Abstract
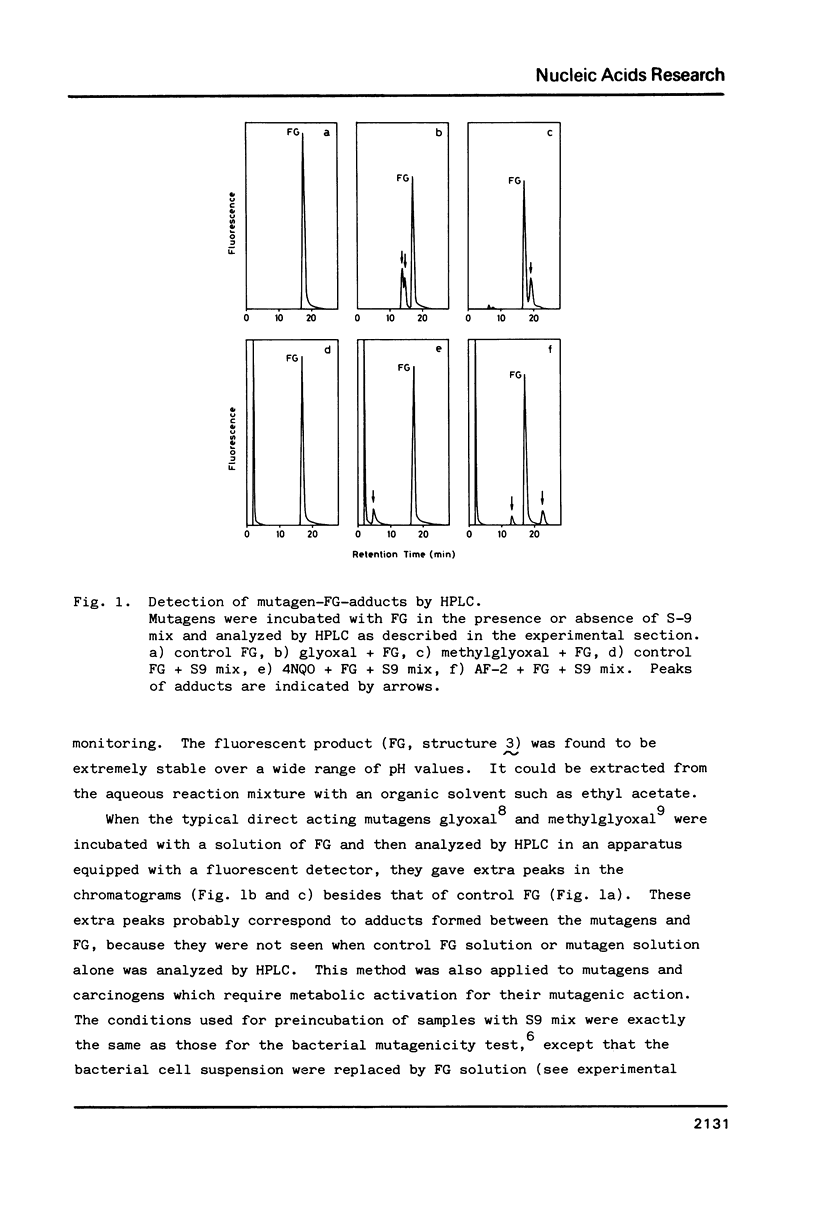
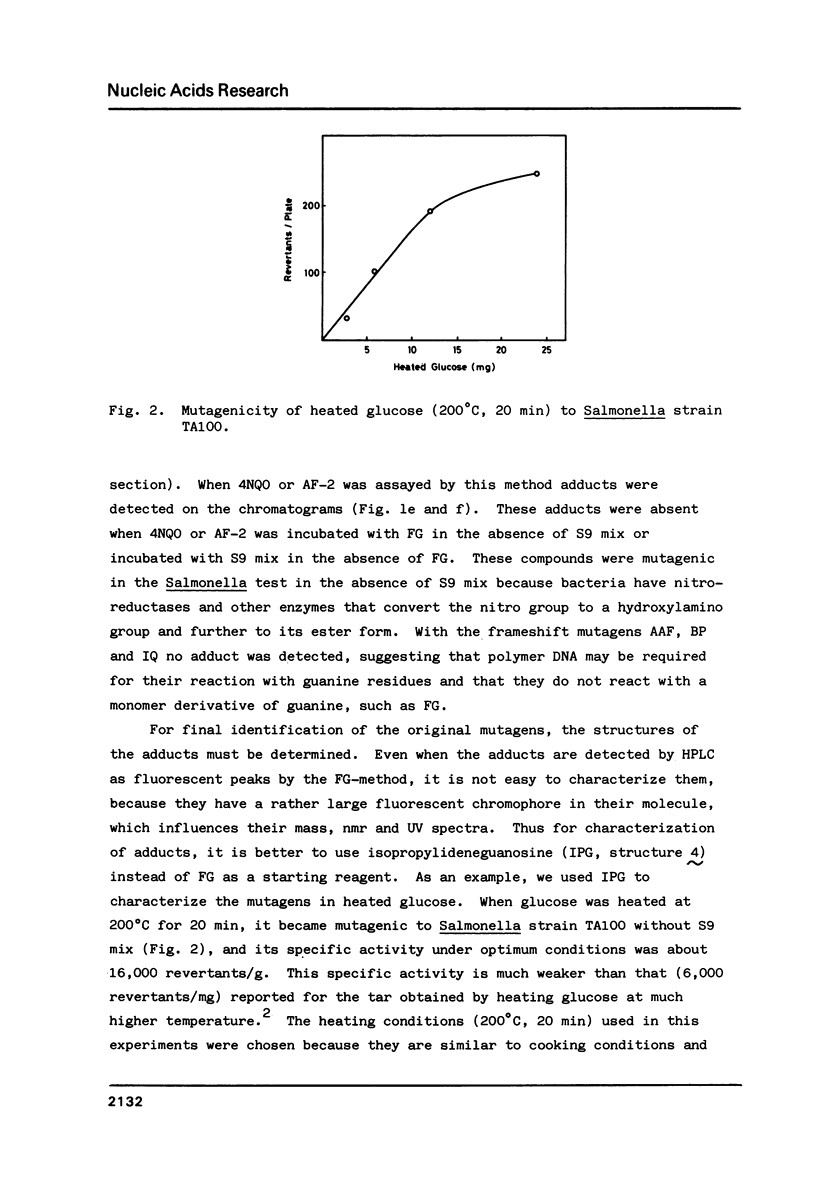
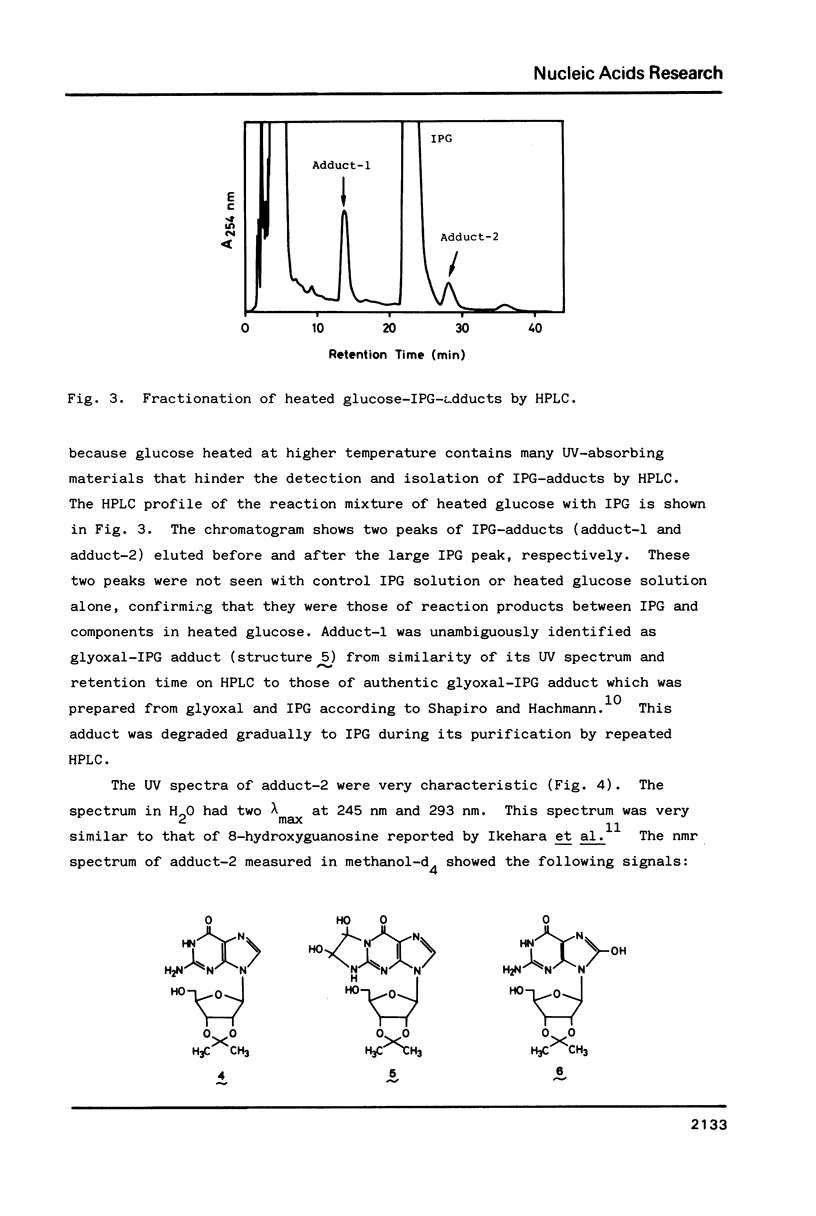
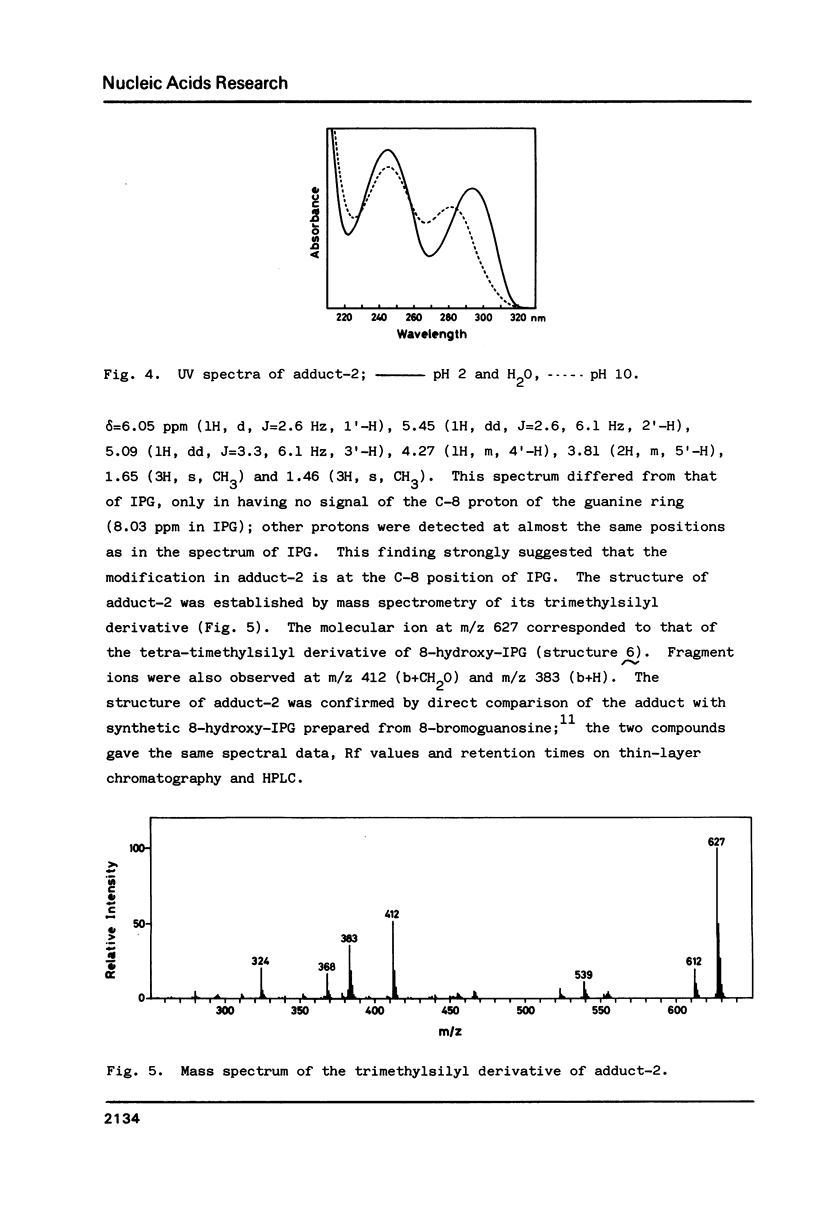
For use in screening for environmental mutagens and carcinogens, a highly fluorescent derivative of guanosine, 2'-deoxy-2'-(2",3"-dihydro-2",4"-diphenyl-2"-hydroxy-3"-oxo-1"-pyrrol yl) guanosine (FG), was synthesized. When incubated with FG in aqueous solution, mutagens form adducts that can be analyzed with an HPLC-fluorescence detector-system. By this method, mutagens such as glyoxal, methylglyoxal, 2-(2-furyl)-3-(5-nitrofuryl) acrylamide and 4-nitroquinoline-N-oxide, used as model compounds, were detected rapidly with high sensitivity. Reaction with isopropylideneguanosine (IPG), followed by isolation and characterization of the mutagen-IPG-adduct was found to be a useful method for identifying unknown mutagens in crude samples. This method was successfully applied in identification of the mutagens in heated glucose (200 degrees C, 20 min); glyoxal-IPG and 8-hydroxy-IPG were identified in the reaction mixture.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N., Mccann J., Yamasaki E. Methods for detecting carcinogens and mutagens with the Salmonella/mammalian-microsome mutagenicity test. Mutat Res. 1975 Dec;31(6):347–364. doi: 10.1016/0165-1161(75)90046-1. [DOI] [PubMed] [Google Scholar]
- Bjeldanes L. F., Chew H. Mutagenicity of 1,2-dicarbonyl compounds: maltol, kojic acid, diacetyl and related substances. Mutat Res. 1979 Aug;67(4):367–371. doi: 10.1016/0165-1218(79)90034-x. [DOI] [PubMed] [Google Scholar]
- Ikehara M., Tada H., Muneyama K. Synthesis of 8-hydroxypurine nucleosides. Chem Pharm Bull (Tokyo) 1965 Sep;13(9):1140–1142. doi: 10.1248/cpb.13.1140. [DOI] [PubMed] [Google Scholar]
- Kasai H., Kumeno K., Yamaizumi Z., Nishimura S., Nagao M., Fujita Y., Sugimura T., Nukaya H., Kosuge T. Mutagenicity of methylglyoxal in coffee. Gan. 1982 Oct;73(5):681–683. [PubMed] [Google Scholar]
- McCloskey J. A., Lawson A. M., Tsuboyama K., Krueger P. M., Stillwell R. N. Mass spectrometry of nucleic acid components. Trimethylsilyl derivatives of nucleotides, nucleosides, and bases. J Am Chem Soc. 1968 Jul 17;90(15):4182–4184. doi: 10.1021/ja01017a062. [DOI] [PubMed] [Google Scholar]
- Randerath K., Reddy M. V., Gupta R. C. 32P-labeling test for DNA damage. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6126–6129. doi: 10.1073/pnas.78.10.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro R., Hachmann J. The reaction of guanine derivatives with 1,2-dicarbonyl compounds. Biochemistry. 1966 Sep;5(9):2799–2807. doi: 10.1021/bi00873a004. [DOI] [PubMed] [Google Scholar]
- Singer B., Kuśmierek J. T. Chemical mutagenesis. Annu Rev Biochem. 1982;51:655–693. doi: 10.1146/annurev.bi.51.070182.003255. [DOI] [PubMed] [Google Scholar]
- Von Minden D. L., McCloskey J. A. Mass spectrometry of nucleic acid components. N,O-Permethyl derivatives of nucleosides. J Am Chem Soc. 1973 Oct 31;95(22):7480–7490. doi: 10.1021/ja00803a044. [DOI] [PubMed] [Google Scholar]
- Weigele M., De Bernardo S., Leimgruber W. Fluorescent labeling of proteins. A new methodology. Biochem Biophys Res Commun. 1973 Oct 1;54(3):899–906. doi: 10.1016/0006-291x(73)90779-1. [DOI] [PubMed] [Google Scholar]
- Wislocki P. G., Borchert P., Miller J. A., Miller E. C. The metabolic activation of the carcinogen 1'-hydroxysafrole in vivo and in vitro and the electrophilic reactivities of possible ultimate carcinogens. Cancer Res. 1976 May;36(5):1686–1695. [PubMed] [Google Scholar]



