Abstract
Summary
Background and objectives
Autosomal dominant polycystic kidney disease (ADPKD) is associated with a substantial cardiovascular disease burden including early onset hypertension, intracranial aneurysms, and left ventricular hypertrophy (LVH). A 41% prevalence of LVH has been reported in ADPKD, using echocardiographic assessment of LV mass (LVM). The HALT PKD study was designed to assess the effect of intensive angiotensin blockade on progression of total kidney volume and LVM. Measurements of LVM were performed using cardiac magnetic resonance (MR).
Design, setting, participants, & measurements
Five hundred forty-three hypertensive patients with GFR >60 ml/min per 1.73 m2 underwent MR assessment of LVM at baseline. LVM was adjusted for body surface area and expressed as LVM index (LVMI; g/m2).
Results
Baseline BP was 125.1 ± 14.5/79.3 ± 11.6 mmHg. Average duration of hypertension was 5.79 years. Prior use of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers was present in 59.5% of patients. The prevalence of LVH assessed using nonindexed LVM (g) was 3.9% (n = 21, eight men and 13 women) and 0.93% (n = 5, one man and four women) using LVMI (g/m2). In exploratory analyses, the prevalence of LVH using LVM indexed to H2.7, and the allometric index ppLVmassHW, ranged from 0.74% to 2.23% (n = 4 to 12). Multivariate regression showed significant direct associations of LVMI with systolic BP, serum creatinine, and albuminuria; significant inverse associations with LVMI were found with age and female gender.
Conclusions
The prevalence of LVH in hypertensive ADPKD patients <50 years of age with short duration of hypertension, and prior use of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers is low. Early BP intervention in ADPKD may have decreased LVH and may potentially decrease cardiovascular mortality.
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is the most common hereditary kidney disease, characterized by increased kidney volume resulting from the development and expansion of multiple cysts throughout the kidney parenchyma. The typical progression pattern results in ESRD by the 6th decade for approximately half of individuals (1). In addition to progressive loss of kidney function, ADPKD is associated with a substantial cardiovascular disease burden including onset of hypertension early in the disease, cardiac valve abnormalities, and left ventricular enlargement beginning in childhood, progressing to overt left ventricular hypertrophy (LVH) in adulthood (2). The cumulative cardiovascular burden undoubtedly contributes to the substantial cardiovascular morbidity and mortality observed in ADPKD both before and after the onset of ESRD (3,4).
Studies of BP and cardiac structure in ADPKD have revealed higher levels of BP and left ventricular mass (LVM) in affected children, before the development of overt hypertension (5). The prevalence of hypertension and LVH is increased in affected adolescents (6). By the 5th decade, overt hypertension and LVH are well established. Chapman et al. reported a 41% prevalence of LVH in 116 consecutive ADPKD patients with a mean age of 41 (7). Associations with LVH in affected hypertensive individuals versus affected normotensive patients included older age, higher serum creatinine, higher levels of mean arterial pressure, higher serum uric acid levels, and longer duration of hypertension (7). Total kidney volume did not correlate with LVH. Nonetheless, hypertension was present in 61% of affected individuals without LVH, but these individuals were younger with shorter duration of hypertension.
The HALT-PKD population constitutes the largest cohort of systematically studied hypertensive ADPKD patients (558 study A and 486 study B) to date. This study was designed to determine the effect of intensive blockade of the renin-angiotensin-aldosterone system (RAAS) and intensive BP control on total kidney volume (TKV) and cardiovascular disease progression (8). Magnetic resonance (MR) assessment of LVM was performed in 543 hypertensive patients with preserved kidney function (GFR >60 ml/min per 1.73 m2) at baseline, before study intervention. This study represents the largest cardiac MR assessment of LVM in individuals with ADPKD and one of the largest cardiac MR studies in any hypertensive population. Variables potentially related to onset or progression of LVH were evaluated to determine the relationship to LVM.
Materials and Methods
Study Population
The design and implementation of the HALT PKD study and the baseline characteristics of this population have been reported in detail (8,9). Briefly, the HALT PKD trials are prospective randomized double-blind placebo-controlled multicenter interventional trials testing whether multilevel blockade of the RAAS using angiotensin-converting enzyme inhibitors (ACEIs) plus angiotensin receptor blockers (ARBs) (lisinopril plus telmisartan) combination therapy will delay progression of renal disease compared with ACEI (lisinopril plus placebo) monotherapy in studies A and B and whether low BP control (95 to 100/60 to 75 mmHg) will delay progression as compared with standard control (120 to 130/70 to 80 mmHg) in study A. In study A, patients are 15 to 49 years with eGFR >60 ml/min per 1.73 m2, whereas in study B, patients are 18 to 64 years with estimated GFR (eGFR) 25 to 60 ml/min per 1.73 m2.
All patients undergo a formal screening visit to verify eligibility, diagnosis of ADPKD, and assignment to study A or study B, based on eGFR. All HALT participants are hypertensive as defined by current use of antihypertensive medications for BP control or systolic BP of ≥130 mmHg and/or a diastolic BP of ≥80 mmHg on three separate readings within the past year. Patients return to the study center within 10 weeks of the screening visit. Existing antihypertensive medications are gradually tapered over a 2- to 4-week washout period and BP controlled to a target of 140/90 mmHg or less with labetolol or clonidine before the baseline visit. Study A participants undergo MR assessment of LVM, renal blood flow, and total kidney volume at the baseline visit, before study intervention. This article will focus on HALT study A participants as MR imaging was not performed in study B patients.
Cardiac Magnetic Resonance Imaging Methods
The cardiac magnetic resonance imaging (MRI) protocol was standardized and implemented in 1.5-T MRI scanners. In each patient, electrocardiogram (ECG) pads and a phased-array surface coil were placed over the chest. Following scout images to determine the position and orientation of the heart, MR images of the left ventricle were acquired using ECG-gated, breath-hold 2D true-FISP (FIESTA) imaging sequences. The images consisted of a stack of 10-mm-thick contiguous slices covering the left ventricle from base to apex in the cardiac short-axis orientation. The field of view was maintained between 25 and 32 cm. The scanning would require 10 to 15 breath-holds to encompass the entire left ventricle depending on the size of the left ventricle.
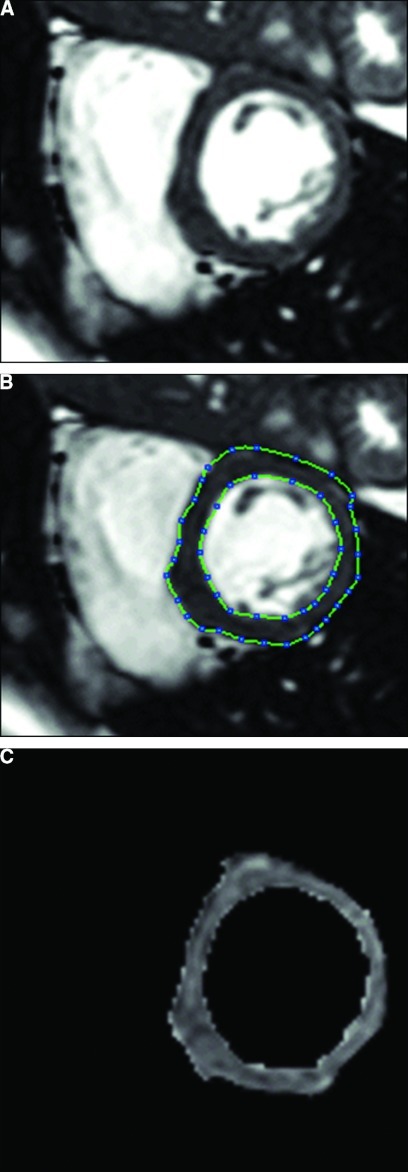
Image data sets were deidentified and transferred to the central image analysis center for the HALT study. Image analysis was implemented and performed in the Analyze software system (Mayo Foundation, Biomedical Imaging Resource, Rochester, MN). At each slice location of the left ventricle, end-diastole image was selected from the ECG-gated cine data set. Left ventricular epicardial and endocardial borders were carefully traced using both semiautomated and manual editing tools of the software (Figure 1). Papillary muscles were excluded from the endocardial border tracing and the myocardial area calculation. Left ventricular volume was computed by the sum of the myocardial area (the difference between endocardial and epicardial contour) over the entire ventricle times slice thickness. LVM was determined by the product of the volume and the specific gravity of myocardium (1.05 g/ml).
Figure 1.
Magnetic resonance image of the heart. Image in short axis at the mid left ventricular level at end diastole demonstrating (A) before and (B) after the delineation of epicardial and endocardial borders, and (C) segmented left ventricular area.
Measures of reliability were obtained by computing inter- and intrarater reliability coefficients. A random sample of 36 participants was selected, and two separate LVM measurements were performed on each participant by two different observers (rater A and rater B). This was also stratified by participants with small, medium, and large LVM values (based on tertiles). The inter-rater reliability was 0.925 comparing raters A and B. When this was stratified by size, the reliability values were 0.886, 0.760, and 0.702, respectively, for small, medium, and large. The intrarater reliability for rater B was 0.922. When stratified by size, the coefficients were 0.751, 0.777, and 0.765, respectively, for small, medium, and large LVM values. All of these reliability measures indicate at least acceptable reproducibility (10).
Statistical Analyses
Descriptive statistics and graphical displays were used to assess normality in unadjusted LVM as well as body surface area (BSA)–adjusted LVM. BSA (m2) was calculated using the formula of Dubois (11). Group comparisons were made using two-sample t tests, whereas measures of association were reported using Pearson correlation coefficients. For each index, multiple regression models were built to examine how baseline covariates predicted baseline LVM. Predictor variables for each of the multiple regression models were chosen based on univariate correlations with the outcome that was significant at the 0.10 level. Multicollinearity among potential predictors was investigated based on variance inflation factors. Stepwise regression, with probabilities to enter and remove as 0.05 and 0.10, respectively, was used for model building (full model). Only variables with P-values <0.05 were further considered for the final models. Standardized regression coefficients (betas) are presented to facilitate the comparison of predictor variables. Because of the exploratory nature of the analyses, adjustments for multiplicity were not performed. All analyses were conducted with STATA/SE 11.1 (College Station, TX), whereas all tables were created using SAS 9.2 (Cary, NC).
Definition of Left Ventricular Hypertrophy
The normal range of LVM by MR is lower than that reported by echocardiography (Table 1) (12–15). The normal range for LVM by cardiac MR has been extensively explored in the Multi-Ethnic Study of Atherosclerosis (MESA) using an index population of 822 normal weight, normotensive, nondiabetic participants aged 45 to 84 years (12). This group also defined two new allometric indices for LVM: percent predicted LVM based on height and gender (ppLVmassH) and percent predicted LVM based on height, weight, and gender (ppLVmassHW). The 95th percentile of LVM reported by Brumback et al. (12) was utilized as the upper limit of normal for the present study. LVM parameters determined by cardiac MR are summarized in Table 1.
Table 1.
Upper limit of normal (95th percentile) for LV mass
| Estimate | Cardiac MR |
Ultrasound |
||
|---|---|---|---|---|
| Women | Men | Women | Men | |
| LVM | 140.3 | 203.5 | ||
| LVMI | 84.6 | 106.2 | 110 | 125 |
| LVMI(H2.7) | 38.0 | 45.1 | ||
| ppLVmassH | 1.33 | 1.33 | ||
| ppLVmassHW | 1.31 | 1.31 | ||
The upper limit of normal (95th percentile) for LVM determined by cardiac MR is reported as nonindexed (in grams) and indexed to body surface area (m2), height (H in meters), and/or weight (W in kg) (12). The upper limit of normal (95th percentile) for LVI mass determined by echocardiography from earlier studies in autosomal dominant polycystic kidney disease is shown for comparison (7,16). LV, left ventricular; MR, magnetic resonance; LVM, LV mass; LVMI, LVM index; ppLVmassH, percent predicted LVM based on height and gender; ppLVmassHW, percent predicted LVM based on height, weight, and gender.
Results
Baseline Characteristics of the HALT PKD Study A Population
Baseline characteristics of the HALT study A participants have been published (9) and are shown in Supplemental Table 1. Fifty-one percent of the patients were men. Statistically significant differences between men and women were noted for age, height, weight, body surface area, body mass index (BMI), and TKV. Men had significantly greater home and office systolic and mean arterial BP. Women were older. All measures of LVM except for ppLVmassH and ppLVmassHW were significantly higher in men; ppLVmassH and ppLVmassHW were not different between the genders. Serum creatinine values were greater in men but eGFR was not different between men and women. Men exhibited greater urinary excretion of sodium and potassium. Urinary aldosterone excretion was greater in women. Urinary albumin excretion was similar for men and women.
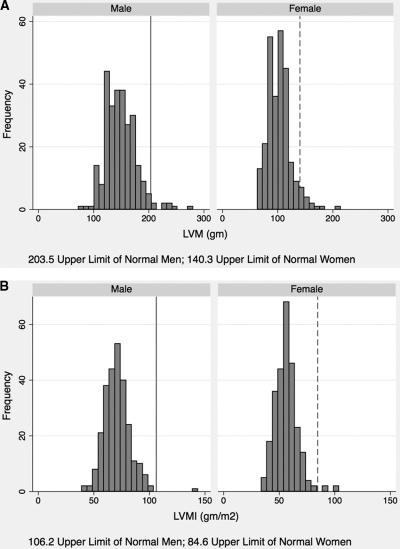
Figure 2 shows the distribution of unadjusted LVM (Figure 2A), and LVM adjusted for body surface area (LVMI) (Figure 2B). LVH was more common in women than in men, although the differences were not statistically significant whether assessed using nonindexed LVM (P = 0.27) or LVMI (P = 0.21). The prevalence of LVH assessed using nonindexed LVM was 3.9% (n = 21, eight men and 13 women), and 0.93% (n = 5, one man and four women) using LVMI. With use of ppLVmassHW, it was determined that one (0.4%) man and three (1.1%) women had LVH. The prevalence of LVH by LVM indexed to H2.7, and the allometric indices ppLVmassH and ppLVmassHW, ranged from 0.74% to 2.23% (n = 4 to 12; data not shown).
Figure 2.
Frequency distribution of left ventricular mass (LVM) for men and women. (A) Frequency distribution of nonindexed LVM for men and women. The upper limit of normal for LVM in men is 203.5 g, shown as solid line. The upper limit of normal for LVM in women is 140.3, shown as dashed line. (B) Frequency distribution of LVMI for men and women. The upper limit of normal for LVMI in men is 106.2 g/m2, shown as solid line. The upper limit of normal for LVMI in women is 84.6 g/m2, shown as dashed line.
Correlation of LVM Indices with Height and Weight
To address whether the indices adequately accounted for body size, we calculated Pearson correlation coefficients of each of the LVM indices with height and weight by gender. A low correlation of the index with body size is anticipated for an index that adequately accounts for body size. These results are shown in Table 2. The indices with the strongest correlation with height and weight by gender are unindexed LVM and LVM(H2.7). LVMI had low and statistically nonsignificant correlations with height and weight by gender. Unexpectedly, ppLVmassHW was highly significantly correlated with weight for men and women (P < 0.001) in contrast to the performance of this index in a normotensive population (12). Despite this association, ppLVmassHW and LVMI were highly correlated (r = 0.847; P < 0.001) (Supplementary Figure 1). Subsequent analyses focused on LVMI.
Table 2.
Pearson correlation coefficient of LV mass indices with height and weight
| Estimate | Height |
Weight |
||
|---|---|---|---|---|
| Women | Men | Women | Men | |
| ppLVmassH | −0.08 (P = 0.20) | −0.10 (P = 0.10) | 0.36 (P < 0.001)a | 0.26 (P < 0.001)a |
| ppLVmassHW | 0.004 (P = 0.96) | 0.02 (P = 0.77) | −0.25 (P < 0.001)a | −0.21 (P = 0.001)a |
| LVMI | 0.03 (P = 0.66) | 0.05 (P = 0.45) | −0.05 (P = 0.41) | −0.05 (P = 0.47) |
| LVMI(H2.7) | −0.23 (P < 0.001)a | −0.31 (P < 0.001)a | 0.31 (P < 0.001)a | 0.18 (P = 0.004)a |
| LVM | 0.29 (P < 0.001)a | 0.37 (P < 0.001)a | 0.44 (P < 0.001)a | 0.42 (P < 0.001)a |
LV, left ventricular; LVM, LV mass; LVMI, LVM index; ppLVmassH, percent predicted LVM based on height and sex; ppLVmassHW, percent predicted LVM based on height, weight, and gender.
P < 0.05.
Associations of Baseline Parameters with LVMI (Table 3)
Table 3.
Correlation of LVMI with baseline characteristics
| Men |
Women |
Both |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| N | r | P | N | r | P | N | r | P | |
| Age (years) | 262 | −0.16 | 0.01a | 264 | −0.14 | 0.02a | 526 | −0.19 | <0.01a |
| Height (cm) | 262 | 0.05 | 0.45 | 264 | 0.03 | 0.66 | 526 | 0.42 | <0.01a |
| Weight (kg) | 262 | −0.05 | 0.47 | 264 | −0.05 | 0.41 | 526 | 0.21 | <0.01a |
| BSA (m2) | 262 | −0.02 | 0.76 | 264 | −0.04 | 0.51 | 526 | 0.32 | <0.01a |
| BMI (kg/m2) | 262 | −0.08 | 0.22 | 264 | −0.06 | 0.34 | 526 | −0.01 | 0.79 |
| Duration of hypertension (years) | 258 | −0.06 | 0.33 | 261 | 0.15 | 0.02a | 519 | 0.04 | 0.43 |
| Systolic BP measured at office (mmHg) | 261 | 0.11 | 0.08 | 264 | 0.25 | <0.01a | 525 | 0.23 | <0.01a |
| Diastolic BP measured at office (mmHg) | 261 | −0.09 | 0.15 | 264 | 0.14 | 0.03a | 525 | 0.05 | 0.25 |
| MAP measured at office (mmHg) | 261 | −0.01 | 0.82 | 264 | 0.19 | 0.02a | 525 | 0.13 | 0.03a |
| TKV (ml) | 258 | 0.14 | 0.03a | 261 | 0.06 | 0.36 | 519 | 0.24 | <0.01a |
| lnTKV | 258 | 0.12 | 0.05 | 261 | 0.12 | 0.06 | 519 | 0.27 | <0.01a |
| Serum creatinine (mg/dl) | 262 | 0.10 | 0.10 | 261 | 0.10 | 0.10 | 523 | 0.42 | <0.01a |
| eGFR (ml/min per 1.73 m2) (MDRD) | 262 | −0.02 | 0.80 | 264 | −0.02 | 0.76 | 526 | −0.02 | 0.73 |
| eGFR (ml/min per 1.73 m2) (CKD-EPI) | 262 | −0.02 | 0.78 | 264 | −0.02 | 0.79 | 526 | −0.05 | 0.25 |
| Serum sodium (mEq/L) | 262 | 0.09 | 0.17 | 264 | −0.01 | 0.84 | 526 | 0.04 | 0.32 |
| Serum potassium (mEq/L) | 262 | −0.02 | 0.72 | 264 | 0.06 | 0.36 | 526 | 0.11 | 0.01a |
| Urine volume (ml) | 254 | −0.02 | 0.78 | 254 | 0.08 | 0.19 | 508 | 0.06 | 0.16 |
| Urine sodium (mEq/24 h) | 236 | 0.01 | 0.86 | 250 | −0.03 | 0.69 | 486 | 0.13 | 0.05a |
| Urine potassium (mEq/24 h) | 234 | 0.05 | 0.44 | 247 | 0.07 | 0.25 | 481 | 0.17 | <0.01a |
| Urine aldosterone (μg/24 h) | 201 | −0.02 | 0.74 | 216 | 0.24 | <0.01a | 417 | −0.04 | 0.44 |
| Urine albumin (mg/24 h) | 236 | 0.29 | <0.01a | 250 | 0.12 | 0.06 | 486 | 0.12 | 0.01a |
LVMI, left ventricular mass index; BSA, body surface area; BMI, body mass index; MAP, mean arterial pressure; TKV, total kidney volume; eGFR, estimated GFR; MDRD, Modification of Diet in Renal Disease; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration.
P < 0.05.
For the combined groups, LVMI was inversely correlated with age and directly associated with height, weight, BSA, office systolic and mean BP, TKV, lnTKV, serum creatinine, serum and urine potassium, urine sodium, and urine albumin excretion. For men, LVMI was inversely correlated with age and directly associated with TKV and urine albumin excretion. For women, LVMI was also inversely correlated with age and directly associated with duration of HTN and office systolic, diastolic, and mean BP, and urine aldosterone excretion. Associations of baseline parameters with nonindexed LVM are shown in Supplemental Table 2.
Multivariate Associations of Baseline Parameters with LVM (Table 4)
Table 4.
Predictors of LVMI in study A
| Full Model |
Final Model |
|||||
|---|---|---|---|---|---|---|
| Difference | 95% CI | Beta | Difference | 95% CI | Beta | |
| Age (years) | −0.28c | [−0.40, −0.16] | −0.18c | −0.25c | [−0.34, −0.13] | −0.16c |
| Systolic BP measured at office (mmHg) | 0.12c | [0.05, 0.18] | 0.13c | 0.12c | [0.05, 0.18] | 0.13c |
| TKV (ml) | 0.0009 | [−0.0006, 0.002] | 0.05 | |||
| Serum creatinine (mg/dl) | 6.63 | [−0.08, 13.33] | 0.10 | 7.45a | [1.00, 13.91] | 0.11a |
| Serum potassium (mEq/L) | 0.98 | [−1.18, 3.14] | 0.03 | |||
| Urine sodium (mEq/24 h) | −0.01 | [−0.02, 0.01] | −0.04 | |||
| Urine potassium (mEq/24 h) | 0.04 | [−0.004, 0.08] | 0.08 | |||
| Urine albumin (mg/24 h) | 0.006 | [−0.0004, 0.01] | 0.07 | 0.007a | [0.0004, 0.01] | 0.08a |
| Female | −11.30c | [−13.95, −8.64] | −0.43c | −11.54c | [−14.10, −8.97] | −0.44c |
| Constant | 52.01c | [37.56, 66.47] | 56.40c | [45.88, 66.92] | ||
| Observations | 471 | 482 | ||||
| R2 | 0.38 | 0.36 | ||||
Height and weight were excluded from the model. LVMI, left ventricular mass index; CI, confidence interval; TKV, total kidney volume.
P < 0.05.
P < 0.01.
P < 0.001.
Multiple regression demonstrated independent associations of age, BSA, BMI, office systolic and diastolic BP, serum creatinine, and female gender with nonindexed LVM (Supplemental Table 3). For LVMI, age, office systolic BP, serum creatinine, urine albumin, and female sex were independently associated. Associations with ppLVmassHW (data not shown) were similar to those for LVMI: age, office systolic BP, serum creatinine, and urine albumin were independently associated with ppLVmassHW; in addition, urine aldosterone was directly associated.
Formal comparison of the baseline characteristics of the patients with LVH to those with normal LVM is not meaningful because of the small number of patients with LVH. However, it is notable that urinary aldosterone excretion in female patients with LVH using nonindexed LVM (n = 12) exceeded those without LVH (n = 207) by nearly twofold (mean ± SD: normal LVM: 15.0 ± 10.5; LVH: 26.7 ± 25.1). This difference was even more pronounced when we compared female patients with LVH using LVMI (n = 4; 49.5 ± 35.0) and those without LVH (n = 215; 15.0 ± 10.3).
Discussion
The HALT PKD study population represents the largest cardiac MR assessment of LVM in hypertensive individuals with ADPKD and one of the largest cardiac MR studies in any hypertensive population. Prior studies of LVM in individuals with ADPKD were done using echocardiography. Analysis of indices of LVM determined by cardiac MR and associations with baseline parameters yield a number of novel and important insights.
Prevalence of LVH
The prevalence of LVH in this hypertensive population was unexpectedly low, ranging from 0.7% to 3.9%, in contrast to prior reports (7,16). The highest prevalence of 3.9% was found using nonindexed LVM, with lower values obtained when LVM was indexed by height, weight, or a combination of height and weight. LVH was more prevalent in women than in men. Several factors may account for these results. HALT participants were younger (men 35.2; women 37.2; excluded >50 years old) than those described in prior studies of individuals with ADPKD (7,16) (mean age 40 to 42 years). The prevalence of LVH in the HALT study was higher in women than in men, which may have been due to the older age of women in this study.
Prior use of ACEI or ARB as antihypertensive medication was common in the HALT study A population. Two hundred fifty-one (45.0%) of the 558 patients were taking at least an ACEI and 90 (16.1%) were taking at least an ARB before study enrollment (RAAS: n = 332 (59.5%); beta blockers: n = 73 (13.1%); diuretic: n = 52 (9.3%). There were no differences in mean LVMI between participants taking ACEI/ARB medications and those who were not (63.73 ± 12.89 versus 63.62 ± 14.32; P = 0.94). The level of BP control before enrollment is not known; however, it is possible that this cohort of individuals with ADPKD were treated to a more aggressive BP target than those from the era from which the earlier studies derive (7,16). The Joint National Committee (JNC) and Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines for levels of BP control that are considered adequate have changed dramatically over this time period. Patients with ADPKD from a more recent cohort (1992 through 2001) were found to have been treated to a lower BP target and with higher use of ACEI than an earlier cohort (1985 through 1992) (17). Decreased LVM with more rigorous BP control, or use of ACEI versus amlodipine, has been demonstrated in ADPKD (16). It is possible that the low prevalence of LVH in this study population reflects a benefit from improved BP control and more common use of angiotensin-blocking agents in recent clinical practice.
Indices of LVM
We utilized the definitions of LVH from indices of LVM derived from a large (n = 822) normal weight, normotensive, nondiabetic, multiethnic population 45 to 84 years of age (12). This population is older than the HALT study population; however, there is little variation in normals of cardiac MR LVM indexed to body size for age between 20 and 80 years (13,14,18,19). LVM determined from cardiac MR is lower than that determined by M-mode echocardiography because of greater accuracy and the lack of need for assumptions about cardiac geometry (18). For example, the echocardiographic definition of LVH used in earlier studies of ADPKD patients (7,16) is >125 g/m2 in men and >110 g/m2 in women as compared with 106.2 g/m2 for men and 84.6 g/m2 for women in the MESA population (12). The mean and 95th percentile of indexed LVM from MESA are similar (13–15) or slightly greater (20,21) than those derived from other cardiac MR studies of healthy individuals. It is unlikely that ethnicity had any effect on the LVMI values for the HALT study A population. African Americans and Hispanics have similar and Asian Americans have lower LVMI than Caucasians; (18) however, the HALT study A population is primarily Caucasian with only 2.5% Asian-American and 2.5% African-American participants (9).
Factors Associated with LVMI
Only systolic BP measured at office, serum creatinine, and urine albumin directly associated with LVMI; age and female gender were inversely associated. Gender had the strongest association; age, serum creatinine, and systolic BP measured at office had weaker associations. Albuminuria had the smallest effect but nonetheless was significant. The associations of elevated BP and gender with LVM are well established. Women are known to have lower LVM than men and LVM is directly related to body size.
The significant association of serum creatinine level with LVMI is unexpected. One would anticipate that this association was the result of LVMI correlating with eGFR, gender, or body size. Although LVMI was related to sex, there was no relationship with eGFR (either between or within genders). The primary reason serum creatinine shows up as a significant predictor is not clinically based, but rather a statistical artifact of combining men and women together. In Table 3, it is evident that LVMI and serum creatinine are correlated across genders, but not within genders. As can be seen in Supplemental Figure 2, combining both genders gives the false impression that there is an overall positive relationship between LVMI and serum creatinine. When the final model in Table 4 is run separately for each gender, independent predictors of LVMI in women are age and systolic BP whereas LVMI in men is predicted by age and urine albumin.
It is notable that the allometric index of LVM, ppLVmassHW, did not perform similarly to that reported for the normotensive, nonobese MESA population. There was a significant correlation of ppLVmassHW with weight in the HALT study A population, which would not be anticipated for an index that adequately accounts for weight. It is unclear whether the behavior of ppLVmassHW in the hypertensive ADPKD population is a function of hypertension, the additional body weight resulting from large kidneys (1 to 2 kg) or overweight, or a different relationship between LVM and body weight in individuals with ADPKD. MESA participants had BMI <25 kg/m2 and had a much narrower weight range than HALT patients, with greater similarity in weight for men and women. Nonetheless, variables predictive of increased LVM expressed either as LVMI or ppLVmassHW were substantially the same and LVMI and ppLVmassHW were highly correlated.
In conclusion, this analysis provides an excellent foundation for the longitudinal intervention study to document efficacy of multilevel angiotensin blockade in preventing the onset or the progression of LVH in a hypertensive ADPKD population with preserved GFR and relatively normal LVM.
Disclosures
None.
Supplementary Material
Acknowledgments
The HALT-PKD study is supported by cooperative agreements from the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases (DK62408, DK62401, DK62410, DK62402, and DK62411). The HALT-PKD study and this publication were also supported by Grants from the National Center for Research Resources (RR000039 Emory, RR000051 Colorado, RR00585 Mayo, RR000054 Tufts Medical Center, and RR23940 Kansas and UL1 RR025008 Emory, UL1 RR025780 Colorado, UL1 RR024150 Mayo, UL1 RR025752 Tufts, and UL1 RR024992 Washington University). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCRR. Study medication for both trials was provided by Boehringer-Ingelheim Pharmaceuticals (telmisartan and matched placebo) and Merck & Co. (lisinopril). The Polycystic Kidney Disease Foundation provided financial support and recruitment assistance for the enrollment phase of HALT PKD. The investigators thank Gigi Flynn and Robin Woltman and all of the clinical coordinators at each clinical site for their perseverance and hard work in implementing HALT PKD.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental information for this article is available online at www.cjasn.org
References
- 1. Hateboer N, v Dijk M, Bogdanova N, Coto E, Saggar-Malik A, San Millan J, Torra R, Breuning M, Ravine D: Comparison of phenotypes of polycystic kidney disease types 1 and 2. European PKD1-PKD2 Study Group Lancet 353(9147): 103–107, 1999 [DOI] [PubMed] [Google Scholar]
- 2. Schrier R: Renal volume, renin-angiotensin-aldosterone system, hypertension, and left ventricular hypertrophy in patients with autosomal dominant polycystic kidney disease. J Am Soc Nephrol 20: 1888–1893, 2009 [DOI] [PubMed] [Google Scholar]
- 3. Perrone R, Ruthazer R, Terrin N: Survival after end-stage renal disease in autosomal dominant polycystic kidney disease: Contribution of extrarenal complications to mortality. Am J Kidney Dis 38: 777–784, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Fick G, Johnson A, Hammond W, Gabow P: Causes of death in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 5: 2048–2056, 1995 [DOI] [PubMed] [Google Scholar]
- 5. Ivy D, Shaffer E, Johnson A, Kimberling W, Dobin A, Gabow P: Cardiovascular abnormalities in children with autosomal dominant polycystic kidney disease. J Am Soc Nephrol 5: 2032–2036, 1995 [DOI] [PubMed] [Google Scholar]
- 6. Zeier M, Geberth S, Schmidt K, Mandelbaum A, Ritz E: Elevated blood pressure profile and left ventricular mass in children and young adults with autosomal dominant polycystic kidney disease. J Am Soc Nephrol 3: 1451–1457, 1993 [DOI] [PubMed] [Google Scholar]
- 7. Chapman A, Johnson A, Rainguet S, Hossack K, Gabow P, Schrier R: Left ventricular hypertrophy in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 8: 1292–1297, 1997 [DOI] [PubMed] [Google Scholar]
- 8. Chapman A, Torres V, Perrone R, Steinman T, Bae K, Miller J, Miskulin D, Rahbari Oskoui F, Masoumi A, Hogan M, Winklhofer F, Braun W, Thompson P, Meyers C, Kelleher C, Schrier R: The HALT polycystic kidney disease trials: Design and implementation. Clin J Am Soc Nephrol 5: 102–109, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Torres V, Chapman A, Perrone R, Bae K, Abebe K, Bost J, Miskulin D, Steinman T, Braun W, Winklhofer F, Hogan M, Oskoui F, Kelleher C, Masoumi A, Glockner J, Halin N, Martin D, Remer E, Patel N, Pedrosa I, Wetzel L, Thompson P, Miller J, Meyers C, Schrier R, HPS Group: The HALT Polycystic Kidney Disease Trials - Analysis of baseline parameters: Effect of gender and developmental programming in autosomal dominant polycystic kidney disease. Kidney Int, submitted, 2011 [Google Scholar]
- 10. Fleiss J: The design and analysis of clinical experiments, New York, Wiley, 1999 [Google Scholar]
- 11. Dubois D, Dubois E: A formula to estimate the approximate surface if height and weight are known. Arch Intern Med 17: 863–871, 1916 [Google Scholar]
- 12. Brumback L, Kronmal R, Heckbert S, Ni H, Hundley W, Lima J, Bluemke D: Body size adjustments for left ventricular mass by cardiovascular magnetic resonance and their impact on left ventricular hypertrophy classification. Int J Cardiovasc Imaging 26: 459–468, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Salton C, Chuang M, O'Donnell C, Kupka M, Larson M, Kissinger K, Edelman R, Levy D, Manning W: Gender differences and normal left ventricular anatomy in an adult population free of hypertension. A cardiovascular magnetic resonance study of the Framingham Heart Study Offspring cohort. J Am Coll Cardiol 39: 1055–1060, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Sandstede J, Lipke C, Beer M, Hofmann S, Pabst T, Kenn W, Neubauer S, Hahn D: Age- and gender-specific differences in left and right ventricular cardiac function and mass determined by cine magnetic resonance imaging. Eur Radiol 10: 438–442, 2000 [DOI] [PubMed] [Google Scholar]
- 15. Marcus J, DeWaal L, Götte M, van der Geest R, Heethaar R, Van Rossum A: MRI-derived left ventricular function parameters and mass in healthy young adults: Relation with gender and body size. Int J Card Imaging 15: 411–419, 1999 [DOI] [PubMed] [Google Scholar]
- 16. Schrier R, McFann K, Johnson A, Ecder T, Tison L: Cardiac and renal effects of standard versus rigorous blood pressure control in autosomal dominant polycystic kidney disease: Results of a seven-year prospective randomized study. J Am Soc Nephrol 13: 1733–1739, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Schrier R, McFann K, Johnson A: Epidemiological study of kidney survival in autosomal dominant polycystic kidney disease. Kidney Int 63: 678–685, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Natori S, Lai S, Finn J, Gomes A, Hundley W, Jerosch-Herold M, Pearson G, Sinha S, Arai A, Lima J, Bluemke D: Cardiovascular function in multi-ethnic study of atherosclerosis: Normal values by age, sex, and ethnicity. AJR 186 [6 Suppl 2]: S357–S365, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Cain P, Ahl R, Hedstrom E, Ugander M, Allansdotter-Johnsson A, Friberg P, Arheden H: Age and gender specific normal values of left ventricular mass, volume and function for gradient echo magnetic resonance imaging: A cross sectional study. BMC Med Imaging 9: 2, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Alfakih K, Plein S, Thiele H, Jones T, Ridgway J, Sivananthan M: Normal human left and right ventricular dimensions for MRI as assessed by turbo gradient echo and steady-state free precession imaging sequences. J Magn Reson Imaging 17: 323–329, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Lorenz C, Walker E, Morgan V, Klein S, Graham T, Jr.: Normal human right and left ventricular mass, systolic function, and gender differences by cine magnetic resonance imaging. J Cardiovasc Magn Reson 1: 7–21, 1999 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.