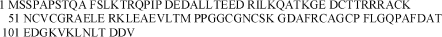Abstract
Background
For the diagnosis of visceral leishmaniasis (VL), rK39 antigen-based rapid test is widely used. Unfortunately, up to 32% healthy individuals from endemic region test positive with this antigen. There is an urgent need to search for a more specific antigen with sensitivity similar to rK39.
Methods
We identified a Leishmania donovani-specific 12.6-kDa (BHUP3) soluble promastigote antigen through sensitive western blot technique. The identified protein was partially purified from sodium dodecyl sulfate–polyacrylamide gel electrophoresis and the antigenic response of eluted protein was determined by western blot with different groups of individual sera. The diagnostic potential was further validated by enzyme-linked immunosorbent assay using serum of 100 VL patients, 93 nonendemic healthy control individuals, 110 endemic healthy control individuals, and 110 disease control individuals. Further, it was characterized by two-dimensional gel electrophoresis followed by matrix-assisted laser desorption/ionization-time-of-flight analysis.
Results
On blotting, antibody against this protein was recognized by all (9/9) VL patient's sera, but it was absent in every control group (nonendemic healthy control and endemic healthy control). Sensitivity of the enzyme-linked immunosorbent assay was 88% (89/101), whereas the specificity for endemic healthy, nonendemic healthy, and different disease groups were 96% (106/110), 100% (93/93), and 97% (107/110), respectively. The two-dimensional gel electrophoresis showed a single spot, and matrix-assisted laser desorption/ionization-time-of-flight analysis revealed that it is a 113-amino-acid-long putative uncharacterized protein of 12.6-kDa anamorsin homolog matched completely with Leishmania major (GenBank accession number: Q4QIS1).
Conclusion
Despite marginally lower sensitivity of BHUP3, excellent specificity warrants its further development as a tool for diagnosis of VL.
Key Words: Diagnosis, ELISA, MALDI-TOF, Visceral leishmaniasis, Western blotting
Introduction
Visceral leishmaniasis (VL), commonly known as kala-azar, is a severe systemic infection and is caused by the parasite Leishmania donovani. In India, the parasite is transmitted by the female sand fly, Phlebotomus argentipes. The VL by L. donovani is a major public health problem in the state of Bihar and adjoining areas of states such as West Bengal, Jharkhand, and Uttar Pradesh in India (Choudhry et al. 1990, Bora 1999). In India, about 100,000 cases of VL have been estimated to occur annually, and of these, Bihar accounts for >90% of VL cases (Bora 1999). Diagnosis of VL relies on the demonstration of amastigotes in the splenic or bone marrow aspirates through invasive procedures. Early case detection followed by adequate treatment is central to the VL elimination program (Bhattacharya et al. 2006). For the diagnosis of rK39 antigen (Burns et al. 1993), based rapid test is most widely used, which utilizes a recombinant protein of 39 amino acids conserved in the kinesin region of L. chagasi. This test has excellent sensitivity (93%–100%) and specificity (97%–98%), but up to 32% healthy individuals living in endemic areas test positive with this test (Sundar et al. 1998). In the proposed study, we identified and characterized a 12.6-kDa L. donovani-specific soluble antigen for serodiagnosis of Indian VL. The preliminary result with this antigen showed that it could be a potential serological biomarker that can overcome the problems associated with the existing biomarkers.
Materials and Methods
The study was conducted at the Infectious Disease Research Laboratory of Banaras Hindu University, Varanasi, Uttar Pradesh, and its field site at Kala-Azar Medical Research Center, Muzaffarpur, which is highly endemic for VL. The study was approved by the Ethical Committee of Institute of Medical Sciences, Banaras Hindu University, Varanasi. Informed written consent was obtained from all participating volunteers.
Patients
Serum samples were collected from parasitologically confirmed VL patients (n=101), healthy individuals (n=110) living in endemic regions (with no history of kala-azar; endemic healthy control [EHC]), healthy individuals (n=93) from nonendemic areas (nonendemic healthy control [NEHC]), and patients representing different diseases (n=110), respectively. The patients with different diseases were suffering from amoebic liver abscess, aplastic anemia, aplastic anemia with nephrotic syndrome, viral fever, tuberculosis, and malaria diseases.
Crude soluble antigen (CSA) preparation
The 1×108 stationary-phase promastigote culture was harvested in cold 1×phosphate-buffered saline (PBS) at pH 7.2 for CSA preparation. After washing and centrifugation, pellet was resuspended in 1×PBS and equal volume of complete protease inhibitor cocktail (Sigma) was added. For complete lysis, the parasite cells were frozen (at −70°C) and thawed (at room temperature) by six alternate cycles followed by sonication (Soniprep MES 150; Sanyo). Finally, the cells were centrifuged (Allegra X-22R; Beckman Coulter) at 4000 rpm for 10 min and the supernatant containing the crude soluble protein was collected (Kelemen and Csati 1955). Protein was quantified using a bicinchoninic acid kit (Thermo Scientific) (Smith et al. 1985).
SDS-PAGE
Crude soluble antigen extract was electrophoresed on 12% polyacrylamide gel following the method of Laemmli (1970).
Western blotting
CSA (45 μg/well) of L. donovani (Kelemen and Csati 1955) was subjected to 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). Crude soluble antigens were then immunoblotted, according to Towbin et al. (1979), with few modifications in western blotting (Bio-Rad Mini-Protean II; Multi Screen), to a PVDF (polyvinylidene difluoride) membrane (0.45 μm pore size; Millipore) at 20 V for 30 min. The membrane was further treated with sera (1:100 in PBS) of different study groups for 1 h at room temperature. Alkaline phosphatase conjugated with goat anti-human IgG (1:1000) was used as secondary antibody. At the end, color was developed using 5-bromo-4-chloro-3-indolylphosphate+nitro blue thiazole as a substrate (Promega). The obtained bands were analyzed by Alpha Imager (Alpha Inno. Tech.).
Partial purification of protein from SDS-PAGE gel
The 12.6-kDa protein bands corresponding to protein marker were directly excised with a sterile scalpel from the SDS-PAGE gel, crushed, and incubated overnight in an elution buffer (50 mM Tris-HCl, 150 mM NaCl, and 0.1 mM EDTA [pH 7.5]) at 37°C. The solution was centrifuged at 10,000 rpm (10°C) for 20 min and the obtained supernatant was quantified for protein by bicinchoninic acid method.
Enzyme-linked immunosorbent assay
The assay was done as described elsewhere with some modifications (Hommel et al. 1978). Microtiter plates (Nunc) were coated with eluted BHUP3 protein (100 ng/well) of L. donovani as a target antigen in carbonate buffer (pH 9.6) for overnight at 4°C and then the plates were blocked with 1% bovine serum albumin in 1×PBS for 2 h at room temperature to prevent nonspecific binding. Sera (1:100 dilution) of different sets were added and incubated at 25°C for 1 h. The serum antibody titers were measured with horseradish peroxidase–conjugated goat anti-human IgG (1:8000) secondary antibody, using trimethylene benzidine (Promega) as a substrate. The reaction was stopped by addition of 1 N H2SO4, and OD was measured at 450 nm by an enzyme-linked immunosorbent assay (ELISA) plate reader (Spectromax 190; Molecular Device). The absorbance was expressed as mean±standard deviations in all groups. The cutoff was calculated as mean±2 standard deviations of nonendemic healthy control.
Two-dimensional gel electrophoresis
Isoelectric focusing was done in immobilized pH gradient gel strips (IPG strips; Bio-Rad) with pH range of 3–10. Five micrograms of eluted BHUP3 protein was applied in 125 μL of rehydration buffer per IPG strip. The sample containing rehydration buffer was loaded overnight at room temperature by gel reswelling under mineral oil to prevent oxidation of protein and drying of the gel strip. The loaded IPG strip was connected with the electrode of protein isoelectric focusing cell (Bio-Rad), with the electric parameters set as follows: 20 min, 100 V and 50 μA; 30 min, 250 V and 50 μA; 2 h, 4000 V; and 3 h, 10,000 V. The IPG strip was then equilibrated in equilibrium buffer and then run for second dimension on the resolving gel of SDS-PAGE. The gel was stained using a highly sensitive silver staining kit (Pierce® Silver stain kit; Thermo Scientific) according to the manufacturer's instructions.
Mass spectrometry
The protein spot after silver staining was excised and subjected to protein sequencing analysis by matrix-assisted laser desorption/ionization-time-of-flight (MALDI-TOF) at the Molecular Biophysics Unit of Indian Institute of Science, Bangalore.
Statistical analysis
Data analysis was done through SPSS 16.0 software. The comparative evaluation was done using a nonparametric t-test. The peak lists of the mass spectra were used for peptide mass fingerprint (PMF) analysis with the Mascot software (Matrix Science; www.matrixscience.com/search_form_select.html) together with NCBI sequence database. Protein was identified using the following parameters: database: eukaryote (eukaryotes); enzyme: trypsin; variable modification: oxidation (M); fixed modification: Carbamidomethyl (C); mass value: monoisotopic; protein mass: unrestricted; peptide mass tolerance:±100 ppm; peptide charge state: +1; maximum missed cleavages: 1. Analyses of the post source decay (PSD) datasets were done using PMF with Mascot.
Results
Western blotting
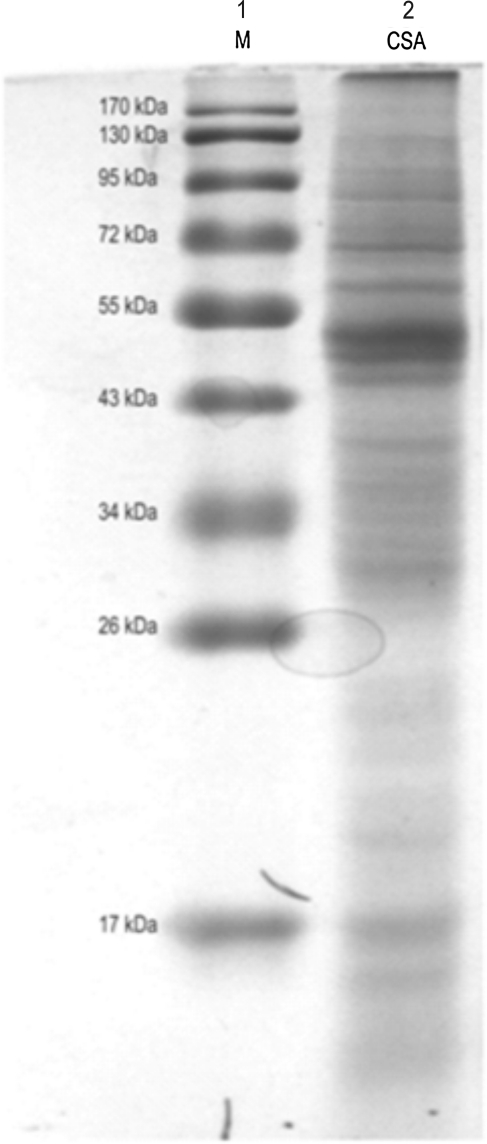
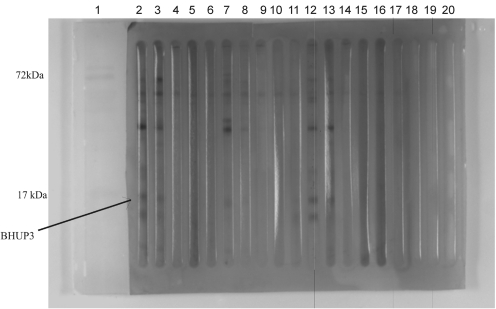
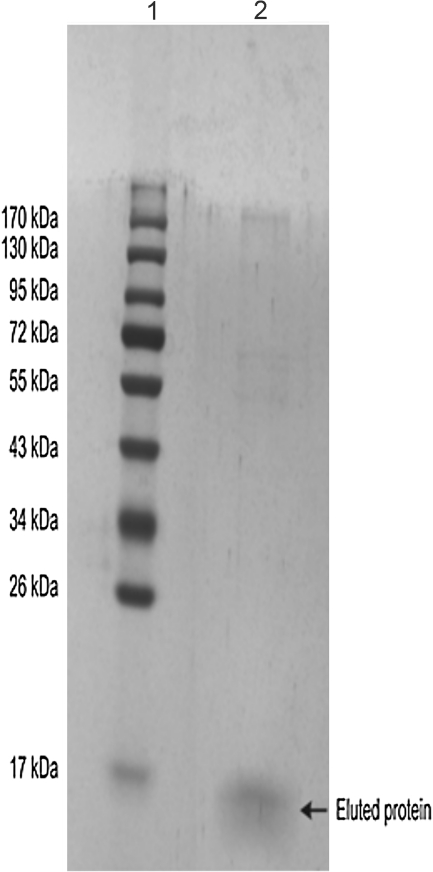
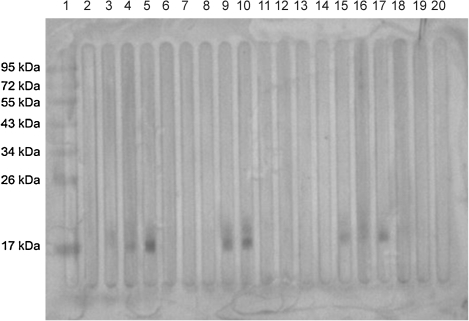
A number of L. donovani-specific protein bands of different molecular weights were observed after separation on a 12% SDS-PAGE gel and staining the gel with Coomassie Brilliant Blue (Fig. 1; Subodh et al., unpublished data). Antibody reactivity against soluble L. donovani antigen was tested with pooled sera of different groups. Western blotting result showed that the antibody against 12.6-kDa (BHUP3) protein was recognized in all pre- (9/9) and post-treated (9/9) VL patients sera, whereas no reaction was observed in endemic healthy control and nonendemic healthy control groups (Fig. 2). Only three (3/9) 6-month follow-up patients' sera were positive for the 12.6-kDa (BHUP3) protein. After identification, the 12.6-kDa protein was directly eluted from SDS-PAGE through a specific gel elution method (Fig. 3). Western blotting result with eluted protein showed the same antigenic response as with crude soluble antigen (Kelemen and Csati 1955) (Fig. 4).
FIG. 1.
Protein profile of crude soluble antigen of Leishmania donovani promastigotes separated on 12% SDS-PAGE. Lane 1, protein marker; lane 2, CSA. SDS-PAGE, sodium dodecyl sulfate–polyacrylamide gel electrophoresis.
FIG. 2.
Western blotting profile of crude soluble antigen of L. donovani promastigotes hybridized with the sera of different groups. Lane 1, protein marker; lane 2, 7, 12 denotes day O sera; 3, 8, 13, discharge sera; 4, 9, 14 6 months follow up; 5, 10, 15 endemic healthy control; 6, 11, 16 non-endemic healthy control. Arrow indicates BHUP3 protein, which was recognized by the sera of pre- and post-treated VL patients but not with our control groups (EHC and NEHC). EHC, endemic healthy control; NEHC, nonendemic healthy control; VL, visceral leishmaniasis.
FIG. 3.
Eluted protein shows as a single band when stained with sensitive silver stain. Lane 1, protein marker; lane 2, eluted protein.
FIG. 4.
Western blotting result of eluted BHUP3 protein. The eluted protein reacts with all the pre- and post-treated VL (lanes 3, 4, 9, 10, 15, and 16) patients' sera. Of three, this eluted protein reacted with only two 6-month (lanes 5 and 17) follow-up patients' sera. None of the sera of endemic controls (lanes 6, 12, and 18), nonendemic controls (lanes 7, 13, and 19), and different diseases (lanes 8, 14, and 20) reacted with the eluted protein.
ELISA
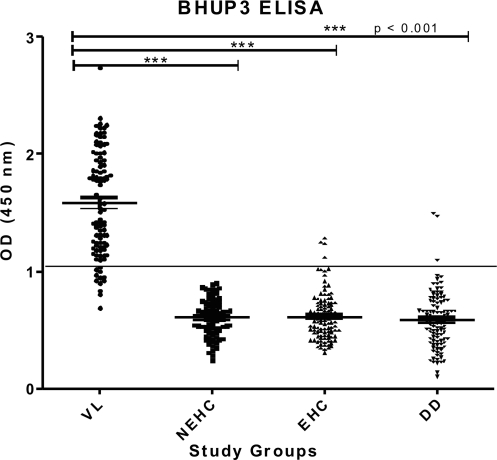
Western blotting result showed the antigenic property of 12.6-kDa BHUP3 protein, which was further validated by a sensitive ELISA method. We have validated our ELISA for 100 parasitologically confirmed VL patients, 93 NEHC, 110 EHC, and 110 disease control. ELISA sensitivity was found to be 88% (89/101), whereas the specificities in terms of NEHC, EHC, and disease control were found to be 100% (93/93), 96% (106/110), and 97% (107/110), respectively (Table 1, Fig. 5). The diagnostic accuracy of BHUP2 protein was evaluated by calculating the receiver operating characteristic value, which was 0.99.
Table 1.
Sensitivity and Specificity of BHUP3 Enzyme-Linked Immunosorbent Assay Results with Different Study Groups
| |
ELISA reactivity of BHUP3 |
|
|---|---|---|
| Subjects | Positive n (%; 95% CI) | Negative n (%; 95% CI) |
| Nonendemic healthy control, n=93 | — | 93 (100; 96.1–100) |
| Confirmed VL, n=101 | 89 (88; 80.1–93.7) | — |
| Endemic healthy control n=110 | — | 106 (96; 90.9–99) |
| Different diseases (malaria, tuberculosis, etc.), n=110 | — | 107 (97; 92.2–99.4) |
CI, confidence interval; ELISA, enzyme-linked immunosorbent assay; VL, visceral leishmaniasis.
FIG. 5.
ELISA reactivity of BHUP3 protein with different groups' sera. Full line represents the cutoff value. Each dot represents individual subject. ELISA, enzyme-linked immunosorbent assay. ***p<0.001.
Two-dimensional gel electrophoresis and MALDI-TOF characterization
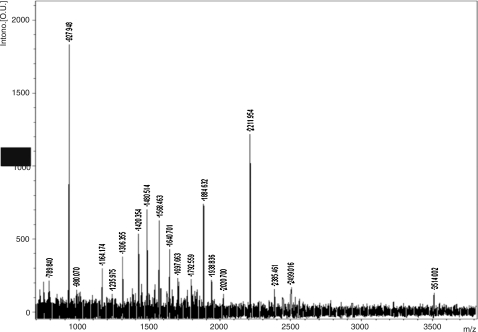
BHUP3 protein was subjected to two-dimensional (2D) gel electrophoresis for further characterization, wherein the eluted protein was separated according to their pI (isoelectric point) and molecular weight. 2D gel electrophoresis result revealed a single protein spot. The recognized protein spot was excised from 2D gel for MALDI-TOF characterization. The peak lists of the MALDI-TOF were used for PMF analysis with the Mascot software of the Matrix Science together with NCBI sequence database (Fig. 6). The protein sequence obtained from mass spectrometry analysis was recognized as an L. donovani-specific anamorsin protein of 12.6 kDa (Fig. 7), which we designated as BHUP3 protein.
FIG. 6.
MALDI-TOF spectra of the eluted BHUP3 protein. The protein spot in the silver-stained gel that had been assigned to antigen spot in the two-dimensional gel was excised, distained, and incubated with trypsin. The resulting fragment was analyzed by MALDI-TOF-MS. MALDI-TOF-MS, matrix-assisted laser desorption/ionization-time-of-flight analysis-mass spectrometry.
FIG. 7.
Protein sequence of the identified BHUP3 protein. NCBI Blast search result suggests that it is a 113-amino-acid-long putative uncharacterized protein of 12.6-kDa anamorsin homolog matched completely with Leishmania major (GenBank accession number: Q4QIS1).
Discussion
Through a series of experiments, we identified a 12.6-kDa L. donovani promastigote antigen, which was found to be an anamorsin-like protein matched with Leishmania major (GenBank accession number: Q4QIS1) by MALDI-TOF characterization and PMF analysis with Blast search in the NCBI protein sequence database. The literature suggests that it is a cytoplasmic protein having antiapoptotic effect in the cell. The ELISA result for the 12.6-kDa antigenic protein has slightly lower sensitivity (88%) but excellent specificity (95%). The healthy subjects living in endemic area have been so far never targeted for treatment, as their role in disease transmission dynamics is poorly understood. The healthy individuals of endemic area usually outnumber the number of clinical cases, with ratios of 10:1 in India and Nepal (Ostyn et al. 2009). The identified antigen shows only 4% false-positivity with healthy subjects living in endemic area, which is comparatively very less than any other existing diagnostic marker. It shows only 3% cross-reactivity with those suffering from diseases other than kala-azar, such as malaria and tuberculosis mostly. These experiments with the 12.6-kDa protein were done with partially purified antigen eluted from SDS-PAGE gel; a recombinant antigen devoid of impurities might provide better results. As the antibody response against the antigen persists in a significant proportion of patients, it does not have a prognostic value and cannot be used to detect relapses; however, high specificity is its highlight.
There are several molecular tools, such as polymerase chain reaction and polymerase chain reaction–restriction fragment length polymorphism, for the identification of L. donovani. In case of L. donovani, a particular restriction fragment length polymorphism pattern of cysteine protease B gene acts as a molecular marker for the identification and discrimination of L. donovani complex (Hide and Banuls 2006, Oshaghi et al. 2009).
To summarize, the result of this study showed that crude antigen gave encouraging results with ELISA in terms of antibody reactivity. However, the main limitation of crude antigens is their cross-reactivity with other diseases, which often occur in VL endemic areas. Our protein shows high specificity, which is one of the highest of the entire existing antigens used in the diagnosis of leishmaniasis.
Acknowledgments
The authors thank all the staff of Kala Azar Medical Research Centre, a unit of Sitaram Memorial Trust, for their assistance in collection of samples used in the experiments. This work was partially supported by the National Institute of Allergy and Infectious Disease, National Institutes of Health (DMID funding mechanism: Tropical Medicine Research Center Grant Number: 1P50AI074321). Subodh Kumar and Dinesh kumar thank Council of Scientific and Industrial Research, New Delhi, India, for providing fellowship.
Disclosure Statement
No competing financial interests exist. This article was not presented in any conference or meeting.
References
- Bhattacharya SK. Sur D. Sinha PK. Karbwang J. Elimination of leishmaniasis (kala-azar) from the Indian subcontinent is technically feasible & operationally achievable. Indian J Med Res. 2006;123:195–196. [PubMed] [Google Scholar]
- Bora D. Epidemiology of visceral leishmaniasis in India. Natl Med J India. 1999;12:62–68. [PubMed] [Google Scholar]
- Burns JM., Jr. Shreffler WG. Benson DR. Ghalib HW. Badaro R. Reed SG. Molecular characterization of a kinesin-related antigen of Leishmania chagasi that detects specific antibody in African and American visceral leishmaniasis. Proc Natl Acad Sci USA. 1993;90:775–779. doi: 10.1073/pnas.90.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhry A. Guru PY. Saxena RP. Tandon A. Saxena KC. Enzyme-linked immunosorbent assay in the diagnosis of kala-azar in Bhadohi (Varanasi), India. Trans R Soc Trop Med Hyg. 1990;84:363–366. doi: 10.1016/0035-9203(90)90319-a. [DOI] [PubMed] [Google Scholar]
- Hommel M. Peters W. Ranque J. Quilici M. Lanotte G. The micro-ELISA technique in the serodiagnosis of visceral leishmaniasis. Ann Trop Med Parasitol. 1978;72:213–218. doi: 10.1080/00034983.1978.11719308. [DOI] [PubMed] [Google Scholar]
- Hide M. Banuls AL. Species-specific PCR assay for L. infantum/L. donovani discrimination. Acta Trop. 2006;100:241–245. doi: 10.1016/j.actatropica.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Kelemen E. Csati M. Case of kala-azar in an adult in szeged; recovery following splenectomy. Magy Belorv Arch. 1955;8:129–131. [PubMed] [Google Scholar]
- Laemmli VK. Cleavage of structural protein during the assembly of the head of baceriophase T4. Nature. 1970;227:682–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Oshaghi MA. Ravasan NM. Hide M. Javadian EA. Rassi Y. Sedaghat MM. Mohebali M. Hajjaran H. Development of species-specific PCR and PCR-restriction fragment length polymorphism assays for L.infantum/L.donovani discrimination. Exp Parasitol. 2009;122:61–65. doi: 10.1016/j.exppara.2009.01.015. [DOI] [PubMed] [Google Scholar]
- Ostyn B. Gidwani K. Picado A. Chappuis F. Khanal B. Singh S. Rijal S. Sundar S. Boelaert M. Incidence of asymptomatic infection with L. donovani and their evolution in high endemic villages in India and Nepal. Trop Med Int Health. 2009;14:60–61. Abstract No. T4P6-03. [Google Scholar]
- Smith P. Krohn RI. Hermenson GT. Mallia AK. Gartner FH. Provenzano MD. Fujimoto EK. Goeke NM. Olson BJ. Klenk DC. Measurement of protein using bicinchoninic acid. Anal BioChem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Sundar S. Reed SG. Singh VP. Kumar PC. Murray HW. Rapid accurate field diagnosis of Indian visceral leishmaniasis. Lancet. 1998;351:563–565. doi: 10.1016/S0140-6736(97)04350-X. [DOI] [PubMed] [Google Scholar]
- Towbin H. Staehelin T. Gordon J. Electrophoretic transfer of polyacrylamide gels to nitrocellulose sheet: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]