Abstract
Cancer treatments can be detrimental to fertility; recent literature has focused on the efforts of fertility preservation for this patient population. It should be recognized, however, that several nonmalignant medical conditions and therapeutic interventions could be similarly hazardous to fertility. Some of these nonmalignant diseases and their treatments that can adversely impact the reproductive axis are gastrointestinal diseases, rheumatologic disorders, nonmalignant hematologic conditions, neurologic disorders, renal disorders, gynecologic conditions, and metabolic diseases. Their negative effects on reproductive function are only now being appreciated and include impaired ovarian function, endocrine function, or sexual function and inability to carry a pregnancy to term. Complications and comorbidities associated with certain diseases may limit the success of established fertility preservation options. Recent advances in fertility preservation techniques may provide these patients with new options for childbearing. Here, we review several fertility-threatening conditions and treatments, describe current established and experimental fertility preservation options, and present three initiatives that may help minimize the adverse reproductive effects of these medical conditions and treatments by raising awareness of the issues and options: (1) increase awareness among practitioners about the reproductive consequences of specific diseases and treatments, (2) facilitate referral of patients to fertility-sparing or restorative programs, and (3) provide patient education about the risk of infertility at the time of diagnosis before initiation of treatment.
Introduction
Given the dramatic increase in the number of cancer survivors during the last few decades, the reproductive effects of cancer treatments and efforts to preserve fertility have received significant attention in the recent literature. A survey of patients undergoing bone marrow transplant found that 62% were willing to accept a 10% risk of treatment-related mortality but only 50% believed that infertility was an acceptable side effect of treatment.1 Cancer treatments are a well-established cause of premature ovarian insufficiency (POI). Less attention has been focused on noncancer populations at risk for adverse reproductive effects from either medical conditions or their treatments. As fertility preservation methods become more widely available and their success rates improve, patients with nonmalignant conditions may become candidates. Some of the nonmalignant diseases and treatments that can impact reproductive function include gastrointestinal diseases, rheumatologic disorders, nonmalignant hematologic diseases, renal disease, neurologic disorders, gynecologic conditions, and metabolic disorders. It is important to discuss all family planning options with patients, including fertility preservation options, use of donor gametes, adoption, and choosing not to have children. In addition, contraception needs to be discussed at the initial consultation, as ovarian dysfunction may be neither immediate nor absolute in most patients.
The objective of this review is to highlight some of the noncancer causes of subfertility and discuss potential fertility preservation strategies for women. In highlighting these diseases, the goal is to enhance the information that is presented to patients rather than to cause alarm in patients or providers. In many cases, the absolute number of patients with these conditions who are at risk of infertility is low. Nevertheless, it is important for clinicians to understand the risks and be able to help guide their patients who are at risk to reproductive specialists.
Premature Ovarian Insufficiency
POI is defined as ovarian failure before the age of 40 and is one significant metric of subfertility used to gauge the fertility risk of a particular disease or treatment. POI or subfertility relates to the size and quality of the remaining pool of ovarian follicles, also called the ovarian reserve. The follicle is the functional unit of the ovary, each containing a single oocyte with the potential to grow, mature, and be fertilized. Thus, the ovarian reserve is a measure of the functional status of the ovary. Standardized ovarian function tests to quantify the ovarian reserve do not exist. The current gold standard for measuring fertility is live birth, yet most literature on treatment-related POI is based on surrogate measures, which often differ from study to study, further limiting this type of analysis. The clinical surrogate for ovarian reserve is usually based on menstrual cyclicity, antral follicle count by transvaginal ultrasound, or concentrations of hormones, such as follicle-stimulating hormone (FSH) and anti-müllerian hormone (AMH). None of these is a perfect correlate of fertility, and there is still no universal surrogate measurement reported in the literature.
Ovarian function encompasses both fertility and the endocrine benefits of ovarian steroids throughout the body. The loss of ovarian function can negatively impact bone health, cardiovascular health, and sexual function, which have been extensively reviewed elsewhere.2,3 We focus this review on fertility preservation, but it is imperative that physicians remain cognizant of the scope of ovarian function and systemic impact of disease-related or treatment-related POI.
Fertility-Threatening Medical Conditions and Treatments Beyond Cancer
Table 1 presents gonadotoxic side effects of treatments for nonmalignant conditions.
Table 1.
Highlighted Treatments with Known Gonadotoxic Side Effects
| Immunosuppressive agentsa,b |
| Alkylating chemotherapy, i.e., cyclophosphamide |
| Mitoxantrone |
| High-dose chemotherapy and radiation before bone marrow transplantation |
| Postsurgical concerns |
| Distortion of anatomy; e.g., fallopian tubes |
| Adhesive disease |
| Impaired ovarian blood supply |
| Damage to normal ovarian parenchyma from electrosurgery |
| Autologous transfusion-related |
| Hemochromatosis from multiple transfusions |
Risk calculator available at www.fertilehope.org/tool-bar/risk-calculator.cfm
Autoimmune diseases treated with gonadotoxic therapies
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease with characteristic exacerbations and remissions that affects multiple organ systems. The Lupus Foundation of America estimates that 1.5–2 million Americans have lupus, of whom >90% are women. Moreover, this disease is particularly common in women of childbearing age.6 Patients experience pain, vaginal ulcers, fatigue, and depression. SLE can be associated with recurrent pregnancy loss, particularly in patients who also have antiphospholipid antibody syndrome.5 Other SLE-related factors that may impact sexual function have not been studied and deserve greater attention.6
The predominant mechanism of SLE-related infertility is drug related rather than disease related.9,10 The prognosis of patients with SLE has improved with the advent of more aggressive therapies.10 Severe manifestations of SLE, such as lupus nephritis, are often treated with cyclophosphamide, which greatly improves the prognosis in these patients.11–13 However, cyclophosphamide is an alkylating chemotherapeutic agent known to cause POI.6 The incidence of amenorrhea following cyclophosphamide treatment for SLE ranges from 27% to 60%, with 80% of these patients experiencing sustained amenorrhea for longer than 1 year, as noted by elevated gonadotropin levels.6,9–11,14,15 The Euro-Lupus protocol of cyclophosphamide therapy (500 mg given every 2 weeks for six doses of 500 mg cyclophosphamide) has not been associated to date with infertility.16 The exact cyclophosphamide dose that causes POI is not known; Ioannidis et al.17 showed that in women >32 years of age, 50% experienced amenorrhea at 8 g/m2 and 90% experienced it at 12 g/m2. As the optimal therapy for lupus nephritis is still debated, some argue for the use of mycophenolate mofetil (MMF) instead of cyclophosphamide, particularly in young women wishing to conserve their fertility.18 Following 6 months of MMF therapy, those who enter remission can be maintained on MMF, with cyclophosphamide reserved for more aggressive disease.18,19 Given the teratogenicity associated with MMF, most rheumatologist and nephrologists would change to azathioprine or other agents, such as hydroxychloroquine and sulfasalazine, as necessary for maintenance or treatment in the setting of pregnancy.20 In addition, long-term follow-up of younger patients is crucial, as some patients treated with gonadotoxic agents with previously normal ovarian function have been found to experience early menopause.19,21
Risk factors for POI secondary to cyclophosphamide therapy include age, cumulative dose, history of thyroid disease, disease duration, and the presence of anti-Ro and anti-U1RNP antibodies.6,10–15,17,22 Cytochrome P450 polymorphisms among patients with SLE can also explain the range of ovarian function after cyclophosphamide exposure. In retrospective analyses, the presence of the CYP2C19*2 polymorphism variant allele was associated with a lower risk of ovarian toxicity from cyclophosphamide exposure.23,24 The effects of other medications used for treating rheumatic diseases on fertility, pregnancy, and lactation are reviewed elsewhere.25
Reproductive dysfunction can be seen in patients with juvenile SLE during times of active disease or as a deleterious consequence of steroid treatment.26 Antiphospholipid antibodies, autoimmune oophoritis, and exposure to alkylating drugs, particularly cyclophosphamide, can impact fertility.26 The frequency of impaired fertility, as defined by amenorrhea, after cyclophosphamide exposure in these patients has been reported as high as 31%.27 Cumulative dose, age, and treatment after puberty are all associated with treatment-related infertility in juvenile SLE.26–28 One study found that even if immediate effects are not seen, idiopathic POI, defined as amenorrhea in the presence of elevated gonadotropin evaluation, is more likely to be seen in juvenile SLE patients than in the general population.29
Multiple sclerosis (MS) affects twice as many women as men, particularly individuals of reproductive age. The fertility risk related to MS is multifactorial. It encompasses issues surrounding sexual dysfunction, a decreased desire to have children, fear of transmitting MS, and delayed childbearing to complete treatment regimens.30 MS symptoms tend to decrease during pregnancy but increase postpartum and during the perimenopausal period, suggesting a protective role of reproductive hormones.30 Although there is some suggestion that fertility may be decreased in MS patients, most believe this is a concern only for those undergoing immunosuppressive therapy.
Although the majority of MS patients do not require gonadotoxic therapy, subsets of patients with progressive MS or disease refractory to other treatments will receive gonadotoxic therapy and are, therefore, at risk for treatment-related ovarian insufficiency. The frequency and risk factors of mitoxantrone–induced amenorrhea multiple sclerosis (FEMIMS) study retrospectively explored the incidence of chemotherapy-induced amenorrhea in MS patients taking mitoxantrone and found that 26% of patients had amenorrhea after treatment. The probability of amenorrhea increased by 18% with each year of age (mean age at study start was 37 years) but decreased with concurrent hormonal therapy.31 Ovarian endocrine function may be adversely impacted by mitoxantrone therapy, which may remove the protective effect of reproductive hormones and exacerbate MS-related causes of infertility. Further investigation is needed to define these relationships in order to appropriately counsel patients on their options for family planning.
Chronic kidney disease (CKD) is associated with infertility as a result of hypogonadotropic hypogonadism and not POI, yet the treatment of CKD depends on the etiology and can involve the use of alkylating based chemotherapy, known to be gonadotoxic. The etiology of CKD may include immunologically mediated glomerulonephritities, such as SLE. Glomerulonephritis can be treated with cyclophosphamide, which, as discussed, negatively impacts fertility.32 Although dialysis does not improve reproductive function, the recent literature has reported improvement in fertility for patients on nocturnal hemodialysis compared with conventional hemodialysis.33 This study retrospectively identified patients who conceived and delivered a live infant while on nocturnal hemodialysis and compared their cohort to historic controls, thereby limiting the strength of their conclusions. Further complicating the issue of infertility in patients with CKD is the prevalence of sexual dysfunction from uremia and psychogenic, neurogenic, vascular, or disease-related comorbidities.34–38
Postsurgical etiologies
Inflammatory bowel disease (IBD) is one of the most common gastrointestinal illnesses associated with treatment-related infertility. Because IBD often affects women in the reproductive years, it is particularly important to be aware of the reproductive risks of surgical treatment for IBD in this population.39 Although the exact etiology of IBD-associated infertility is not clear, the primary cause may be the extensive abdominal and pelvic surgery required for treatment in some patients. Although the majority of patients with IBD do not require surgery, at least 30% will undergo surgical therapy, and these women are at increased risk for development of treatment-related infertility.40,41
Higher rates of infertility occur in patients of advanced age, in patients needing a two-stage surgical procedure, and in patients requiring a blood transfusion. These clinical markers may reflect the degree of surgical difficulty, adhesion formation, or nutritional status.42 Although age is an independent risk factor for decreased fertility, the mean age of patients studied is often <30 years.43
Patients with ulcerative colitis (UC) and familial adenomatous polyposis (FAP) may need to undergo definitive surgical treatment for their disease. One common surgical treatment for these patients is ileal pouch-anal anastomosis (IPAA) with restorative proctocolectomy, which has been associated with decreased postoperative fertility.44 Changes in the anatomic location of the posterior vaginal wall after removal of the rectum and the development of postoperative tubal adhesions are also believed to contribute to decreased fertility in these patients.44 Postoperative abnormalities on hysterosalpingography, an imaging study looking for patency of the fallopian tubes, were seen in 67% of 21 patients after IPAA, although preoperative assessment was not performed in the study.45 In a study of UC patients undergoing IPAA, 83% were pregnant in the year before diagnosis, compared to 75% in a reference group, but then dropped to 18% after surgery.46 A meta-analysis of patients with UC reported infertility rates of 14.6% after medical treatments compared with a 48% risk of infertility after surgical intervention.47 Although these studies have limitations, including imprecise definitions of infertility, inconsistent assessment of menstrual status or hormone measures, inconsistent baseline fertility rates, and retrospective design, there still appears to be a link between IPAA surgical intervention for gastrointestinal disease and infertility.
An alternative surgical procedure for patients with IBD, colectomy with ileorectal anastomosis, is less deleterious to fertility, as measured by spontaneous pregnancy rates, perhaps because the surgery has less pelvic involvement.48 However, a criticism of this study is that it lacked a control group and instead compared surgical outcomes to historic controls who underwent IPAA. Because colectomy with ileorectal anastomosis does not involve excision of the rectum, patients are at higher risk for subsequent rectal carcinoma compared with patients undergoing the standard surgical approach. Despite this risk, some patients with minimal rectal disease who are interested in future fertility may be candidates for this less aggressive approach.47,48 Additional strategies for fertility preservation in these patients include intraoperative interventions to displace the ovaries outside of the surgical field (but still accessible for subsequent oocyte retrieval in the context of in vitro fertilization [IVF]), use of adhesion barriers to prevent adhesions, and use of minimally invasive surgical techniques, although the efficacy of these approaches remains unproven.44
Endometriomas are the result of endometriotic lesions on the ovary that form cystic structures. When they become >3 cm, removing them has been shown to improve pregnancy rates over simple excision and drainage,28,49–51 yet surgical removal of the cysts can damage the surrounding ovarian parenchyma and reduce ovarian reserve.52–54 Thus, surgery may cause iatrogenic ovarian insufficiency. Benaglia et al.55 prospectively followed patients with endometriomas diagnosed by ultrasound to determine the side of ovulation by serial ultrasounds on days 6–10 of the cycle. They concluded that the presence of the endometrioma alone may lead to anovulation and decreased fertility regardless of if it is surgically treated. Irrespective of the mechanism of decreased ovarian reserve, in vitro follicle maturation techniques may eventually allow the use of secondary follicles from either the parenchyma outside the lesion or the contralateral ovary in patients with endometriomas.
Transplantation/transfusion-related disorders
A variety of disorders may be treated with bone marrow transplantation or hematopoietic stem cell transplant with preconditioning alkylating chemotherapy and radiation, including thalassemia major, sickle cell anemia, aplastic anemia, Fanconi anemia, and myeloproliferative diseases.56 The severe gonadotoxic effects of treatment regimens for these diseases are well documented.57,58 The combination of radiation and high-dose chemotherapy required before bone marrow transplant makes recovery of ovarian function in this patient population unlikely, and spontaneous recovery of ovarian function, as defined by a return of menstrual cycles, has been found to occur in only 6% of patients.4 The type of and dose of chemotherapy, use of radiation, and the need for bone marrow transplant also affect the risk of reproductive dysfunction.5,57,59 Some chemotherapeutic agents are less gonadotoxic than others; hydroxyurea and anagrelide do not affect fertility, whereas busulfan and cyclophosphamide are quite deleterious to fertility.4 Unfortunately, the available data on the gonadotoxicity of specific chemotherapeutic agents are inadequate, and studies in this area are limited by such confounding factors as age, dose of chemotherapeutics, and use of multiagent treatment regimens.5,60
Patients with irreversible, severe, end-stage renal disease (ESRD) may be candidates for renal transplantation. After transplant, the majority of women begin to ovulate, with only approximately 16% remaining amenorrheic for more than 1 year.61,62 It is generally recommended that patients wait 1–2 years after transplant before attempting pregnancy to minimize the obstetric risks to the mother, growth-restricted risks to the fetus, and rejection-related risks to the graft.61,62 Favorable outcomes of pregnancy can be expected in the majority of patients posttransplant who are followed closely by both transplant physicians and high-risk obstetric services.62–64 Similar recommendations exist for liver and pancreas transplant patients.63,64
Hemochromatosis is also associated with treatment-induced infertility. Hemochromatosis is a condition of excessive iron accumulation caused either by an inherited defect in iron metabolism or by excessive blood transfusion therapy for such diseases as thalassemia, chronic hemolytic anemia, and sickle cell anemia. Specifically, the accumulation of iron deposits in the anterior pituitary can cause reproductive dysfunction.65 Although iron deposition is seen in the testes, it is not known if deposits form in ovarian tissue.66 Nonetheless, there is evidence to suggest that ovarian follicular function may be impaired in patients with hemochromatosis.67 Patients do respond to gonadotropin stimulation, however, that is, superovulation, which is the process of using exogenous FSH and luteinizing hormone (LH) to recruit multiple follicles for approximately 10 days before oocyte retrieval in a standard IVF cycle.68,69 Iron depletion has been shown in a few cases to reverse gonadal damage in men,70,71 but similar results have not been reported in women.66
Genetic diseases
The most common conditions associated with POI are Turner syndrome (XO karyotype) and fragile X syndrome. Turner syndrome is the most common sex chromosome disorder among women, affecting 1 in 2000 liveborn girls.72 At least 30% of Turner patients have mosaicism, meaning they have a mixture of normal cells and cells with only one X chromosome.73 The main features of Turner syndrome are short stature and a failure to enter puberty because of an accelerated rate of atresia of ovarian follicles, leading to gonadal insufficiency and failure.74 Despite this, spontaneous ovulation and healthy pregnancies have been reported, usually in patients with mosaic Turner syndrome.74 Recent literature from Europe supports the use of oocyte cryopreservation after ovarian stimulation in these patients, although no pregnancies have been reported.75–77 Two of these initial reports included women with mosaic Turner syndrome in their early 20s who underwent conventional ovarian stimulation and oocyte retrieval to cryopreserve oocytes.75,76 After ovarian reserve testing, it was thought that the patients had a good chance of responding to gonadotropin stimulation. One additional report describes a patient who had previously undergone ovarian tissue cryopreservation at the age of 17 and then subsequently underwent oocyte cryopreservation in her mid-20s.77 For adult Turner syndrome patients, oocyte cryopreservation performed on a case-by-case basis has been advocated and is relatively straightforward. Ovarian tissue cryopreservation for adolescent patients with Turner syndrome may be more challenging. Ovarian tissue cryopreservation is considered to be most beneficial in prepubertal mosaic Turner syndrome patients because this cohort is believed to have the greatest number of available follicles.78 The age to recommend ovarian tissue cryopreservation is far from being standardized, however, and the psychologic impact and consent issues remain quite complicated. Thus, application of this fertility preservation technology to adolescent women with mosaic Turner syndrome is still in its infancy.
Patients with Turner syndrome often have renal and cardiac anomalies that can complicate pregnancies, and they are at particularly high risk of aortic dissection.74 Moreover, the risk for aortic dissection or rupture during pregnancy may be 2% or higher, and the risk of death during pregnancy is increased as much as 100-fold.79–81 Women with baseline or increased aortic root dilation on imaging are believed to be at highest risk, although dissection can even occur in women in the absence of aortic dilation.79–81 Prepregnancy cardiac evaluation is advised,74 and most patients will require gestational carriers. Until the technology of ovarian tissue cryopreservation matures, these patients will require egg donors. Adoption is also an option for these patients.
Other genetic conditions are associated with POI, such as fragile X permutation82 and X chromosome deletions.83 Because prenatal genetic diagnostic techniques are increasingly being used, individuals with X chromosome deletions may be diagnosed in utero. These patients may be candidates for fertility preservation procedures when they grow up, and genetic counseling should include a discussion of the risk of transmission to biologic offspring.
Metabolic conditions
Metabolic disorders have been associated with POI. Galactosemia is an autosomal recessive disorder of galactose metabolism caused by deficiency of the galactose-1-phosphate uridylyltransferase (GALT) enzyme, and POI is seen in 70%–80% of patients with galactosemia.84–86 The most recent study was a retrospective cross-sectional analysis of 53 females with a mean age of 13.3 years that defined POI by age-appropriate abnormal hormonal evaluations.84 The exact mechanism of POI in these women is not known and is likely related a particular inherited genotypic mutation.84 Interestingly, carriers do not appear to show reduced ovarian reserve or a decreased age at menopause.87 Young female patients may benefit from discussions of fertility preservation and hormone replacement therapy to prevent hypoestrogenic comorbidities. Some have suggested frequent blood draws and hormone assays for early identification of girls at risk for ovarian insufficiency in order to facilitate prompt referral to reproductive specialists,84 whereas others believe frequent monitoring can be detrimental and can mislead individuals, resulting in unwanted pregnancies because of lack of a perceived need for contraception.88 Other metabolic conditions known to impact fertility include diabetes and polycystic ovary syndrome; infertility related to these conditions has been reviewed extensively elsewhere.89–92
Current Fertility Preservation Options for Women
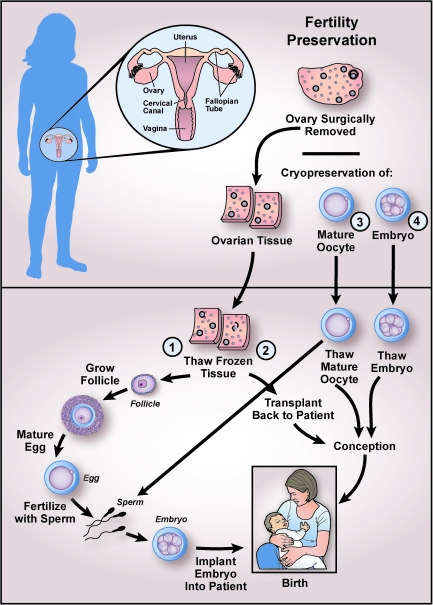
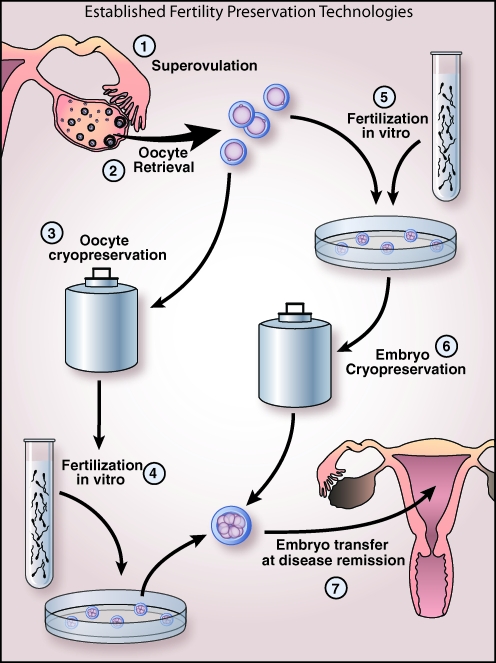
Fertility preservation options are widely available and are used predominantly in women diagnosed with cancer. Options include minimizing use of gonadotoxic chemotherapy, ovarian transposition to minimize radiation exposure, egg or embryo cryopreservation (Figs. 1 and 2), and ovarian tissue cryopreservation (Fig. 1). Embryo cryopreservation after IVF is the most widely available and well-established fertility preservation strategy (Fig. 2). Reported survival rates per thawed embryo range from 35% to 90%, and implantation rates are between 8% and 30%.59
FIG. 1.
Pathways to fertility preservation. Although not shown, immature follicles can be retrieved from ovarian tissue at the time of surgery and cryopreserved for later growth and maturation in vitro. Fertility preservation techniques can be divided into the various pathways of embryo cryopreservation, mature oocyte cryopreservation, and ovarian tissue cryopreservation, followed by either ovarian tissue transplantation or in vitro growth of follicles and maturation of oocytes. Except for embryo cryopreservation (4), all of these procedures are experimental and require patient consent and Institutional Review Board (IRB) approval. Of note, collection of mature oocytes occurs after hormonal stimulation. These oocytes can either be cryopreserved or fertilized to create embryos for cryopreservation. (1) Steps of in vitro follicle growth and maturation. After ovarian tissue harvest or after thawing of previously cryopreserved ovarian tissue, secondary follicles are retrieved and placed in culture. Mature follicles are removed from culture, and the oocytes are isolated for in vitro maturation to create fertilizable oocytes. These mature oocytes can be cryopreserved or fertilized. Fertilization is achieved using standard in vitro fertilization (IVF) techniques or intracytoplasmic sperm injection (ICSI), and the resulting embryos are transferred into the uterus. The success of in vitro follicle maturation has been limited to animal models and is thus considered experimental and conducted only as part of IRB-approved research protocols. (2) Alternatively, thawed ovarian tissue strips can be transplanted back to the patient to restore ovarian function and achieve conception, as has been documented in approximately 20 patients worldwide. (3) Mature oocytes obtained after hormone stimulation can be cryopreserved for later fertilization followed by embryo cryopreservation.
FIG. 2.
Established fertility preservation technologies. (1) Superovulation with gonadotropins (or aromatase inhibitors for patients with estrogen-sensitive disease). (2) Oocytes are retrieved by transvaginal aspiration. (3) At this stage, a patient can choose to cryopreserve her oocytes if a partner is not available or (5) undergo fertilization in vitro with known or donor sperm specimen. If fertilization occurs, (6) the embryos will be cryopreserved. When patient's disease is in remission, (4) previously cryopreserved oocytes will be thawed and fertilized in vitro, followed by embryo transfer, or (7) previously cryopreserved embryos will be thawed and transferred to the patient.
Oocyte cryopreservation (Fig. 1) is an alternative option for patients who do not have a partner or who do not wish to use donor sperm, with a mean survival rate of 47%, fertilization rate of 52%, and pregnancy rate per thawed oocyte of 1.52% based on 21 studies.59 Success has been limited by available oocyte freezing techniques, although recent advances have been made with an alternative freezing technique, vitrification, with some groups reporting 81% survival and 45% clinical pregnancy per cycle.93 Oocyte cryopreservation is still considered experimental by the American Society of Reproductive Medicine and, thus, is performed under Institutional Review Board (IRB) protocols at many centers.94
Ovarian tissue cryopreservation for subsequent transplantation or in vitro follicle maturation is an investigational option for patients who do not have a partner, who are unable to undergo ovarian stimulation (e.g., prepubertal female), or who are unable to delay treatment long enough to undergo ovarian stimulation (Fig. 1). Transplantation of thawed ovarian tissue can be orthotopic, meaning that the tissue is placed in the ovarian fossa, or heterotopic, by placing tissue subcutaneously.95 The heterotopic approach has resulted in a human fertilized oocyte that developed into a 4-cell embryo, but to date, successful pregnancy has been achieved only in animal models.96,97 There have been case reports of successful live births after orthotopic ovarian tissue transplantation98–102; however, there remain concerns about reseeding of malignant cells and the long-term viability of the grafts.93 In vitro growth of follicles isolated from cryopreserved ovarian tissue, followed by in vitro maturation to produce oocytes competent for fertilization and implantation, is an active area of investigation103–107 (Fig. 1). Thus far, successful live birth has been achieved in murine models,54 and the technology is being optimized in nonhuman primates.108–110 In vitro maturation of immature eggs from aspirated follicles collected at the time of surgery is also being investigated.
There are insufficient data to support the use of medications (such as oral contraceptives, gonadotropin-releasing hormone [GnRH] agonists, and GnRH antagonists) to protect the ovaries from the gonadotoxic effects of chemotherapy.111–114 The use of GnRH agonists in conjunction with alkylating chemotherapy has shown some benefit in cohort studies of patients with hematologic malignancies, SLE, and glomerulonephritis.32,111–114 Interpretation of these studies has been controversial, however, and implementation of GnRH agonists is still considered experimental, with its own inherent risks.115
Fertility preservation application to medically and treatment-induced infertility
Using rheumatologic disease as an illustrative example, we explore the application of family planning/fertility preservation strategies to the management of a patient with a nonmalignant condition. The initial step is awareness on the part of the rheumatologist or primary care physician of the potential gonadotoxic effects from medication, such as cytoxan, used in treating connective tissue disorders. It is important to communicate to the patient that she is at risk for future infertility but is not currently infertile, so that contraception is still employed until pregnancy is desired. It has been found that in the transplant population, unplanned pregnancy occurs at a significantly higher rate (>90%) than in the normal population.34–36,62 This higher rate of unplanned pregnancies may also be true in rheumatologic patients.
Before treatment, when the risks and benefits of therapy are discussed, the impact of treatments on future fertility needs to be addressed. A patient's future family planning desires need to be discussed, including the option of not having children, use of donor gametes, and adoption, as well as having genetically related children. It needs to be explained to patients that the adoption process can be difficult for people with chronic diseases.116
If patients are interested in fertility preservation, they can be directed to the National Physician Cooperative (NPC), a National Institutes of Health (NIH)-funded initiative that has created a network of specialized centers with reproductive endocrinologists who are trained and aware of the fertility preservation treatments that are currently available. There are limited data on the safety and efficacy of fertility preservation procedures in patients with nonmalignant diseases. Working with NPC reproductive specialists, the treating physician is able to have an informed, open discussion with patients about fertility preservation options, weighing the potential fertility risks against the benefits of treatment. This multidisciplinary approach is instrumental in successful treatment of these patients while allowing them to be informed of their reproductive options. For example, the addition of psychosocial counseling can be invaluable to ensure that the patient has decision-making capacity and is making a reasonably informed choice about fertility preservation.116
Patients also should be referred to websites, such as myoncofertility.org and fertilehope.org, for more patient-directed information about fertility preservation options (Table 2). Another key lesson that has been learned through implementation of the NPC is the importance of using a patient navigator to help facilitate various appointments with reproductive specialists and act as a patient advocate.
Table 2.
Additional Educational Resources
| For patients |
| Fertility preservation website: Although intended for cancer patients, this provides patient-focused information on reproduction, fertility treatments, patient stories, and further resources: www.myoncofertility.org |
| Fertility preservation website: Designed for cancer patients, provides information on particular treatment effects to fertility: www.fertilehope.org |
| “I'm too young for this!” Age-appropriate information and support for young cancer patients that may also be relevant for patients with chronic disease, particularly in reference to issues about fertility: i2y.com |
| For healthcare professionals |
| Oktay K, Oktem O. Review: Fertility preservation medicine: A new field in the care of young cancer survivors. Pediatr Blood Cancer 2009;53:267–273. Review by leaders in field of fertility preservation focused on causes and treatment of reproductive dysfunction in the context of cancer. Information can be extended to noncancer gonadotoxic treatments, as a specific review is absent from the literature. |
| Woodruff TK, Snyder KA, eds. Oncofertility: Fertility Preservation for Cancer Survivors. New York: Springer, 2007. One of the first textbooks dedicated to fertility preservation. It includes information on fertility risk and treatment options, techniques and research, healthcare decision making, ethical and psychosocial impacts, and patient stories. |
| American Society of Clinical Oncology. Clinical guidelines for fertility preservation: www.asco.org/ascov2/Practice+&+Guidelines/Guidelines |
| American Society of Reproductive Medicine. Fertility preservation and reproduction in cancer patients: www.asrm.org/publications/detail.aspx?id=613 |
Continuing with our illustrative example, rheumatologic diseases are chronic diseases with periods of exacerbation and remission. Gamete banking after ovarian stimulation is still an option, but it must be done in a closely monitored setting. Oophorectomy for subsequent ovarian tissue cryopreservation may be appropriate, but at this time, it is best reserved for prepubertal girls requiring extensive alkylating chemotherapy. For treatments in which the exact trajectory of gonadotoxic effect is less known, the exact time to initiate fertility preservation needs to be individualized. However, studies comparing IVF outcomes in patients before and after chemotherapy have found that IVF efficacy is dramatically reduced even after one cycle of chemotherapy, demonstrating the need to complete the IVF cycle before initiating chemotherapy whenever possible.117
Pregnancy in a patient with complicated medical comorbidities must be managed in a truly multidisciplinary fashion, with the inputs of high-risk obstetricians, subspecialty medicine physicians, reproductive endocrinologists, and primary care physicians. Some patients may require gestational carriers. In general, good control of rheumatologic diseases, such as SLE, and close monitoring of fetal complications allow for a healthy pregnancy for most patients.7,14,118,119 Lastly, and particularly in the context of patients with chronic disease, the risks of infertility treatments must be thoroughly discussed. For instance, ovarian stimulation for IVF is complicated by the possibility of estrogen-mediated SLE exacerbation. Furthermore, exogenous estrogen increases the risk of thrombosis in a patient population already at higher risk of developing a venous thrombotic event.
Conclusions
Fertility preservation offers young patients standard and experimental techniques provided at specialized centers. Even though the absolute number of patients at risk of POI secondary to nonmalignant diseases or their treatments is low, the ability to identify those at higher risk for iatrogenic infertility can lead to personalized care and timely referral to a reproductive specialist. When considering treatment strategies, practitioners should also consider the impact of the loss of ovarian endocrine function on bone health, cardiovascular health, and sexual function in young women. Awareness among internists and surgeons about the reproductive consequences of medical conditions and their treatments, appropriate and timely referral of patients to fertility preservation programs, and comprehensive patient education are three of the major actions needed to ensure that patients have the best chance to achieve future reproductive success. The paucity of data on fertility preservation in these patient populations makes counseling patients difficult, and a cross-disciplinary counseling strategy may be useful. Further research is urgently needed in this area.
Acknowledgments
We thank Drs. Helen Kim, Candace Tingen, Kate Timmerman, and Stacey Tobin for critical review of the manuscript. We thank Kristin Smith for critical review of the manuscript and assistance with Table 1 and Dr. Francesca Duncan for assistance with Table 1. This work is supported by NIH/NICHD Structure-Function Relationships in Reproductive Science, U54 HD041857; and the Oncofertility Consortium, UL1DE019587, RL1HD058295, and KL1CA133839; and R01HD062797.
Disclosure Statement
No competing financial interests exist.
References
- 1.Chakrabarti S. Bareford D. A survey on patient perception of reduced-intensity transplantation in adults with sickle cell disease. Bone Marrow Transplant. 2007;39:447–451. doi: 10.1038/sj.bmt.1705622. [DOI] [PubMed] [Google Scholar]
- 2.De Vos M. Devroey P. Fauser BC. Primary ovarian insufficiency. Lancet. 2010;376:911–921. doi: 10.1016/S0140-6736(10)60355-8. [DOI] [PubMed] [Google Scholar]
- 3.Kodaman PH. Early menopause: Primary ovarian insufficiency and surgical menopause. Semin Reprod Med. 2010;28:360–369. doi: 10.1055/s-0030-1262895. [DOI] [PubMed] [Google Scholar]
- 4.Griesshammer M. Bergmann L. Pearson T. Fertility, pregnancy and the management of myeloproliferative disorders. Baillieres Clin Haematol. 1998;11:859–874. doi: 10.1016/s0950-3536(98)80043-7. [DOI] [PubMed] [Google Scholar]
- 5.Meirow D. Ovarian injury and modern options to preserve fertility in female cancer patients treated with high dose radio-chemotherapy for hemato-oncological neoplasias and other cancers. Leuk Lymphoma. 1999;33:65–76. doi: 10.3109/10428199909093726. [DOI] [PubMed] [Google Scholar]
- 6.Mok CC. Lau CS. Wong RW. Risk factors for ovarian failure in patients with systemic lupus erythematosus receiving cyclophosphamide therapy. Arthritis Rheum. 1998;41:831–837. doi: 10.1002/1529-0131(199805)41:5<831::AID-ART9>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 7.Gayed M. Gordon C. Pregnancy and rheumatic diseases. Rheumatology (Oxf) 2007;46:1634–1640. doi: 10.1093/rheumatology/kem156. [DOI] [PubMed] [Google Scholar]
- 8.Tarnacka B. Rodo M. Cichy S. Czlonkowska A. Procreation ability in Wilson's disease. Acta Neurol Scand. 2000;101:395–398. doi: 10.1034/j.1600-0404.2000.90140a.x. [DOI] [PubMed] [Google Scholar]
- 9.Martin F. Lauwerys B. Lefebvre C. Devogelaer JP. Houssiau FA. Side-effects of intravenous cyclophosphamide pulse therapy. Lupus. 1997;6:254–257. doi: 10.1177/096120339700600307. [DOI] [PubMed] [Google Scholar]
- 10.Huong DL. Amoura Z. Duhaut P, et al. Risk of ovarian failure and fertility after intravenous cyclophosphamide. A study in 84 patients. J Rheumatol. 2002;29:2571–2576. [PubMed] [Google Scholar]
- 11.Medeiros MM. Silveira VA. Menezes AP. Carvalho RC. Risk factors for ovarian failure in patients with systemic lupus erythematosus. Braz J Med Biol Res. 2001;34:1561–1568. doi: 10.1590/s0100-879x2001001200008. [DOI] [PubMed] [Google Scholar]
- 12.Boumpas DT. Austin HA., 3rd Vaughan EM. Yarboro CH. Klippel JH. Balow JE. Risk for sustained amenorrhea in patients with systemic lupus erythematosus receiving intermittent pulse cyclophosphamide therapy. Ann Intern Med. 1993;119:366–369. doi: 10.7326/0003-4819-119-5-199309010-00003. [DOI] [PubMed] [Google Scholar]
- 13.Wetzels JF. Cyclophosphamide-induced gonadal toxicity: A treatment dilemma in patients with lupus nephritis? Neth J Med. 2004;62:347–352. [PubMed] [Google Scholar]
- 14.Park MC. Park YB. Jung SY. Chung IH. Choi KH. Lee SK. Risk of ovarian failure and pregnancy outcome in patients with lupus nephritis treated with intravenous cyclophosphamide pulse therapy. Lupus. 2004;13:569–574. doi: 10.1191/0961203304lu1063oa. [DOI] [PubMed] [Google Scholar]
- 15.Katsifis GE. Tzioufas AG. Ovarian failure in systemic lupus erythematosus patients treated with pulsed intravenous cyclophosphamide. Lupus. 2004;13:673–678. doi: 10.1191/0961203304lu2012oa. [DOI] [PubMed] [Google Scholar]
- 16.Houssiau FA. Vasconcelos C. D'Cruz D, et al. The 10-year follow-up data of the Euro-Lupus Nephritis Trial comparing low-dose and high-dose intravenous cyclophosphamide. Ann Rheum Dis. 2010;69:61–64. doi: 10.1136/ard.2008.102533. [DOI] [PubMed] [Google Scholar]
- 17.Ioannidis JP. Katsifis GE. Tzioufas AG. Moutsopoulos HM. Predictors of sustained amenorrhea from pulsed intravenous cyclophosphamide in premenopausal women with systemic lupus erythematosus. J Rheumatol. 2002;29:2129–2135. [PubMed] [Google Scholar]
- 18.Boumpas DT. Sidiropoulos P. Bertsias G. Optimum therapeutic approaches for lupus nephritis: What therapy and for whom? Nat Clin Pract Rheumatol. 2005;1:22–30. doi: 10.1038/ncprheum0016. [DOI] [PubMed] [Google Scholar]
- 19.Fine DM. Pharmacological therapy of lupus nephritis. JAMA. 2005;293:3053–3060. doi: 10.1001/jama.293.24.3053. [DOI] [PubMed] [Google Scholar]
- 20.Temprano KK. Bandlamudi R. Moore TL. Antirheumatic drugs in pregnancy and lactation. Semin Arthritis Rheum. 2005;35:112–121. doi: 10.1016/j.semarthrit.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 21.Gourley MF. Austin HA., 3rd Scott D, et al. Methylprednisolone and cyclophosphamide, alone or in combination, in patients with lupus nephritis. A randomized, controlled trial. Ann Intern Med. 1996;125:549–557. doi: 10.7326/0003-4819-125-7-199610010-00003. [DOI] [PubMed] [Google Scholar]
- 22.Appenzeller S. Blatyta PF. Costallat LT. Ovarian failure in SLE patients using pulse cyclophosphamide: Comparison of different regimes. Rheumatol Int. 2008;28:567–571. doi: 10.1007/s00296-007-0478-3. [DOI] [PubMed] [Google Scholar]
- 23.Singh G. Saxena N. Aggarwal A. Misra R. Cytochrome P450 polymorphism as a predictor of ovarian toxicity to pulse cyclophosphamide in systemic lupus erythematosus. J Rheumatol. 2007;34:731–733. [PubMed] [Google Scholar]
- 24.Takada K. Arefayene M. Desta Z, et al. Cytochrome P450 pharmacogenetics as a predictor of toxicity and clinical response to pulse cyclophosphamide in lupus nephritis. Arthritis Rheum. 2004;50:2202–2210. doi: 10.1002/art.20338. [DOI] [PubMed] [Google Scholar]
- 25.Janssen NM. Genta MS. The effects of immunosuppressive and anti-inflammatory medications on fertility, pregnancy, and lactation. Arch Intern Med. 2000;160:610–619. doi: 10.1001/archinte.160.5.610. [DOI] [PubMed] [Google Scholar]
- 26.Silva CA. Brunner HI. Gonadal functioning and preservation of reproductive fitness with juvenile systemic lupus erythematosus. Lupus. 2007;16:593–599. doi: 10.1177/0961203307077538. [DOI] [PubMed] [Google Scholar]
- 27.Riley P. Maillard SM. Wedderburn LR. Woo P. Murray KJ. Pilkington CA. Intravenous cyclophosphamide pulse therapy in juvenile dermatomyositis. A review of efficacy and safety. Rheumatology (Oxf) 2004;43:491–496. doi: 10.1093/rheumatology/keh082. [DOI] [PubMed] [Google Scholar]
- 28.Brunner HI. Bishnoi A. Barron AC, et al. Disease outcomes and ovarian function of childhood-onset systemic lupus erythematosus. Lupus. 2006;15:198–206. doi: 10.1191/0961203306lu2291oa. [DOI] [PubMed] [Google Scholar]
- 29.Packham JC. Hall MA. Premature ovarian failure in women with juvenile idiopathic arthritis (JIA) Clin Exp Rheumatol. 2003;21:347–350. [PubMed] [Google Scholar]
- 30.Ghezzi A. Zaffaroni M. Female-specific issues in multiple sclerosis. Expert Rev Neurother. 2008;8:969–977. doi: 10.1586/14737175.8.6.969. [DOI] [PubMed] [Google Scholar]
- 31.Cocco E. Sardu C. Gallo P, et al. Frequency and risk factors of mitoxantrone-induced amenorrhea in multiple sclerosis: The FEMIMS study. Mult Scler. 2008;14:1225–1233. doi: 10.1177/1352458508094882. [DOI] [PubMed] [Google Scholar]
- 32.Cigni A. Faedda R. Atzeni MM, et al. Hormonal strategies for fertility preservation in patients receiving cyclophosphamide to treat glomerulonephritis: A nonrandomized trial and review of the literature. Am J Kidney Dis. 2008;52:887–896. doi: 10.1053/j.ajkd.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 33.Barua M. Hladunewich M. Keunen J, et al. Successful pregnancies on nocturnal home hemodialysis. Clin J Am Soc Nephrol. 2008;3:392–396. doi: 10.2215/CJN.04110907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anantharaman P. Schmidt RJ. Sexual function in chronic kidney disease. Adv Chronic Kidney Dis. 2007;14:119–125. doi: 10.1053/j.ackd.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 35.Levy DP. Giatras I. Jungers P. Pregnancy and end-stage renal disease—Past experience and new insights. Nephrol Dial Transplant. 1998;13:3005–3007. doi: 10.1093/ndt/13.12.3005. [DOI] [PubMed] [Google Scholar]
- 36.Guazzelli CA. Torloni MR. Sanches TF. Barbieri M. Pestana JO. Contraceptive counseling and use among 197 female kidney transplant recipients. Transplantation. 2008;86:669–672. doi: 10.1097/TP.0b013e3181817e7d. [DOI] [PubMed] [Google Scholar]
- 37.Kettas E. Cayan F. Akbay E. Kiykim A. Cayan S. Sexual dysfunction and associated risk factors in women with end-stage renal disease. J Sex Med. 2008;5:872–877. doi: 10.1111/j.1743-6109.2007.00664.x. [DOI] [PubMed] [Google Scholar]
- 38.Leavey SF. Weitzel WF. Endocrine abnormalities in chronic renal failure. Endocrinol Metab Clin North Am. 2002;31:107–119. doi: 10.1016/s0889-8529(01)00006-8. [DOI] [PubMed] [Google Scholar]
- 39.Kappelman MD. Rifas-Shiman SL. Kleinman K, et al. The prevalence and geographic distribution of Crohn's disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol. 2007;5:1424–1429. doi: 10.1016/j.cgh.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 40.Cima RR. Pemberton JH. Medical and surgical management of chronic ulcerative colitis. Arch Surg. 2005;140:300–310. doi: 10.1001/archsurg.140.3.300. [DOI] [PubMed] [Google Scholar]
- 41.Langholz E. Munkholm P. Davidsen M. Binder V. Colorectal cancer risk and mortality in patients with ulcerative colitis. Gastroenterology. 1992;103:1444–1451. doi: 10.1016/0016-5085(92)91163-x. [DOI] [PubMed] [Google Scholar]
- 42.Gorgun E. Remzi FH. Goldberg JM, et al. Fertility is reduced after restorative proctocolectomy with ileal pouch anal anastomosis: a study of 300 patients. Surgery. 2004;136:795–803. doi: 10.1016/j.surg.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 43.Johnson P. Richard C. Ravid A, et al. Female infertility after ileal pouch-anal anastomosis for ulcerative colitis. Dis Colon Rectum. 2004;47:1119–1126. doi: 10.1007/s10350-004-0570-7. [DOI] [PubMed] [Google Scholar]
- 44.Stein SL. Michelassi F. How can fecundity be preserved in patients undergoing pelvic surgery? Nat Clin Pract Gastroenterol Hepatol. 2008;5:308–309. doi: 10.1038/ncpgasthep1139. [DOI] [PubMed] [Google Scholar]
- 45.Oresland T. Palmblad S. Ellstrom M. Berndtsson I. Crona N. Hulten L. Gynaecological and sexual function related to anatomical changes in the female pelvis after restorative proctocolectomy. Int J Colorectal Dis. 1994;9:77–81. doi: 10.1007/BF00699417. [DOI] [PubMed] [Google Scholar]
- 46.Ording Olsen K. Juul S. Berndtsson I. Oresland T. Laurberg S. Ulcerative colitis: Female fecundity before diagnosis, during disease, and after surgery compared with a population sample. Gastroenterology. 2002;122:15–19. doi: 10.1053/gast.2002.30345. [DOI] [PubMed] [Google Scholar]
- 47.Waljee A. Waljee J. Morris AM. Higgins PD. Threefold increased risk of infertility: A meta-analysis of infertility after ileal pouch anal anastomosis in ulcerative colitis. Gut. 2006;55:1575–1580. doi: 10.1136/gut.2005.090316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mortier PE. Gambiez L. Karoui M, et al. Colectomy with ileorectal anastomosis preserves female fertility in ulcerative colitis. Gastroenterol Clin Biol. 2006;30:594–597. doi: 10.1016/s0399-8320(06)73233-x. [DOI] [PubMed] [Google Scholar]
- 49.Alborzi S. Momtahan M. Parsanezhad ME. Dehbashi S. Zolghadri J. A prospective, randomized study comparing laparoscopic ovarian cystectomy versus fenestration and coagulation in patients with endometriomas. Fertil Steril. 2004;82:1633–1637. doi: 10.1016/j.fertnstert.2004.04.067. [DOI] [PubMed] [Google Scholar]
- 50.Beretta P. Franchi M. Ghezzi F. Busacca M. Zupi E. Bolis P. Randomized clinical trial of two laparoscopic treatments of endometriomas: Cystectomy versus drainage and coagulation. Fertil Steril. 1998;70:1176–1180. doi: 10.1016/s0015-0282(98)00385-9. [DOI] [PubMed] [Google Scholar]
- 51.Vercellini P. Somigliana E. Vigano P. Abbiati A. Barbara G. Crosignani PG. Surgery for endometriosis-associated infertility: A pragmatic approach. Hum Reprod. 2009;24:254–269. doi: 10.1093/humrep/den379. [DOI] [PubMed] [Google Scholar]
- 52.Busacca M. Vignali M. Endometrioma excision and ovarian reserve: A dangerous relation. J Minim Invasive Gynecol. 2009;16:142–148. doi: 10.1016/j.jmig.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 53.Catenacci M. Sastry S. Falcone T. Laparoscopic surgery for endometriosis. Clin Obstet Gynecol. 2009;52:351–361. doi: 10.1097/GRF.0b013e3181b08cc3. [DOI] [PubMed] [Google Scholar]
- 54.Xu M. Kreeger PK. Shea LD. Woodruff TK. Tissue-engineered follicles produce live, fertile offspring. Tissue Eng. 2006;12:2739–2746. doi: 10.1089/ten.2006.12.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Benaglia L. Somigliana E. Vercellini P. Abbiati A. Ragni G. Fedele L. Endometriotic ovarian cysts negatively affect the rate of spontaneous ovulation. Hum Reprod. 2009;24:2183–2186. doi: 10.1093/humrep/dep202. [DOI] [PubMed] [Google Scholar]
- 56.Tan PL. Wagner JE. Auerbach AD. Defor TE. Slungaard A. Macmillan ML. Successful engraftment without radiation after fludarabine-based regimen in Fanconi anemia patients undergoing genotypically identical donor hematopoietic cell transplantation. Pediatr Blood Cancer. 2006;46:630–636. doi: 10.1002/pbc.20538. [DOI] [PubMed] [Google Scholar]
- 57.Gidoni Y. Holzer H. Tulandi T. Tan SL. Fertility preservation in patients with nononcological conditions. Reprod Biomed Online. 2008;16:792–800. doi: 10.1016/s1472-6483(10)60144-7. [DOI] [PubMed] [Google Scholar]
- 58.Somali M. Mpatakoias V. Avramides A, et al. Function of the hypothalamic-pituitary-gonadal axis in long-term survivors of hematopoietic stem cell transplantation for hematological diseases. Gynecol Endocrinol. 2005;21:18–26. doi: 10.1080/09513590500099255. [DOI] [PubMed] [Google Scholar]
- 59.Sonmezer M. Oktay K. Fertility preservation in female patients. Hum Reprod Update. 2004;10:251–266. doi: 10.1093/humupd/dmh021. [DOI] [PubMed] [Google Scholar]
- 60.Lee SJ. Schover LR. Partridge AH, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24:2917–2931. doi: 10.1200/JCO.2006.06.5888. [DOI] [PubMed] [Google Scholar]
- 61.Ghazizadeh S. Lessan-Pezeshki M. Khatami MR, et al. Infertility among kidney transplant recipients. Saudi J Kidney Dis Transpl. 2007;18:79–82. [PubMed] [Google Scholar]
- 62.Fuchs KM. Wu D. Ebcioglu Z. Pregnancy in renal transplant recipients. Semin Perinatol. 2007;31:339–347. doi: 10.1053/j.semperi.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 63.Christopher V. Al-Chalabi T. Richardson PD, et al. Pregnancy outcome after liver transplantation: A single-center experience of 71 pregnancies in 45 recipients. Liver Transplant. 2006;12:1138–1143. doi: 10.1002/lt.20810. [DOI] [PubMed] [Google Scholar]
- 64.Douglas NC. Shah M. Sauer MV. Fertility and reproductive disorders in female solid organ transplant recipients. Semin Perinatol. 2007;31:332–338. doi: 10.1053/j.semperi.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 65.Tuck SM. Fertility and pregnancy in thalassemia major. Ann NY Acad Sci. 2005;1054:300–307. doi: 10.1196/annals.1345.062. [DOI] [PubMed] [Google Scholar]
- 66.Bradley RJ. Rosen MP. Subfertility and gastrointestinal disease: “Unexplained” is often undiagnosed. Obstet Gynecol Surv. 2004;59:108–117. doi: 10.1097/01.OGX.0000109223.04391.9D. [DOI] [PubMed] [Google Scholar]
- 67.Reubinoff BE. Har-El R. Kitrossky N, et al. Increased levels of redox-active iron in follicular fluid: A possible cause of free radical-mediated infertility in beta-thalassemia major. Am J Obstet Gynecol. 1996;174:914–918. doi: 10.1016/s0002-9378(96)70325-3. [DOI] [PubMed] [Google Scholar]
- 68.Farina G. Pedrotti C. Cerani P, et al. Successful pregnancy following gonadotropin therapy in a young female with juvenile idiopathic hemochromatosis and secondary hypogonadotropic hypogonadism. Haematologica. 1995;80:335–337. [PubMed] [Google Scholar]
- 69.Meyer WR. Hutchinson-Williams KA. Jones EE. DeCherney AH. Secondary hypogonadism in hemochromatosis. Fertil Steril. 1990;54:740–742. doi: 10.1016/s0015-0282(16)53842-4. [DOI] [PubMed] [Google Scholar]
- 70.Kelly TM. Edwards CQ. Meikle AW. Kushner JP. Hypogonadism in hemochromatosis: Reversal with iron depletion. Ann Intern Med. 1984;101:629–632. doi: 10.7326/0003-4819-101-5-629. [DOI] [PubMed] [Google Scholar]
- 71.Hamer OW. Gnad M. Scholmerich J. Palitzsch KD. Successful treatment of erectile dysfunction and infertility by venesection in a patient with primary haemochromatosis. Eur J Gastroenterol Hepatol. 2001;13:985–988. doi: 10.1097/00042737-200108000-00021. [DOI] [PubMed] [Google Scholar]
- 72.Gravholt CH. Juul S. Naeraa RW. Hansen J. Prenatal and postnatal prevalence of Turner's syndrome: A registry study. BMJ. 1996;312:16–21. doi: 10.1136/bmj.312.7022.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hanson L. Bryman I. Barrenas ML, et al. Genetic analysis of mosaicism in 53 women with Turner syndrome. Hereditas. 2001;134:153–159. doi: 10.1111/j.1601-5223.2001.00153.x. [DOI] [PubMed] [Google Scholar]
- 74.Bondy CA. Care of girls and women with Turner syndrome: A guideline of the Turner Syndrome Study Group. J Clin Endocrinol Metab. 2007;92:10–25. doi: 10.1210/jc.2006-1374. [DOI] [PubMed] [Google Scholar]
- 75.Balen AH. Harris SE. Chambers EL. Picton HM. Conservation of fertility and oocyte genetics in a young woman with mosaic Turner syndrome. Br J Obstet Gynaecol. 2010;117:238–242. doi: 10.1111/j.1471-0528.2009.02423.x. [DOI] [PubMed] [Google Scholar]
- 76.Kavoussi SK. Fisseha S. Smith YR. Smith GD. Christman GM. Gago LA. Oocyte cryopreservation in a woman with mosaic Turner syndrome: A case report. J Reprod Med. 2008;53:223–226. [PubMed] [Google Scholar]
- 77.El-Shawarby SA. Sharif F. Conway G. Serhal P. Davies M. Oocyte cryopreservation after controlled ovarian hyperstimulation in mosaic Turner syndrome: Another fertility preservation option in a dedicated UK clinic. Br J Obstet Gynaecol. 2010;117:234–237. doi: 10.1111/j.1471-0528.2009.02422.x. [DOI] [PubMed] [Google Scholar]
- 78.Abir R. Fisch B. Nahum R. Orvieto R. Nitke S. Ben Rafael Z. Turner's syndrome and fertility: Current status and possible putative prospects. Hum Reprod Update. 2001;7:603–610. doi: 10.1093/humupd/7.6.603. [DOI] [PubMed] [Google Scholar]
- 79.Practice Committee American Society of Reproductive Medicine. Increased maternal cardiovascular mortality associated with pregnancy in women with Turner syndrome. Fertil Steril. 2005;83:1074–1075. doi: 10.1016/j.fertnstert.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 80.Karnis MF. Zimon AE. Lalwani SI. Timmreck LS. Klipstein S. Reindollar RH. Risk of death in pregnancy achieved through oocyte donation in patients with Turner syndrome: A national survey. Fertil Steril. 2003;80:498–501. doi: 10.1016/s0015-0282(03)00974-9. [DOI] [PubMed] [Google Scholar]
- 81.Lin AE. Lippe BM. Geffner ME, et al. Aortic dilation, dissection, and rupture in patients with Turner syndrome. J Pediatr. 1986;109:820–826. doi: 10.1016/s0022-3476(86)80700-4. [DOI] [PubMed] [Google Scholar]
- 82.Streuli I. Fraisse T. Ibecheole V. Moix I. Morris MA. de Ziegler D. Intermediate and premutation FMR1 alleles in women with occult primary ovarian insufficiency. Fertil Steril. 2009;92:464–470. doi: 10.1016/j.fertnstert.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 83.Toniolo D. Rizzolio F. X chromosome and ovarian failure. Semin Reprod Med. 2007;25:264–271. doi: 10.1055/s-2007-980220. [DOI] [PubMed] [Google Scholar]
- 84.Guerrero NV. Singh RH. Manatunga A. Berry GT. Steiner RD. Elsas LJ., 2nd Risk factors for premature ovarian failure in females with galactosemia. J Pediatr. 2000;137:833–841. doi: 10.1067/mpd.2000.109148. [DOI] [PubMed] [Google Scholar]
- 85.Kaufman FR. Kogut MD. Donnell GN. Goebelsmann U. March C. Koch R. Hypergonadotropic hypogonadism in female patients with galactosemia. N Engl J Med. 1981;304:994–998. doi: 10.1056/NEJM198104233041702. [DOI] [PubMed] [Google Scholar]
- 86.Waggoner DD. Buist NR. Donnell GN. Long-term prognosis in galactosaemia: Results of a survey of 350 cases. J Inherit Metab Dis. 1990;13:802–818. doi: 10.1007/BF01800204. [DOI] [PubMed] [Google Scholar]
- 87.Knauff EA. Richardus R. Eijkemans MJ, et al. Heterozygosity for the classical galactosemia mutation does not affect ovarian reserve and menopausal age. Reprod Sci. 2007;14:780–785. doi: 10.1177/1933719107308614. [DOI] [PubMed] [Google Scholar]
- 88.Gubbels CS. Kuppens SM. Bakker JA, et al. Pregnancy in classic galactosemia despite undetectable anti-mullerian hormone. Fertil Steril. 2009;91:e1213–1296. doi: 10.1016/j.fertnstert.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 89.Hirschberg AL. Polycystic ovary syndrome, obesity and reproductive implications. Womens Health (Lond) 2009;5:529–540. doi: 10.2217/whe.09.39. [DOI] [PubMed] [Google Scholar]
- 90.Livshits A. Seidman DS. Fertility issues in women with diabetes. Womens Health (Lond) 2009;5:701–707. doi: 10.2217/whe.09.47. [DOI] [PubMed] [Google Scholar]
- 91.Nader S. Reproductive endocrinology: Live birth prediction in polycystic ovary syndrome. Nat Rev Endocrinol. 2010;6:64–66. doi: 10.1038/nrendo.2009.251. [DOI] [PubMed] [Google Scholar]
- 92.Shayya R. Chang RJ. Reproductive endocrinology of adolescent polycystic ovary syndrome. Br J Obstet Gynaecol. 2010;117:150–155. doi: 10.1111/j.1471-0528.2009.02421.x. [DOI] [PubMed] [Google Scholar]
- 93.Tulandi T. Huang JY. Tan SL. Preservation of female fertility: An essential progress. Obstet Gynecol. 2008;112:1160–1172. doi: 10.1097/AOG.0b013e31818bba31. [DOI] [PubMed] [Google Scholar]
- 94.Ethics Committee of the American Society for Reproductive Medicine. Fertility preservation and reproduction in cancer patients. Fertil Steril. 2005;83:1622–1628. doi: 10.1016/j.fertnstert.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 95.Roberts JE. Oktay K. Fertility preservation: A comprehensive approach to the young woman with cancer. J Natl Cancer Inst Monogr. 2005:57–59. doi: 10.1093/jncimonographs/lgi014. [DOI] [PubMed] [Google Scholar]
- 96.Lee DM. Yeoman RR. Battaglia DE, et al. Live birth after ovarian tissue transplant. Nature. 2004;428:137–138. doi: 10.1038/428137a. [DOI] [PubMed] [Google Scholar]
- 97.Oktay K. Buyuk E. Veeck L, et al. Embryo development after heterotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;363:837–840. doi: 10.1016/S0140-6736(04)15728-0. [DOI] [PubMed] [Google Scholar]
- 98.Meirow D. Levron J. Eldar-Geva T, et al. Pregnancy after transplantation of cryopreserved ovarian tissue in a patient with ovarian failure after chemotherapy. N Engl J Med. 2005;353:318–321. doi: 10.1056/NEJMc055237. [DOI] [PubMed] [Google Scholar]
- 99.Demeestere I. Simon P. Emiliani S. Delbaere A. Englert Y. Fertility preservation: Successful transplantation of cryopreserved ovarian tissue in a young patient previously treated for Hodgkin's disease. Oncologist. 2007;12:1437–1442. doi: 10.1634/theoncologist.12-12-1437. [DOI] [PubMed] [Google Scholar]
- 100.Silber SJ. Grudzinskas G. Gosden RG. Successful pregnancy after microsurgical transplantation of an intact ovary. N Engl J Med. 2008;359:2617–2618. doi: 10.1056/NEJMc0804321. [DOI] [PubMed] [Google Scholar]
- 101.Donnez J. Dolmans MM. Demylle D, et al. Live birth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364:1405–1410. doi: 10.1016/S0140-6736(04)17222-X. [DOI] [PubMed] [Google Scholar]
- 102.Donnez J. Silber S. Andersen CY, et al. Children born after autotransplantation of cryopreserved ovarian tissue. A review of 13 live births. Ann Med. 2011;43:437–450. doi: 10.3109/07853890.2010.546807. [DOI] [PubMed] [Google Scholar]
- 103.West-Farrell ER. Xu M. Gomberg MA. Chow YH. Woodruff TK. Shea LD. The mouse follicle microenvironment regulates antrum formation and steroid production: Alterations in gene expression profiles. Biol Reprod. 2009;80:432–439. doi: 10.1095/biolreprod.108.071142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.West ER. Shea LD. Woodruff TK. Engineering the follicle microenvironment. Semin Reprod Med. 2007;25:287–299. doi: 10.1055/s-2007-980222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Xu M. Banc A. Woodruff TK. Shea LD. Secondary follicle growth and oocyte maturation by culture in alginate hydrogel following cryopreservation of the ovary or individual follicles. Biotechnol Bioeng. 2009;103:378–386. doi: 10.1002/bit.22250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xu M. West E. Shea LD. Woodruff TK. Identification of a stage-specific permissive in vitro culture environment for follicle growth and oocyte development. Biol Reprod. 2006;75:916–923. doi: 10.1095/biolreprod.106.054833. [DOI] [PubMed] [Google Scholar]
- 107.Xu M. Woodruff TK. Shea LD. Bioengineering and the ovarian follicle. Cancer Treat Res. 2007;138:75–82. doi: 10.1007/978-0-387-72293-1_6. [DOI] [PubMed] [Google Scholar]
- 108.Jin S. Lei L. Shea LD. Zelinski MB. Stouffer RL. Woodruff TK. Markers of growth and development in primate primordial follicles are preserved after slow cryopreservation. Fertil Steril. 2010;93:2627–2632. doi: 10.1016/j.fertnstert.2009.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Xu M. Barrett SL. West-Farrell E, et al. In vitro grown human ovarian follicles from cancer patients support oocyte growth. Hum Reprod. 2009;24:2531–2540. doi: 10.1093/humrep/dep228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Xu M. West-Farrell ER. Stouffer RL. Shea LD. Woodruff TK. Zelinski MB. Encapsulated three-dimensional culture supports development of nonhuman primate secondary follicles. Biol Reprod. 2009;81:587–594. doi: 10.1095/biolreprod.108.074732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Blumenfeld Z. Shapiro D. Shteinberg M. Avivi I. Nahir M. Preservation of fertility and ovarian function and minimizing gonadotoxicity in young women with systemic lupus erythematosus treated by chemotherapy. Lupus. 2000;9:401–405. doi: 10.1191/096120300678828596. [DOI] [PubMed] [Google Scholar]
- 112.Dooley MA. Nair R. Therapy Insight: Preserving fertility in cyclophosphamide-treated patients with rheumatic disease. Nat Clin Pract Rheumatol. 2008;4:250–257. doi: 10.1038/ncprheum0770. [DOI] [PubMed] [Google Scholar]
- 113.Sinha R. Dionne JM. Should gonadotropin releasing hormone analogue be administered to prevent premature ovarian failure in young women with systemic lupus erythematosus on cyclophosphamide therapy? Arch Dis Child. 2008;93:444–445. doi: 10.1136/adc.2007.131334. [DOI] [PubMed] [Google Scholar]
- 114.Somers EC. Marder W. Christman GM. Ognenovski V. McCune WJ. Use of a gonadotropin-releasing hormone analog for protection against premature ovarian failure during cyclophosphamide therapy in women with severe lupus. Arthritis Rheum. 2005;52:2761–2767. doi: 10.1002/art.21263. [DOI] [PubMed] [Google Scholar]
- 115.Oktay K. Sonmezer M. Oktem O. Fox K. Emons G. Bang H. Absence of conclusive evidence for the safety and efficacy of gonadotropin-releasing hormone analogue treatment in protecting against chemotherapy-induced gonadal injury. Oncologist. 2007;12:1055–1066. doi: 10.1634/theoncologist.12-9-1055. [DOI] [PubMed] [Google Scholar]
- 116.Gardino SL. Jeruss JS. Woodruff TK. Using decision trees to enhance interdisciplinary team work: The case of oncofertility. J Assist Reprod Genet. 2010;27:227–231. doi: 10.1007/s10815-010-9413-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dolmans MM. Demylle D. Martinez-Madrid B. Donnez J. Efficacy of in vitro fertilization after chemotherapy. Fertil Steril. 2005;83:897–901. doi: 10.1016/j.fertnstert.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 118.Mok CC. Wong RW. Pregnancy in systemic lupus erythematosus. Postgrad Med J. 2001;77:157–165. doi: 10.1136/pmj.77.905.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Steen VD. Pregnancy in scleroderma. Rheum Dis Clin North Am. 2007;33:345–358. doi: 10.1016/j.rdc.2007.03.001. [DOI] [PubMed] [Google Scholar]