Abstract
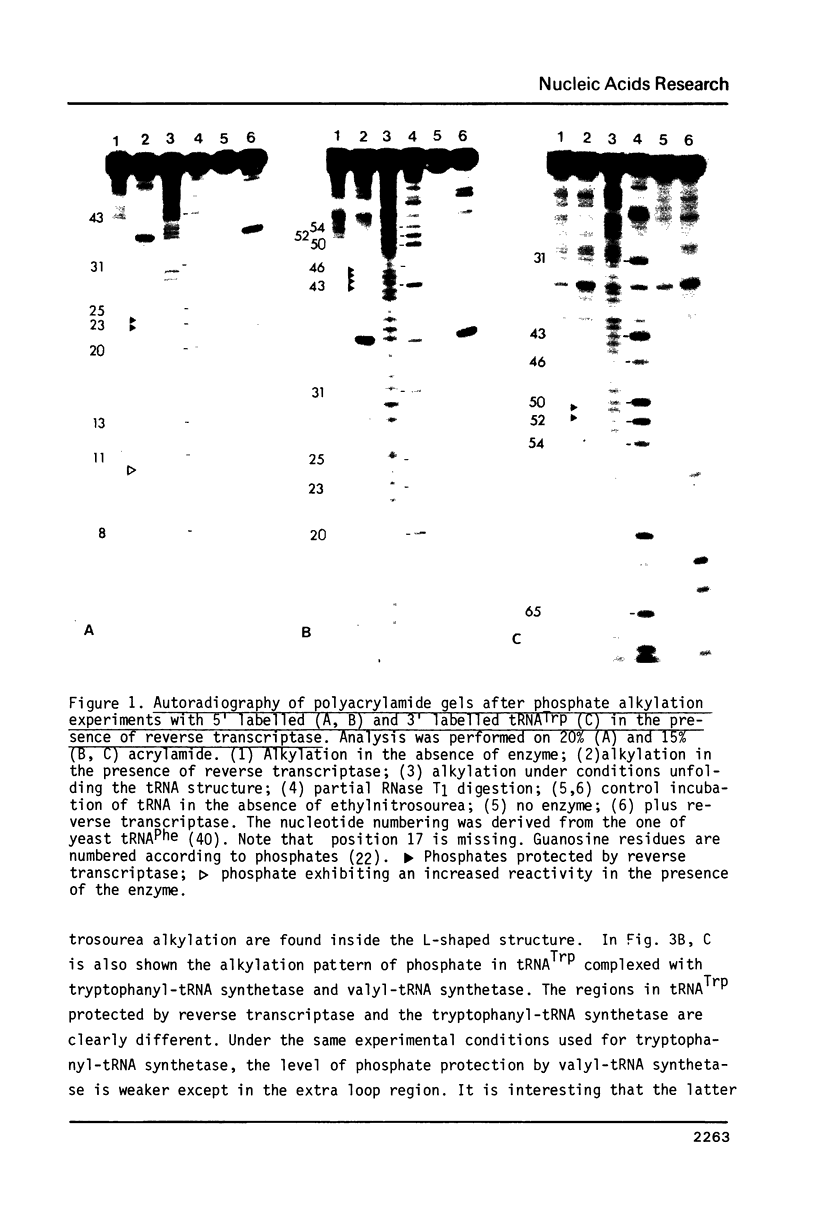
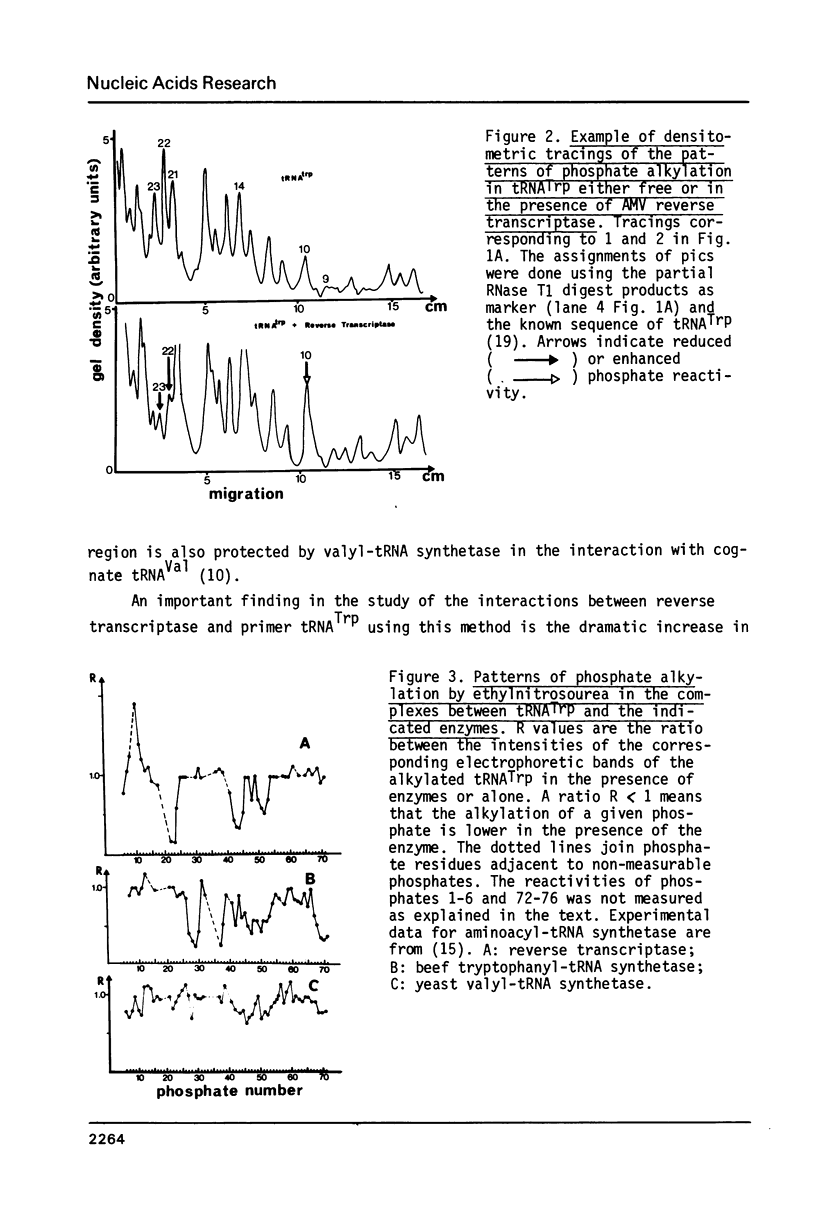
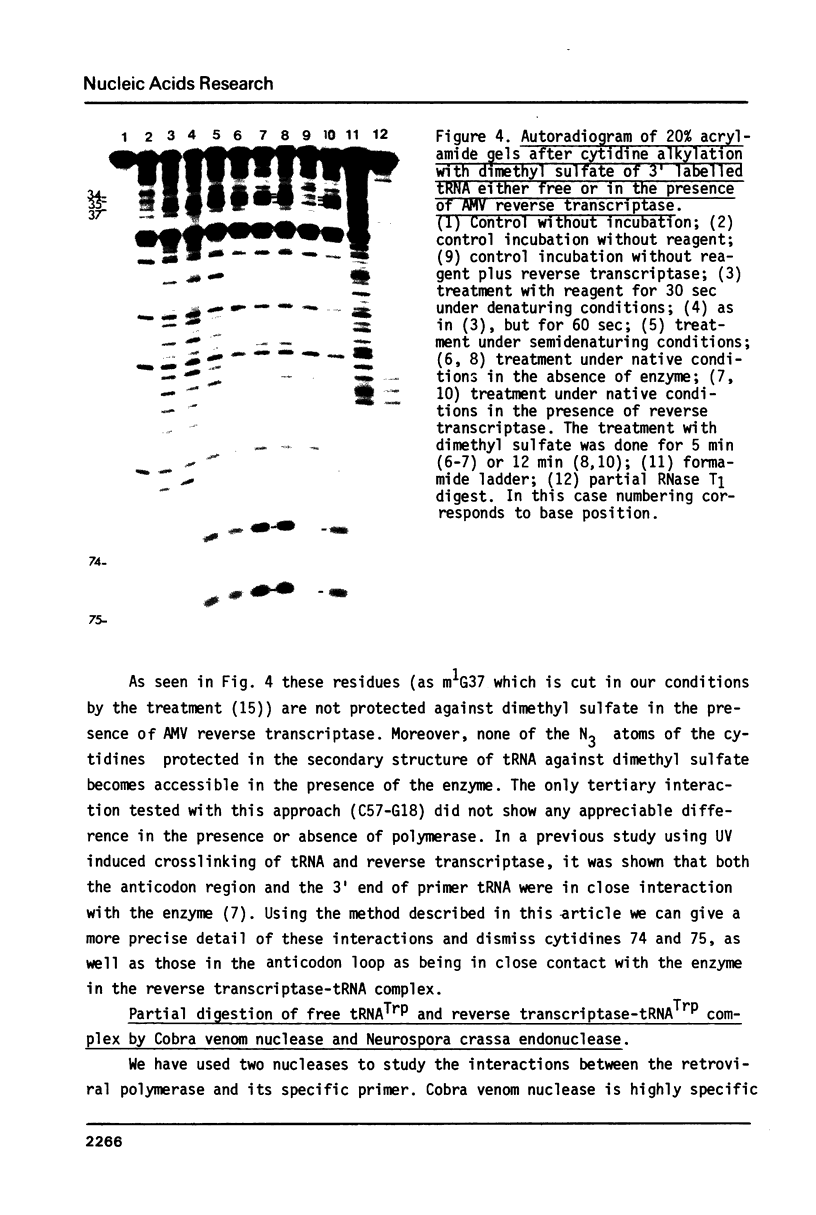
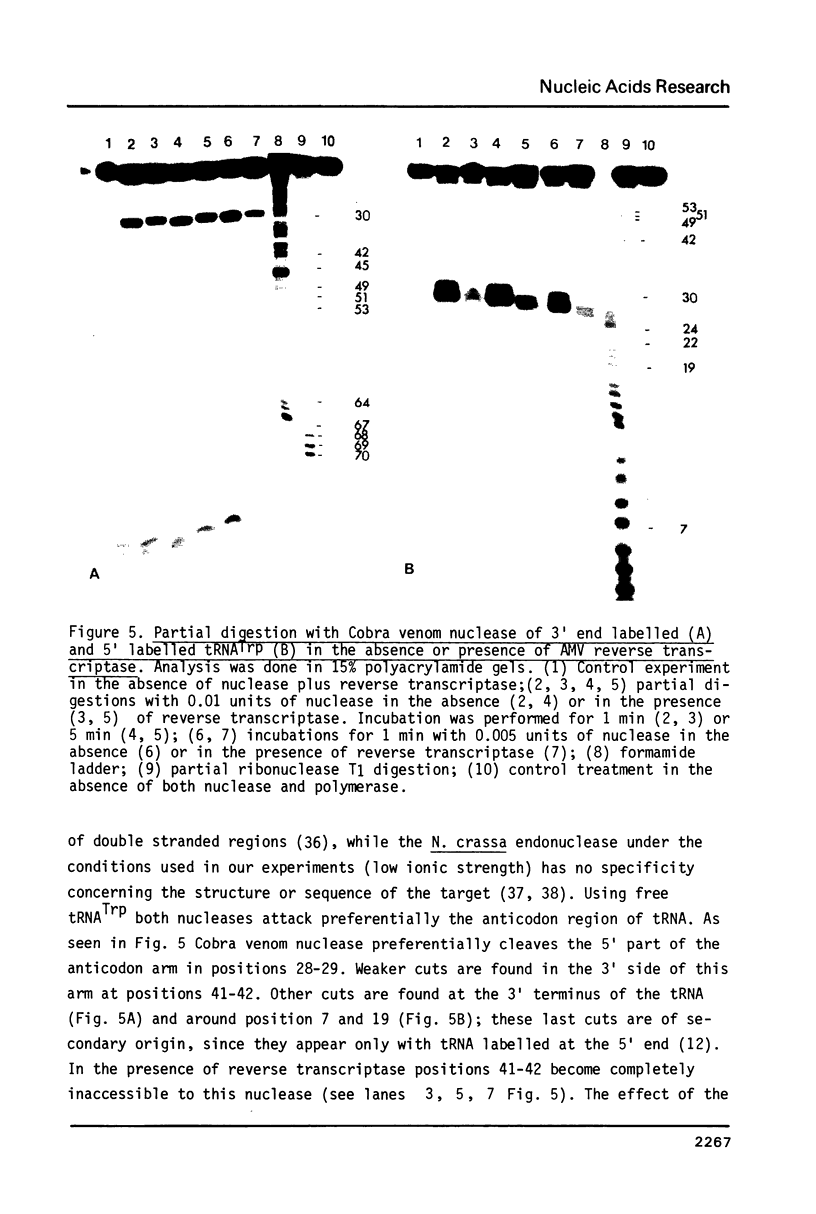
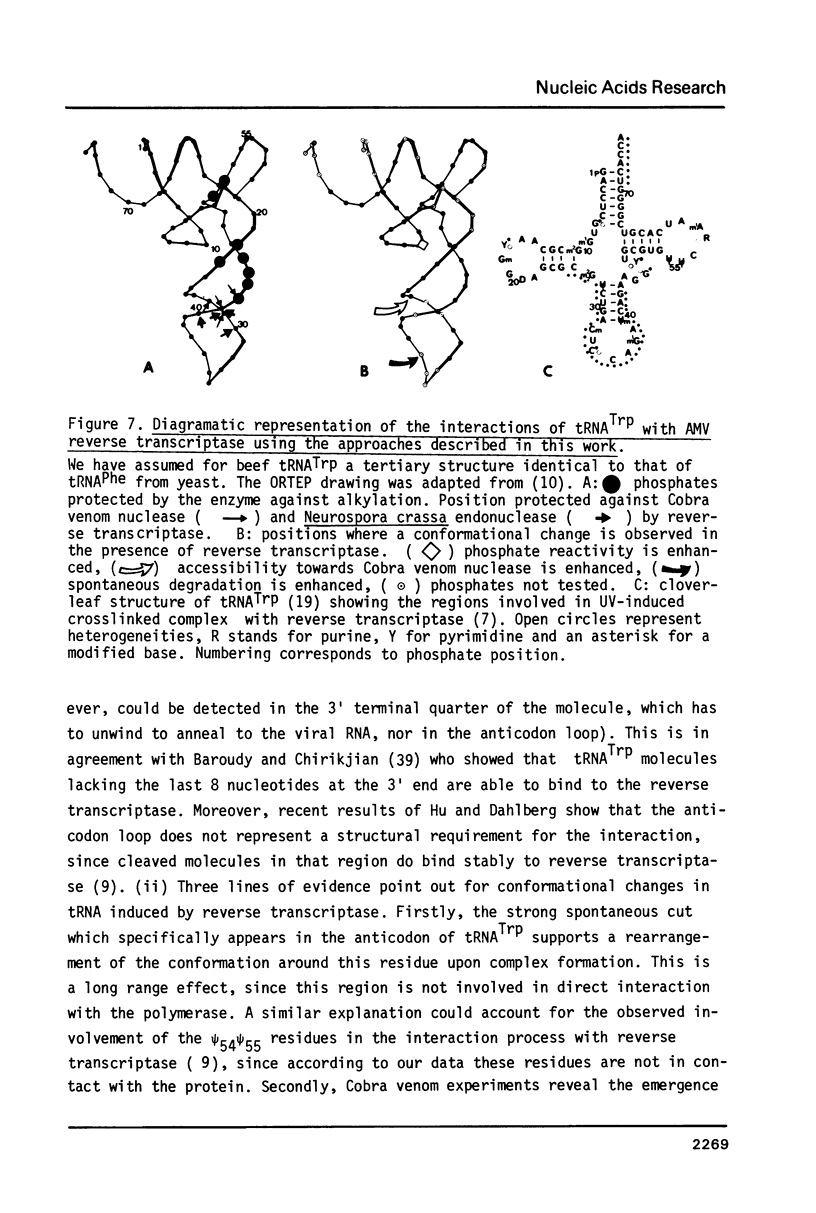
The interactions between beef tRNATrp with avian myeloblastosis reverse transcriptase have been studied by statistical chemical modifications of phosphate (ethylnitrosourea) and cytidine (dimethyl sulfate) residues, as well as by digestion of complexed tRNA by Cobra venom nuclease and Neurospora crassa endonuclease. Results with nucleases and chemicals show that reverse transcriptase interacts preferentially with the D arm, the anticodon stem and the T psi stem. All these regions are located in the outside of the L-shaped structure of tRNA. This domain of interaction is different to that reported previously in the complex of beef tRNA with the cognate aminoacyl-tRNA synthetase (M. Garret et al.; Eur. J. Biochem. In press). Avian reverse transcriptase destabilizes the region of tRNA where most of the tertiary interactions maintaining the structure of tRNA are located.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araya A., Hevia E., Litvak S. Study of the interactions between avian myeloblastosis virus reverse transcriptase and primer tRNA. Affinity labeling and inactivation of the enzyme by periodate-treated tRNATrp. Nucleic Acids Res. 1980 Sep 11;8(17):4009–4020. doi: 10.1093/nar/8.17.4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araya A., Keith G., Fournier M., Gandar J. C., Labouesse J., Litvak S. Photochemical cross-linking studies on the interactions of avian myeloblastosis virus reverse transcriptase with primer tRNATrp and TTP. Arch Biochem Biophys. 1980 Dec;205(2):437–448. doi: 10.1016/0003-9861(80)90127-7. [DOI] [PubMed] [Google Scholar]
- Araya A., Sarih L., Litvak S. Reverse transcriptase mediated binding of primer tRNA to the viral genome. Nucleic Acids Res. 1979 Aug 24;6(12):3831–3843. doi: 10.1093/nar/6.12.3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barciszewski J., Romby P., Ebel J. P., Giegé R. Chemical probes for tRNA tertiary structure. Comparative alkylation of tRNA with methylnitrosourea, ethylnitrosourea and dimethylsulfate. FEBS Lett. 1982 Dec 27;150(2):459–464. doi: 10.1016/0014-5793(82)80789-8. [DOI] [PubMed] [Google Scholar]
- Baroudy B. M., Chirikjian J. G. Structural requirements for binding of bovine tRNATrp with avian myeloblastosis virus DNA polymerase. Nucleic Acids Res. 1980 Jan 11;8(1):57–66. doi: 10.1093/nar/8.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. M. Retroviruses. Annu Rev Biochem. 1978;47:35–88. doi: 10.1146/annurev.bi.47.070178.000343. [DOI] [PubMed] [Google Scholar]
- Boutorin A. S., Clark B. F., Ebel J. P., Kruse T. A., Petersen H. U., Remy P., Vassilenko S. A study of the interaction of Escherichia coli elongation factor-Tu with aminoacyl-tRNAs by partial digestion with cobra venom ribonuclease. J Mol Biol. 1981 Nov 5;152(3):593–608. doi: 10.1016/0022-2836(81)90271-0. [DOI] [PubMed] [Google Scholar]
- Böhlen P., Stein S., Dairman W., Udenfriend S. Fluorometric assay of proteins in the nanogram range. Arch Biochem Biophys. 1973 Mar;155(1):213–220. doi: 10.1016/s0003-9861(73)80023-2. [DOI] [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favorova O. O., Fasiolo F., Keith G., Vassilenko S. K., Ebel J. P. Partial digestion of tRNA--aminoacyl-tRNA synthetase complexes with cobra venom ribonuclease. Biochemistry. 1981 Feb 17;20(4):1006–1011. doi: 10.1021/bi00507a055. [DOI] [PubMed] [Google Scholar]
- Fournier M., Dorizzi M., Sarger C., Labouresse J. Purification of tRNATrp, tRNAVal, and partial purification of tRNAIle and tRNAMfet from beef liver. Biochimie. 1976;58(10):1159–1165. doi: 10.1016/s0300-9084(76)80114-9. [DOI] [PubMed] [Google Scholar]
- Fournier M., Labouesse J., Dirheimer G., Fix C., Keith G. Primary structure of bovine liver tRNATrp. Biochim Biophys Acta. 1978 Nov 21;521(1):198–208. doi: 10.1016/0005-2787(78)90262-9. [DOI] [PubMed] [Google Scholar]
- Gangloff J., Jaozara R., Dirheimer G. Study of the interaction of yeast arginyl-tRNA synthetase with yeast tRNAArg2 and tRNAArg3 by partial digestions with cobra venom ribonuclease. Eur J Biochem. 1983 May 16;132(3):629–637. doi: 10.1111/j.1432-1033.1983.tb07410.x. [DOI] [PubMed] [Google Scholar]
- Gauss D. H., Sprinzl M. Compilation of tRNA sequences. Nucleic Acids Res. 1983 Jan 11;11(1):r1–53. [PMC free article] [PubMed] [Google Scholar]
- Houts G. E., Miyagi M., Ellis C., Beard D., Beard J. W. Reverse transcriptase from avian myeloblastosis virus. J Virol. 1979 Feb;29(2):517–522. doi: 10.1128/jvi.29.2.517-522.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J. C., Dahlberg J. E. Structural features required for the binding of tRNATrp to avian myeloblastosis virus reverse transcriptase. Nucleic Acids Res. 1983 Jul 25;11(14):4823–4833. doi: 10.1093/nar/11.14.4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern D., Dietrich A., Fasiolo F., Renaud M., Giegé R., Ebel J. P. The yeast aminoacyl-tRNA synthetases. Methodology for their complete or partial purification and comparison of their relative activities under various extraction conditions. Biochimie. 1977;59(5-6):453–462. doi: 10.1016/s0300-9084(77)80050-3. [DOI] [PubMed] [Google Scholar]
- LINN S., LEHMAN I. R. AN ENDONUCLEASE FROM NEUROSPORA CRASSA SPECIFIC FOR POLYNUCLEOTIDES LACKING AN ORDERED STRUCTURE. II. STUDIES OF ENZYME SPECIFICITY. J Biol Chem. 1965 Mar;240:1294–1304. [PubMed] [Google Scholar]
- Moras D., Comarmond M. B., Fischer J., Weiss R., Thierry J. C., Ebel J. P., Giegé R. Crystal structure of yeast tRNAAsp. Nature. 1980 Dec 25;288(5792):669–674. doi: 10.1038/288669a0. [DOI] [PubMed] [Google Scholar]
- Mérault G., Graves P. V., Labouesse B., Labouesse J. Influence of magnesium on the steady-state-derived order of substrate addition and product release in tRNATrp aminoacylation by beef pancreas tryptophan: tRNA ligase: significance of the deduced mechanism. Eur J Biochem. 1978 Jul 3;87(3):541–550. doi: 10.1111/j.1432-1033.1978.tb12405.x. [DOI] [PubMed] [Google Scholar]
- Panet A., Weil G., Friis R. R. Binding of tryptophanyl-tRNA to the reverse transcriptase of replication-defective avian sarcoma viruses. J Virol. 1978 Nov;28(2):434–443. doi: 10.1128/jvi.28.2.434-443.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peattie D. A., Gilbert W. Chemical probes for higher-order structure in RNA. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4679–4682. doi: 10.1073/pnas.77.8.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters G. G., Hu J. Reverse transcriptase as the major determinant for selective packaging of tRNA's into Avian sarcoma virus particles. J Virol. 1980 Dec;36(3):692–700. doi: 10.1128/jvi.36.3.692-700.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley G. J., Rich A. Structural domains of transfer RNA molecules. Science. 1976 Nov 19;194(4267):796–806. doi: 10.1126/science.790568. [DOI] [PubMed] [Google Scholar]
- Rabin E. Z., Mustard M., Fraser M. J. Specific inhibition by ATP and other properites of an endonuclease of Neurospora crassa. Can J Biochem. 1968 Oct;46(10):1285–1291. doi: 10.1139/o68-193. [DOI] [PubMed] [Google Scholar]
- Rether B., Bonnet J., Ebel J. P. Studies on tRNA nucleotidyltransferase from baker's yeast. 1. Purification of the enzyme. Protection against thermal inactivation and inhibition by several substrates. Eur J Biochem. 1974 Dec 16;50(1):281–288. doi: 10.1111/j.1432-1033.1974.tb03896.x. [DOI] [PubMed] [Google Scholar]
- Riehl N., Giegé R., Ebel J. P., Ehresmann B. Effect of elongation factor Tu on the conformation of phenylalanyl-tRNAPhe. FEBS Lett. 1983 Apr 5;154(1):42–46. doi: 10.1016/0014-5793(83)80871-0. [DOI] [PubMed] [Google Scholar]
- Scheinker V. S., Beresten S. F., Mashkova T. D., Mazo A. M., Kisselev L. L. Role of exposed cytosine residues in aminoacylation activity of tRNATrp. FEBS Lett. 1981 Sep 28;132(2):349–352. doi: 10.1016/0014-5793(81)81195-7. [DOI] [PubMed] [Google Scholar]
- Simoncsits A., Brownlee G. G., Brown R. S., Rubin J. R., Guilley H. New rapid gel sequencing method for RNA. Nature. 1977 Oct 27;269(5631):833–836. doi: 10.1038/269833a0. [DOI] [PubMed] [Google Scholar]
- Thiyagarajan P., Ponnuswamy P. K. Solvent accessibility study on tRNAPhe. Biopolymers. 1979 Sep;18(9):2233–2247. doi: 10.1002/bip.1979.360180911. [DOI] [PubMed] [Google Scholar]
- Vlassov V. V., Giegé R., Ebel J. P. Tertiary structure of tRNAs in solution monitored by phosphodiester modification with ethylnitrosourea. Eur J Biochem. 1981 Sep;119(1):51–59. doi: 10.1111/j.1432-1033.1981.tb05575.x. [DOI] [PubMed] [Google Scholar]
- Vlassov V. V., Kern D., Giegé R., Ebel J. P. Protection of phosphodiester bonds in yeast tRNAVal by its cognate aminoacyl-tRNA synthetase against alkylation by ethylnitrosourea. FEBS Lett. 1981 Jan 26;123(2):277–281. doi: 10.1016/0014-5793(81)80307-9. [DOI] [PubMed] [Google Scholar]
- Vlassov V. V., Kern D., Romby P., Giegé R., Ebel J. P. Interaction of tRNAPhe and tRNAVal with aminoacyl-tRNA synthetases. A chemical modification study. Eur J Biochem. 1983 May 16;132(3):537–544. doi: 10.1111/j.1432-1033.1983.tb07395.x. [DOI] [PubMed] [Google Scholar]