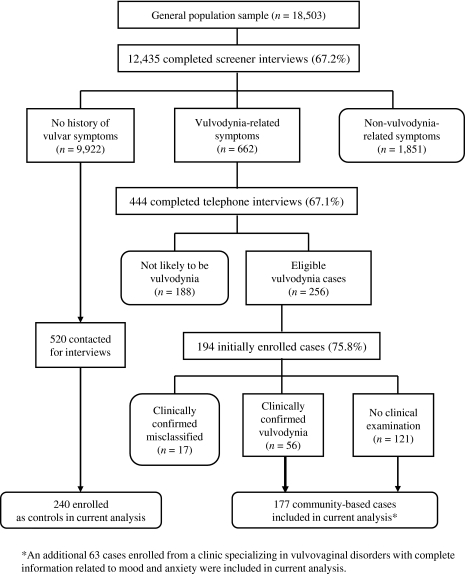
Using Massachusetts Town Books (annual Census publications that list residents by name, age, and address), 450 women, aged 18–64, were randomly selected each month for 55 months from three ethnically diverse Boston neighborhoods (defined geographically by ZIP code) and six suburban communities. These monthly samples were weighted to U.S. Census data by age and community distribution. Only women with a verifiable telephone number were included. A short questionnaire was developed to assess whether women in the general population had experienced chronic vulvar pain (burning, knifelike sharp pain, or pain on contact during intercourse, at the time of tampon insertion, or during a pelvic examination) for 3 months or longer. This questionnaire was mailed to a sample of 24,750 women, and 2,059 questionnaires were returned as undeliverable with no forwarding address. After three mailings and telephone follow-up, 3,443 women could not be confirmed as residing at the mailed household, and 745 women could not complete the questionnaire because of language barriers. Of the remaining 18,503 women, 12,435 (67.2%) completed the initial screening questionnaire (Fig. 1). The response was lower for inner-city neighborhoods (64.6%) compared to west suburban communities (68.2%), but the mean age of responders and nonresponders was identical. Of 12,435 women who returned the initial questionnaire, 662 self-reported chronic vulvar pain and were eligible for further screening as potential vulvodynia cases. There were 1,851 women reporting chronic itching or other nonvulvodynia-related symptoms who were excluded. The 9,922 women with no history of vulvar pain symptoms constituted the pool of eligible controls.
FIG. 1.
Flow chart of general population screening by which cases and controls were identified from the Boston Metropolitan area, 2000–2005.
Selection of cases and controls
Women who reported symptoms consistent with a diagnosis of vulvodynia (n=662) (Fig. 1) were asked to participate in a telephone interview to facilitate ruling out the presence of other vulvar conditions (i.e., sexually transmitted diseases [STD], yeast/bacterial infections, vulvar skin problems, misclassified pelvic disorders, estrogen-related dyspareunia, and inflammatory vaginitis). As shown in Figure 1, 4 potential cases (67.1%) agreed to complete the telephone assessment. Based on the telephone and initial self-administered interviews, 256 women were selected as eligible cases by a clinical expert (E.G.S.). Women manifesting symptoms consistent with conditions other than vulvodynia (e.g., infection, dermatosis) were excluded (n=188). Of 256 eligible cases from the general population, 194 (75.8%) agreed to participate in interviews that assessed the presence of psychiatric illnesses with the SCID. The mean duration of symptoms was 136 months, with <5% of cases reporting symptoms for <1 year and 25% reporting symptoms for >18 years.
A free clinical examination was offered to all cases for diagnosis confirmation and to give recommendations for course of therapy. Clinical confirmation involved a pelvic examination that applied Friedrich's diagnostic criteria.12 For localized vulvodynia, cases were clinically confirmed by absence of signs or symptoms of known causes of vulvar pain and by the presence of pain on touch by a cotton swab in the vestibule. For generalized vulvodynia, cases were clinically confirmed by absence of signs or symptoms of known causes of vulvar pain and by the patient's description of burning, stinging, pain, or rawness with or without allodynia in the affected area.
Localized and generalized cases of vulvodynia were combined for analysis purposes. Seventy-three cases (38%) agreed to the clinical examination and of these women, 56 (77%) were clinically confirmed as having vulvodynia. The remaining 17 women had no disorder (n=3), lichen sclerosis (n=2), vaginal infections (n=2), pelvic pain disorder (n=8), vaginismus (n=1), or irritable bowel syndrome (IBS) (n=1); these women were excluded.
Our study was based on 240 cases and matched controls who had complete information related to mood and anxiety. Among cases, 121 were community based and not clinically confirmed, 56 were community based and clinically confirmed, and 63 were clinic identified and confirmed. Women from similar communities who had no history of chronic vulvar pain were randomly selected within a specific ZIP code and age matched (within 5 years) as potential controls to each of the 240 cases. In order to obtain 240 participants, 520 controls were approached. Once age matched, a reference age was assigned to the control. This reference age, identical to the age at first onset of chronic vulvar pain in her matched case, was used to assign mood and anxiety disorders as antecedent or subsequent to onset of vulvodynia in controls. Matched controls could not be found for 7 cases. Instead, controls were selected with the closest age within a case's ZIP code. Psychiatric disorders were assessed in controls with the SCID. All cases and controls were offered a $25 honorarium for participation in the medical and psychiatric interviews.
Assessment of depression and anxiety
Psychiatric history was assessed using the SCID. The SCID is recognized as the gold standard in diagnosing major axis I psychiatric disorders.11 For the purposes of this study, women were characterized as having a mood disorder if they met DSM criteria for major depressive disorder (MDD) or dysthymia (chronic low levels of depression). Consistent with standard SCID procedures, participants were asked whether they had experienced “a two-week period of feeling depressed or down most of the day nearly every day” or had “ever lost interest or pleasure in things that they usually enjoyed.” If subjects answered yes to either of these two screener questions, seven additional questions pertaining to sleeping habits, restlessness, fatigue, self-worth, ability to concentrate, appetite, and suicidal thoughts were administered. If a participant responded affirmatively to five or more symptoms (including the screening questions), impairment was assessed, and if compromised, the participant was initially defined as having experienced major depression. New onset dysthymia was diagnosed according to DSM criteria as long as the participant met criteria for this disorder over a 2-year period.
Women with scores that indicated a history of panic, general anxiety disorder (GAD), posttraumatic stress disorder (PTSD), obsessive-compulsive disorder (OCD), social phobia, or agoraphobia were categorized as having an anxiety disorder. All interviewers were trained through in-person workshops that included video training procedures. Colleagues in psychiatry with whom B.L.H. has worked for over 15 years in many of his ongoing studies initiated the training and were used to review SCIDs of subjects meeting DSM psychiatric criteria.
By using the SCID, the age at first onset of depression and anxiety, as well as age at all subsequent episodes, was established. This information was used to determine the temporal association between psychiatric disorders and vulvar pain onset. Separately for depression and anxiety, three categorical variables were created: (1) disorder antecedent to age at onset of vulvar pain (for cases) and reference age (for controls), (2) recurrent or new onset of disorder subsequent to vulvar pain or reference age, and (3) among those with no history of disorder, new onset of disorder subsequent to vulvar pain or reference age.
Statistical analysis
Our first analysis used conditional logistic regression models, with matched pairs treated as strata, to estimate the association between history of depression or anxiety and subsequent vulvodynia. Education, race, age at menarche, age at first tampon use, and age at first sexual intercourse were included as covariates. Four separate logistic regression models are presented for each potential precursor to vulvodynia: antecedent mood disorder only (mood), antecedent anxiety disorder only (anxiety), occurrence of both antecedent mood and anxiety disorders (both), and occurrence of mood or anxiety disorder (any).
Since information on diagnosed psychiatric disorders subsequent to the diagnosis of vulvodynia was available, we were able to use the follow-up data as one would in a retrospective cohort study. Our second analysis, therefore, used Cox proportional hazards models to estimate the rate of recurrence or new onset of depression or anxiety in women with vulvodynia relative to the rate in women without vulvodynia. Women accumulated survival time from the age of vulvodynia onset (or reference age for matched controls) until the age at interview. Separate survival models are presented for each potential consequence of vulvodynia: subsequent mood disorder only (mood), subsequent anxiety disorder only (anxiety), occurrence of both subsequent mood and anxiety disorder (both), and occurrence of mood or anxiety disorder (any). Each Cox model was run twice. In Model 1, the outcome was time to recurrent or new onset of mood and anxiety and was adjusted for antecedent depression or anxiety; in Model 2, the analysis was restricted to women with no history of depression or anxiety, and the outcome was time to first onset of any mood or anxiety. Both models were adjusted for age, education, race, age at menarche, age at first tampon use, and age at first sexual intercourse.
Because of potential misclassification of the vulvodynia diagnosis, sensitivity analyses were performed. Both the aforementioned analyses were restricted to clinically confirmed cases and nonclinically confirmed cases.
Results
Women with and without vulvodynia were similar with respect to age (due to matching), education, age at menarche, age at first tampon use, and age at first sexual intercourse (Table 1). In this study, cases were more likely to have Hispanic and white representation, and controls were more likely to have African American and other representation. Women with vulvodynia were significantly more likely to have a history of mood or anxiety disorder. In addition, cases were more likely than controls to develop mood or anxiety disorder at all ages. Demographic variable differences between clinically confirmed case-control pairs (n=119 matched pairs) and nonclinically confirmed case-control pairs (n=121 matched pairs) were similar, except for age at first tampon use. Nonclinically confirmed cases were more likely to start using tampons at a young age in comparison to their matched controls. This difference was not observed between clinically confirmed cases and their matched controls.
Table 1.
Demographic Characteristics of Women in Community-Based Sample (n=480)
| Characteristic | Women with vulvodynia (n=240) | Women without vulvodynia (n=240) |
|---|---|---|
| Age at interviewa | ||
| <30 | 34.6% | 33.3% |
| 30–39 | 27.9% | 28.8% |
| 40–49 | 23.8% | 24.6% |
| >50 | 13.8% | 13.3% |
| Reference agea,b | ||
| <20 | 28.8% | 29.6% |
| 20–24 | 25.8% | 25.0% |
| 25–30 | 23.3% | 24.2% |
| >30 | 22.1% | 21.3% |
| Racec | ||
| White | 90.4% | 85.4% |
| Hispanic | 5.8% | 3.3% |
| African American | 2.1% | 7.5% |
| Other | 1.7% | 3.8% |
| Education | ||
| Some high school | 1.7% | 1.7% |
| High school graduate | 5.8% | 8.3% |
| Some college | 15.4% | 18.8% |
| Vocational/technical | 1.7% | 2.9% |
| College graduate | 44.2% | 38.8% |
| Graduate school | 31.3% | 29.6% |
| Age at menarche | ||
| <12 | 20.4% | 21.3% |
| 12–13 | 52.9% | 55.0% |
| >13 | 26.7% | 23.8% |
| Age at first tampon use | ||
| Never used | 7.5% | 9.2% |
| <15 | 32.1% | 26.3% |
| 15–19 | 46.3% | 52.1% |
| 20–24 | 10.0% | 7.9% |
| >24 | 4.2% | 4.6% |
| Age at first sexual intercourse | ||
| Not sexually active | 0.8% | 2.5% |
| <15 | 7.5% | 7.9% |
| 15–19 | 59.2% | 57.5% |
| 20–24 | 25.0% | 26.7% |
| >24 | 7.5% | 5.4% |
| Age at first onset of antecedent mood or anxietyc | ||
| None | 73.3% | 88.8% |
| <20 | 12.1% | 5.4% |
| 20–24 | 4.6% | 2.9% |
| ≥25 | 10.0% | 2.9% |
Discrepancies are due to late rematching of controls with the closest age within a case's ZIP Code.
Age at first onset of vulvar pain among cases and matched reference age for controls.
McNemar's test: p<0.0001.
Table 2 shows that 26.7% of women with vulvodynia had mood or anxiety disorder before the first onset of vulvar pain symptoms compared to 11.3% of controls during a similar time frame. After adjusting for covariates, women with antecedent mood or anxiety had four times the odds of vulvodynia relative to women without antecedent mood or anxiety. Substantial associations were also observed for mood disorder only, anxiety disorder only, and having both mood and anxiety disorder before the onset of vulvodynia. The analyses of clinically confirmed and nonclinically confirmed subgroups yielded similar results and were not differentially altered by the differential distribution of age at first tampon use (clinically confirmed: any psychiatric disorder, odds ratio [OR] 2.7, 95% confidence interval [CI] 1.3-5.7; nonclinically confirmed: any psychiatric disorder, OR 3.9, 95% CI 1.9-8.1).
Table 2.
Risk of Antecedent of Mood and Anxiety Disorder Among Women With and Without Vulvodynia (n=480)
| Women with vulvodynia (n=240) n (%) | Women without vulvodynia (n=240) n (%) | Crude OR | AdjustedaOR | 95% CI | |
|---|---|---|---|---|---|
| No antecedent history of mood or anxiety (referent) | 176 (73.3) | 213 (88.8) | 1.0 | 1.0 | - |
| Any antecedent history of mood or anxiety | 64 (26.7) | 27 (11.3) | 3.8 | 4.0 | (2.1, 7.5) |
| Antecedent history of mood only | 40 (16.7) | 15 (6.3) | 3.0 | 3.1 | (1.5, 6.5) |
| Antecedent history of anxiety only | 12 (5.0) | 5 (2.1) | 10.0 | 10.0 | (1.2, 87.4) |
| Antecedent history of mood and anxiety | 12 (5.0) | 7 (2.9) | 5.0 | 5.0 | (1.2, 26.7) |
Mood: major depressive disorder or dysthymia. Anxiety: general anxiety disorder, posttraumatic stress disorder, obsessive-compulsive disorder, mania, social phobia, and agoraphobia.
Adjusted for race, education, age at menarche, age at first tampon use, and age at first sexual intercourse.
CI, confidence interval; OR, odds ratio.
Table 3 shows recurrent or new onset of mood or anxiety as a consequence of vulvodynia. Model 1 shows that women with vulvodynia had 1.7 times the rate of new or recurrent mood or anxiety compared to women with no vulvar pain history. When comparing our clinically confirmed pairs to nonclinically confirmed pairs, we again observed similar results that were not differentially altered by the differential distribution of age at first tampon use (clinically confirmed: any psychiatric disorder, OR 2.0, 95% CI 0.9-4.1; nonclinically confirmed: any psychiatric disorder, OR 1.4, 95% CI 0.7-2.6). This association did not appear to be confined specifically to mood disorder or anxiety disorder. However, the greatest association was observed with new or recurrent onset of both mood and anxiety disorder (hazard ratio [HR] 7.1, 95% CI 1.2-41.1). To determine if the association was largely confined to new rather than recurrent onset of mood or anxiety disorder, a second set of analyses was restricted to women without an antecedent history of mood or anxiety. Model 2 shows that women with vulvodynia had rates of new mood and anxiety disorder (Table 3, Both) that were seven times greater than that observed among women with no vulvar pain history (95% CI 1.2-41.1).
Table 3.
Risk of Recurrent or New Onset of Mood or Anxiety as a Consequence of Vulvodynia (n=480)
| |
Recurrent or new onset of mood or anxiety disorders |
||||
|---|---|---|---|---|---|
| Experienced vulvodynia | None n (%) | Any n (%) | Mood only n (%) | Anxiety only n (%) | Both n (%) |
| No (n=240) | 206 (85.8) | 34 (14.2) | 26 (10.8) | 6 (2.5) | 2 (0.8) |
| Yes (n=240) | 185 (77.1) | 55 (22.9) | 35 (14.6) | 10 (4.2) | 10 (4.2) |
| Crude HR (95% CI) | 1.0 (reference) | 1.8 (1.2-2.7) | 1.7 (1.0-2.8) | 2.0 (0.7-5.4) | 6.0 (1.3-27.5) |
| Model 1 HR (95% CI)a | 1.0 (reference) | 1.7 (1.1-2.6) | 1.6 (0.9-2.8) | 0.7 (0.1-3.7) | 7.1 (1.2-41.1) |
| Model 2 HR (95% CI)b | 1.0 (reference) | 1.5 (0.9-2.5) | 1.5 (0.9-2.7) | 0.6 (0.1-3.5) | 7.1 (1.2-41.1) |
Adjusted for antecedent history of mood or anxiety, age, race, education, age at menarche, age at first tampon use, and age at first sexual intercourse.
Restricted to women without antecedent history of mood or anxiety: exposed (n=176) and not exposed (n=213). Adjusted for all covariates in model 1 excluding antecedent history of mood or anxiety.
HR, hazard ratio.
Discussion
To our knowledge, this is the first community-based epidemiologic study in women to demonstrate that DSM-IV-diagnosed antecedent depression and anxiety disorders have an influence on the risk of adult onset vulvodynia. Moreover, we have determined that vulvodynia causes a greater likelihood of both new and recurrent onset of psychopathology.
Our findings are consistent with findings of a few recent studies suggesting that depression or anxiety symptoms are more prevalent in women with than in women without vulvodynia.5–10 In one nested case-control study of 100 women with vulvodynia and 325 matched community-based controls, women who self-identified with depressive symptoms before the onset of vulvar pain were more likely to develop vulvodynia.7 A smaller study that sampled 28 female patients seeking treatment for vulvodynia and 50 healthy controls recruited from a local university suggested that antecedent (trait) anxiety was associated with greater risk of vulvodynia.8 Wylie et al.9 interviewed 164 women (82 with vulvodynia and 82 without) and found that along with depression and anxiety, abnormal behavioral traits, such as OCD and phobia, were significantly more present in women with vulvodynia. The authors suggested that the full spectrum of psychologic disorders might be a factor in the development of vulvar pain. Each of these studies, however, relied heavily on self-reported symptoms rather than diagnosis of psychologic disorders.10,13,14 Only one study to our knowledge used a structured clinical interview (SCID) to establish that the first episode of diagnosed MDD was antecedent to the onset of vulvodynia.10 Their findings, however, cannot be broadly generalized to all women, as the study was based on a small sample of 53 women with vulvodynia who were actively seeking psychologic treatment. By using the SCID in a community-based population, our study was able to confirm the diagnosis of DSM-IV axis I disorders, establish temporality of these psychiatric disorders to vulvodynia, and increase the likelihood of generalizability to other women.
Our findings, combined with findings of previous literature,5–10 negate the belief that depression and anxiety are solely secondary to the diagnosis of vulvodynia. Our results support the hypothesis that psychiatric morbidity plays a significant role in the pathogenesis of vulvodynia.2 Given this premise, a critical question that researchers must answer is how a psychologic experience can lead to chronic vulvar pain. One plausible biologic mechanism involves inflammation. The field of psychoneuroimmunology has provided mechanistic evidence linking the experience of psychologic stressors to physiologic changes in the central nervous, endocrine, and immune systems.15 A review and meta-analysis of 30 experimental studies showed that circulating inflammatory factors are responsive to acute psychologic stress.16 The hypothalamic-pituitary-adrenal (HPA) axis, which regulates the body's cortisol levels, is intertwined with the mediation and control of immune and inflammatory responses of cytokines.17 Chronic stressors can lead to dysregulation of the HPA axis, which in turn can generate inflammation.18 Associations between depression and inflammation have been observed among adults in several community-based studies.19–28 Of specific relevance to the present study, Miller et al.29 measured cytokines after women with and without clinical depression were exposed to a 17-minute experimental stressor. Depressed participants produced more interleukin-6 (IL-6) and tumor necrosis-α (TNF-α) in the presence of dexamethasone, a synthetic anti-inflammatory drug. Depression was thus associated with greater resistance to molecules that normally terminate the inflammatory cascade. Further research should be done to investigate the influence psychologic stressors have on inflammation of the vulvar region.
The results from these analyses should be considered within the context of the limitations of this study. More precise estimates may be obtained through studies using a larger number of cases and controls. We can also not rule out that women with vulvar pain may have differentially recalled their psychiatric history compared to matched controls. However, structured clinical interviews, like the SCID, contribute less recall bias than unstructured interviews and self-reported measures.13,14 There is the potential for recall bias and the inaccuracy of retrospective diagnosis date of vulvodynia in the community-identified cases.
There is the possibility of misclassification of the vulvodynia diagnosis. Of the 240 cases, only 119 were clinically confirmed with the most stringent criteria used for diagnosing vulvodynia (Fig. 1). After performing sensitivity analyses, however, we observed similar results in analyses restricted to subgroups of clinically and nonclinically confirmed cases. This suggests that misclassification is not likely to have accounted for all the observed associations. We do acknowledge the limitations of combining both subgroups. Attempts should be made to include only clinically confirmed cases in future studies.
Conclusions
This study establishes that DSM-IV-diagnosed mood and anxiety may influence the development of unexplained vulvar pain, a chronic disorder that affects millions of women.2 Moreover, this study establishes that the development of vulvodynia may lead to new or recurrent mood or anxiety disorder. The use of a community-based sample of women enhances the likelihood that our findings are generalizable to other women. Our findings corroborate the hypothesis that psychiatric morbidity may have a distinct role in the pathogenesis of vulvodynia, separate from its potential occurrence as a consequence of vulvodynia. A promising area of future research will be to investigate mechanisms linking psychiatric morbidity to the development of vulvodynia, including such biologic mechanisms as inflammation. It is also critical that the temporality between psychiatric disorders and vulvar pain onset be more firmly established. Retrospective cohort or nested case-control studies would be valuable steps in future research. If the present findings are replicated, those involved in the clinical care of women with vulvodynia should be cognizant of the potential mental health consequences of this disorder. In addition, practitioners should be aware that a history of psychiatric morbidity could lead to the onset of vulvodynia, potentially through altered immunoinflammatory response mechanisms.
Acknowledgments
This study was supported by a grant from the National Institutes of Health (R01 HD038428).
Disclosure Statement
The authors have no conflicts of interest to report.
References
- 1.Ridley CM. Vulvodynia: Evolution of classification and management. J Eur Acad Dermatol Venereol. 1996;7:129–134. [Google Scholar]
- 2.Harlow BL. Stewart EG. A population-based assessment off chronic unexplained vulvar pain: Have we underestimated the prevalence of vulvodynia? J Am Med Womens Assoc. 2003;58:82–88. [PubMed] [Google Scholar]
- 3.Gunter J. Vulvodynia: New thoughts on a devastating condition. Obstet Gynecol Surv. 2007;62:812–819. doi: 10.1097/01.ogx.0000290350.14036.d6. [DOI] [PubMed] [Google Scholar]
- 4.Arnold LD. Bachmann GA. Rosen R, et al. Vulvodynia: Characteristics and associations with comorbidities and quality of life. Obstet Gynecol. 2006;107:617–624. doi: 10.1097/01.AOG.0000199951.26822.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stewart DE. Reicher AE. Gerulath AH. Boydell KM. Vulvodynia and psychological distress. Obstet Gynecol. 1994;84:587–590. [PubMed] [Google Scholar]
- 6.Lundqvist EN. Bergdahl J. Vulvar vestibulitis: Evidence of depression and state anxiety in patients and partners. Acta Derm Venereol. 2003;83:369–373. doi: 10.1080/00015550310003764. [DOI] [PubMed] [Google Scholar]
- 7.Arnold LD. Bachman GA. Rosen R, et al. Assessment of vulvodynia symptoms in a sample of US women: A prevalence survey with nested case control study. Am J Obstet Gynecol. 2007:196128e1–128e6. doi: 10.1016/j.ajog.2006.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Granot M. Lavee Y. Psychological factors associated with perception of experimental pain in vulvar vestibulitis syndrome. J Sex Marital Ther. 2005;31:285–302. doi: 10.1080/00926230590950208. [DOI] [PubMed] [Google Scholar]
- 9.Wylie K. Hallam-Jones R. Harrington C. Psychological difficulties within a group of patients with vulvodynia. Psychosom Obstet Gynaecol. 2004;25:257–265. doi: 10.1080/01674820400024463. [DOI] [PubMed] [Google Scholar]
- 10.Masheb RM. Wang E. Lozano C, et al. Prevalence and correlates of depression in treatment-seeking women with vulvodynia. J Obstet Gynaecol. 2005;25:786–791. doi: 10.1080/01443610500328199. [DOI] [PubMed] [Google Scholar]
- 11.First MB. Spitzer RL. Gibbon M, et al. Users guide to the structured clinical interview for DSM-IV axis I disorders. New York: Biometrics Research; 1996. [Google Scholar]
- 12.Friedrich EG., Jr Vulvar vestibulitis syndrome. J Reprod Med. 1987;32:110–114. [PubMed] [Google Scholar]
- 13.Sanchez-Villegas A. Schlatter J. Ortuno F, et al. Validity of a self-reported diagnosis of depression among participants in a cohort study using the Structured Clinical Interview for DSM-IV (SCID-I) BMC Psychiatry. 2008;8:43. doi: 10.1186/1471-244X-8-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Innes JM. Herbertt RM. Structured interview and self-report measures of the type A coronary-prone behavior pattern: Private and public self-consciousness as moderator variables. Pers Individual Differ. 1989;10:475–478. [Google Scholar]
- 15.Glaser R. Kiecolt-Glaser JK. Stress-induced immune dysfunction: Implications for health. Nat Rev Immunol. 2005;5:243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- 16.Steptoe A. Hamer M. Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: A review and meta-analysis. Brain Behav Immun. 2007;21:901–912. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 17.Kemeny ME. Schedlowski M. Understanding the interaction between psychosocial stress and immune-related diseases: A stepwise progression. Brain Behav Immun. 2007;21:1009–1018. doi: 10.1016/j.bbi.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 18.Weiss SJ. Neurobiological alterations associated with traumatic stress. Perspect Psychiatr Care. 2007;43:114–122. doi: 10.1111/j.1744-6163.2007.00120.x. [DOI] [PubMed] [Google Scholar]
- 19.Bremmer MA. Beekman AT. Deeg DJ, et al. Inflammatory markers in late-life depression: Results from a population-based study. J Affect Disord. 2008;106:249–255. doi: 10.1016/j.jad.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 20.Dentino AN. Pieper CF. Rao MK, et al. Association of interleukin-6 and other biological variables with depression in older people living in the community. J Am Geriatr Soc. 1999;47:6–11. doi: 10.1111/j.1532-5415.1999.tb01894.x. [DOI] [PubMed] [Google Scholar]
- 21.Ford DE. Erlinger TP. Depression and C-reactive protein in US adults: Data from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2004;164:1010–1014. doi: 10.1001/archinte.164.9.1010. [DOI] [PubMed] [Google Scholar]
- 22.Liukkonen T. Silvennoinen-Kassinen S. Jokelainen J, et al. The association between C-reactive protein levels and depression: Results from the Northern Finland 1966 Birth Cohort Study. Biol Psychiatry. 2006;60:825–830. doi: 10.1016/j.biopsych.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 23.Ranjit N. Diez-Roux AV. Shea S, et al. Psychosocial factors and inflammation in the multi-ethnic study of atherosclerosis. Arch Intern Med. 2007;167:174–181. doi: 10.1001/archinte.167.2.174. [DOI] [PubMed] [Google Scholar]
- 24.Herbert TB. Cohen S. Depression and immunity: A meta-analytic review. Psychol Bull. 1993;113:472–486. doi: 10.1037/0033-2909.113.3.472. [DOI] [PubMed] [Google Scholar]
- 25.Kiecolt-Glaser JK. Glaser R. Depression and immune function: Central pathways to morbidity and mortality. J Psychosom Res. 2002;53:873–876. doi: 10.1016/s0022-3999(02)00309-4. [DOI] [PubMed] [Google Scholar]
- 26.Zorilla EP. Luborsky L. McKay JR, et al. The relationship of depression and stressors to immunological assays: A meta-analytic review. Brain Behav Immun. 2001;15:199–226. doi: 10.1006/brbi.2000.0597. [DOI] [PubMed] [Google Scholar]
- 27.Cyranowski JM. Marsland AL. Bromberger JT, et al. Depressive symptoms and production of proinflammatory cytokines by peripheral blood mononuclear cells stimulated in vitro. Brain Behav Immun. 2007;21:229–237. doi: 10.1016/j.bbi.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 28.Kuo HK. Yen CJ. Chang CH, et al. Relation of C-reactive protein to stroke, cognitive disorders, and depression in the general population: Systematic review and meta-analysis. Lancet Neurol. 2005;4:371–380. doi: 10.1016/S1474-4422(05)70099-5. [DOI] [PubMed] [Google Scholar]
- 29.Miller GE. Rohleder N. Stetler C, et al. Clinical depression and regulation of the inflammatory response during acute stress. Psychosom Med. 2005;67:679–687. doi: 10.1097/01.psy.0000174172.82428.ce. [DOI] [PubMed] [Google Scholar]