Abstract
Background:
Adenosine and related purines have established roles in inflammation, and elevated airway concentrations are predicted in patients with COPD. However, accurate airway surface purine measurements can be confounded by stimulation of purine release during collection of typical respiratory samples.
Methods:
Airway samples were collected noninvasively as exhaled breath condensate (EBC) from 36 healthy nonsmokers (NS group), 28 healthy smokers (S group), and 89 subjects with COPD (29 with GOLD [Global Initiative for Chronic Obstructive Lung Disease] stage II, 29 with GOLD stage III, and 31 with GOLD stage IV) and analyzed with mass spectrometry for adenosine, adenosine monophosphate (AMP), and phenylalanine, plus urea as a dilution marker. Variable dilution of airway secretions in EBC was controlled using ratios to urea, and airway surface concentrations were calculated using EBC to serum urea-based dilution factors.
Results:
EBC adenosine to urea ratios were similar in NS (0.20 ± 0.21) and S (0.22 ± 0.20) groups but elevated in those with COPD (0.32 ± 0.30, P < .01 vs NS). Adenosine to urea ratios were highest in the most severely affected cohort (GOLD IV, 0.35 ± 0.34, P < .01 vs NS) and negatively correlated with FEV1 (r = −0.27, P < .01). Elevated AMP to urea ratios were also observed in the COPD group (0.58 ± 0.97 COPD, 0.29 ± 0.35 NS, P < .02), but phenylalanine to urea ratios were similar in all groups. Airway surface adenosine concentrations calculated in a subset of subjects were 3.2 ± 2.7 μM in those with COPD (n = 28) relative to 1.7 ± 1.5 μM in the NS group (n = 16, P < .05).
Conclusions:
Airway purines are present on airway surfaces at physiologically significant concentrations, are elevated in COPD, and correlate with markers of COPD severity. Purinergic signaling pathways are potential therapeutic targets in COPD, and EBC purines are potential noninvasive biomarkers.
Purinergic signaling pathways regulate airways defense mechanisms, including mucociliary clearance and inflammatory responses. These pathways are activated by extracellular purines, which include adenosine triphosphate (ATP) and its metabolites adenosine diphosphate, adenosine monophosphate (AMP), and adenosine. Adenosine, in particular, has been linked to inflammatory airways diseases,1 including twofold to fourfold elevations in blood2,3 and BAL fluid4 of subjects with asthma. Similarly, adenosine concentrations in exhaled breath condensate (EBC) are elevated in subjects with stable asthma,5,6 increased with asthma exacerbations,7 and decreased with successful treatment. Findings from animal models, particularly the adenosine deaminase knockout mouse,8,9 further support a link between airway inflammation and lung adenosine.
These data suggest that purinergic signaling pathways may be active in inflammatory lung diseases such as COPD and may be targets for therapeutic intervention.10 Indeed, elevated airway adenosine concentrations in COPD have been inferred from studies of human airway adenosine receptors,11 and several reviews have touted potential benefits of pharmacologic blockade of adenosine receptors.10,12 Although these benefits are a reasonable extrapolation from the animal8,13 and human studies,4,5 a recent investigation suggested that airway adenosine concentrations (as measured in sputum) may actually be lower in COPD.14 However, adenosine concentrations in respiratory samples such as sputum or BAL can be altered unpredictably by extracellular metabolism during processing or by mechanical and osmotic forces that trigger purine release during collection.
To assess airway purine concentrations noninvasively in COPD, we applied a previously reported mass spectrometric method6,15 to measure adenosine, AMP, and phenylalanine, plus urea as a dilution marker, in EBC collected from participants in two multicenter observational studies. Variable dilution of airway secretions within EBC was controlled by assessing biomarkers as ratios to urea or using serum to EBC urea-based dilution factors.16 Data were analyzed relative to GOLD (Global Initiative for Chronic Obstructive Lung Disease) stage17 and lung function (FEV1 % predicted) to provide insights into the role of purinergic signaling in COPD disease progression.
Materials and Methods
Study Subjects
Subjects were participants in one of two observational studies: RES19044 (A Multi-center Cohort Study to Evaluate Radiological, Physiological and Biochemical Biomarkers in Patients with Chronic Obstructive Pulmonary Disease and Age and Gender Matched Controls)18 or ECLIPSE (Evaluation of COPD Longitudinally to Identify Predictive Surrogate End-points) (clinicaltrials.gov; Identifier: NCT00292552).19 RES19044 was approved by Institutional Review Boards at St. Elizabeth’s Medical Center (00195) and the University of Pennsylvania (801096), and details for ECLIPSE are as previously published.19 Subjects were diagnosed based on GOLD criteria (using postbronchodilator FEV1 and FVC) and assigned to one of five cohorts at screening: healthy nonsmokers (NS group), healthy current or former smokers (S group), or one of three COPD cohorts (GOLD stages II, III, or IV). Patients with known respiratory disorders other than COPD were excluded.
Study Design
In both studies, EBC was collected by having subjects exhale through an RTube (Respiratory Research, Inc; Charlottesville, Virginia) using tidal breathing for 10 min, with the chiller held at −70°C until immediately prior to collection. Smokers refrained from smoking ≥ 3 h prior to collection. Samples were stored at −80°C and shipped on dry ice to the University of North Carolina at Chapel Hill for analysis. Samples for RES19044 were collected from 2005 to 2006 and were analyzed in late 2007. Samples for ECLIPSE were collected in 2006 and were analyzed in mid-2009. Demographic characteristics of the subjects in whom EBC biomarkers were successfully measured arein Table 1. Pulmonary function tests were performed as previously described.19 In RES19044, BUN was measured in serum obtained on the day of EBC collection. The samples from ECLIPSE represented a subset of that larger study chosen such that the subject demographics matched those of RES19044.
Table 1.
—Subject Demographics
| Control Subjects |
Subjects With COPD |
||||
| Demographic | NS | S | II | III | IV |
| No. | 36 | 28 | 29 | 29 | 31 |
| Age, y | 60.9 ± 9.4 | 62.5 ± 9.1 | 60.8 ± 8.2 | 61 ± 7.5 | 62 ± 7.6 |
| Gender, male (female) | 18 (18) | 19 (9) | 14 (15) | 18 (11) | 16 (15) |
| FEV1, % predicted | 108.9 ± 12.6 | 107.9 ± 12.9 | 61.9 ± 10.5a | 39.8 ± 8.5a | 24.1 ± 4.1a |
| ICS, % | 3 | 0.0 | 69 | 97 | 81 |
| BUNb | 16.4 ± 6.5 | 18.8 ± 12.4 | 16.1 ± 4.1 | 16.8 ± 5.6 | 17.4 ± 8.6 |
II = GOLD stage II; III = GOLD stage III; IV = GOLD stage IV; GOLD = Global Initiative for Chronic Obstructive Lung Disease; ICS = inhaled corticosteroids; NS = healthy nonsmoker; S = healthy smoker.
P < .01 vs all other cohorts.
BUN was assessed in a subset of subjects (16 NS, nine S, 10 GOLD II, seven GOLD III, 11 GOLD IV).
Mass Spectrometric Analysis
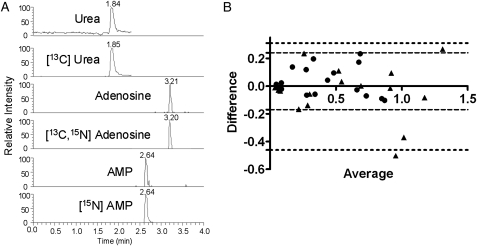
Sixty-one samples from RES19044 and 102 from ECLIPSE were analyzed using a previously published method6,15 (Fig 1A) modified to account for variable volumes. As previously described,15 500 μL of EBC (total volume if < 500 μL) plus a 1:20 ratio of internal standard solution (50 nM [13C, 15N] adenosine, 250 nM [15N] AMP, and 50 μM [13C] plus isotopically labeled amino acids [NSK-A; Cambridge Isotope Laboratories, Inc; Andover, Massachusetts]) were lyophilized, resuspended, and subjected to mass spectrometric analysis of urea, adenosine, and AMP. A second aliquot of up to 500 μL was processed as described here for replicate analysis if sufficient volume was available. Detection of phenylalanine (166→120) and isotopically labeled internal standard (172→126) was added during the course of the study. Peak areas for biomarkers and internal standards were determined via an automated processing program (Xcalibur; ThermoFinnigan; San Jose, California) and concentrations calculated from standard curves run in parallel. The average of duplicate measures was used when available.
Figure 1.
A, Chromatogram demonstrating detection of urea, adenosine, AMP, and isotopically labeled internal standards within an exhaled breath condensate (EBC) from a subject with COPD. B, Bland-Altman plot of replicate analysis of 17 samples demonstrated that most replicates fell within the 95% limits of agreement (circles and dotted lines represent adenosine to urea ratio; triangles and dashed lines represent AMP to urea ratio). AMP = adenosine monophosphate.
Ten samples (three from RES19044, seven from ECLIPSE) were excluded from further analysis: three likely contaminants (adenosine and AMP levels > 3 orders of magnitude above the mean); seven with urea concentrations below reliable quantification threshold of 1 μM as determined in a previous study.15 Samples with subthreshold urea measurements were excluded since we could not determine the extent of dilution of airway secretions within the condensate. Two samples with AMP concentrations below quantifiable limits were assigned a value of one-half of the lowest measured concentration.
Statistical Analysis
EBC and demographic data are reported as mean ± SD. Log transformation of EBC biomarkers was used to yield normal distributions by D’Agostino-Pearson test. To adjust for differences between studies, a two-way analysis of variance (ANOVA) model was used including study and cohort as factors, using the standard F-test to assess cohort differences. For significant results, pairwise differences were investigated with the Tukey-Kramer posttest adjustment. Linear contrasts were used to assess linear trends across cohorts as well as to test various group differences.
Results
Adenosine and Other Biomarkers Measured in EBC
Airway concentrations of purines and other metabolites were successfully measured in EBC from 153 subjects: 36 healthy nonsmokers (NS group), 28 healthy smokers (S group), and 89 subjects with COPD (GOLD stage II, 29; GOLD stage III, 29; and GOLD stage IV, 31). Age and gender distribution was generally similar among cohorts, whereas FEV1 was decreased significantly in COPD (Table 1). Use of inhaled corticosteroids was common in the COPD cohorts (Table 1), as was use of inhaled long-acting β agonists (73% of all subjects with COPD) and inhaled anticholinergics (67%). EBC urea correlated significantly with EBC adenosine and AMP (r = 0.39, 0.41, respectively, by Spearman, P < .001 for both), consistent with urea as a dilution marker. Within-sample reproducibility (accuracy) was assessed through replicate analyses of a subset with sufficient volume (n = 17, 11 control subjects, six subjects with COPD), with data analyzed as ratios to urea.6 Strong correlations were observed between replicate analyses (r = 0.96 for adenosine to urea, r = 0.93 for AMP to urea), with good agreement by Bland-Altman analysis (Fig 1B).
EBC Adenosine Is Elevated in COPD and Correlates With Disease Severity
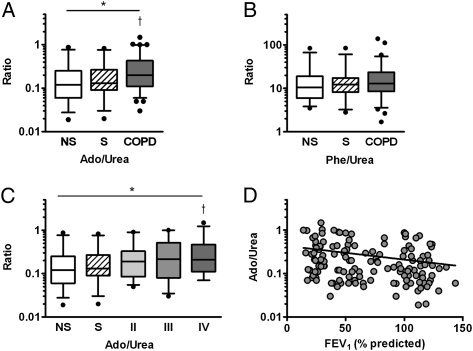
Comparing EBC adenosine to urea ratios in the NS, S, and COPD groups revealed that the ratios differed among the groups (P < .01 by ANOVA), with posttest analyses demonstrating elevations in COPD (0.32 ± 0.30) relative to NS groups (0.20 ± 0.21, P < .01) with a trend toward statistical difference from the S group (0.22 ± 0.20, P = .09) (Fig 2A). In contrast, EBC phenylalanine to urea ratios were similar among the cohorts (Fig 2B). Analysis with COPD groups stratified by GOLD stage revealed that EBC adenosine to urea ratios differed among the cohorts (P < .03 by ANOVA), with the highest ratios present in GOLD stage IV (0.35 ± 0.34, P < .02 vs NS) (Fig 2C) and an increasing linear trend between EBC adenosine to urea ratios and cohort (P < .01). Smoking status was not independently predictive of EBC adenosine to urea ratio (not shown). Consistent with these observations, a modest but statistically significant negative correlation between EBC adenosine to urea ratio and percent predicted FEV1 was observed (r = −0.27, P < .01) (Fig 2D).
Figure 2.
EBC Ado was successfully measured in 36 healthy NS subjects (NS group), 28 healthy S subjects (S group), and 89 subjects with COPD (29 GOLD II, 29 GOLD III, 31 GOLD IV) and reported as ratios of Ado (nM) to urea (μM) to control for variable dilution of airway secretions. A, Ado to urea ratios differed among the cohorts and were elevated in subjects with COPD relative to NS. B, EBC Phe was measured from EBC from 26 NS group subjects, 29 S group subjects, and 78 subjects with COPD; the Phe to urea ratio (μM Phe to μM urea) was similar among all cohorts. C, Analysis of Ado to urea ratio with subjects with COPD divided by GOLD stage; cohorts differed by analysis of variance (ANOVA) with Ado to urea ratio elevated in GOLD III and GOLD IV subjects relative to NS group subjects. D, A modest correlation (r = −0.27, P = .002) was noted between EBC Ado to urea ratio and FEV1 % predicted. *P < .05 by ANOVA, †P < .05 vs NS by linear contrast with Tukey-Kramer adjustment. Ado = adenosine; GOLD = Global Initiative for Chronic Obstructive Lung Disease; NS = nonsmoker; Phe = phenylalanine; S = smoker. See Figure 1 legend for expansion of other abbreviation.
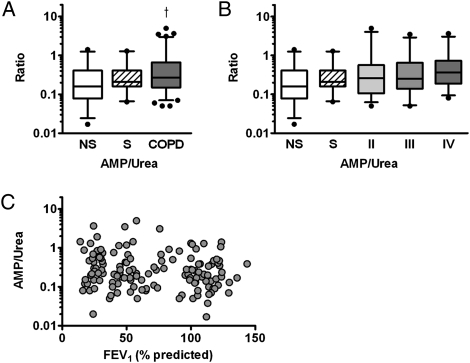
Extracellular adenosine and other purines can be derived from metabolism of extracellular ATP,20 and concentrations of ATP have paralleled its metabolite AMP in previous studies.21 Therefore, we hypothesized that extracellular AMP concentrations would be elevated if ATP was the source of elevated purines (our assay was insufficiently sensitive to measure ATP directly). Consistent with this hypothesis, we observed increased EBC AMP to urea ratios in COPD relative to NS groups (0.58 ± 0.97 COPD, 0.29 ± 0.35 NS, P < .01). With the subjects with COPD stratified by GOLD stage, EBC AMP to urea ratio differed among the cohorts and was significantly elevated in GOLD stage IV (0.61 ± 0.85 GOLD IV, P < .05 vs NS) (Fig 3A), with an increasing linear trend between EBC AMP to urea ratio and cohort (P < .02). A weak but statistically significant correlation between EBC AMP to urea ratio and FEV1 % predicted was also observed (r = −0.17, P < .05) (Fig 3C).
Figure 3.
EBC AMP was measured and assessed as ratios of AMP (nM) to urea (μM). A, A trend toward group differences in NS, S, and COPD groups was observed (P = .056 by ANOVA), and EBC AMP to urea ratio was elevated in subjects with COPD vs NS group subjects. B, Analysis of AMP to urea ratio with subjects with COPD stratified by GOLD stage. Cohorts did not differ significantly by ANOVA (P = .18), but a linear trend was noted between EBC AMP to urea ratio and cohort (r = 0.132, P < .05). C, A weak but statistically significant correlation between EBC AMP to urea ratio and percent predicted FEV1 was noted (r = −0.17, P < .05). †P < .05 vs NS by linear contrast with Tukey-Kramer adjustment. See Figure 1 and 2 legends for expansion of abbreviation.
Airway Surface Concentrations and Dilution in EBC
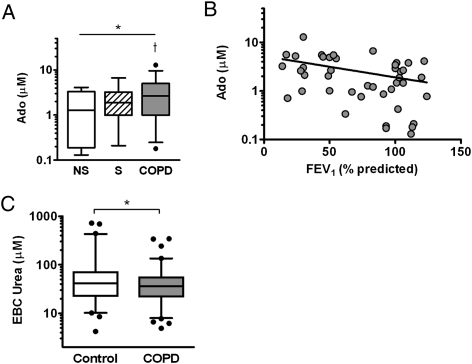
To assess airway surface concentrations of adenosine, we used a previously described method to calculate dilution factors based on ratios of urea EBC to urea in serum,16,22 measured in a subset of subjects (16 NS group subjects, nine S group subjects, 10 with GOLD stage II, seven with GOLD stage III, and 11 with GOLD stage IV). Serum urea did not vary significantly among cohorts (see Table 1). Airway surface adenosine concentrations differed among the groups and were elevated in COPD cohorts (3.2 ± 2.7 μM COPD, 1.7 ± 1.5 μM NS, P < .05) (Fig 4A) and negatively correlated to FEV1 % predicted (r = −0.39, P < .01) (Fig 4C). No statistically significant differences in corrected AMP concentrations were observed within this subset.
Figure 4.
A, Concentrations of Ado in airway surface liquid were calculated using EBC to serum urea-based dilution factors. Airway surface Ado concentrations differed among the cohorts and were higher in subjects with COPD relative to NS group subjects. *P < .05 by analysis of variance, †P < .05 vs NS. B, Airway Ado concentrations correlated with FEV1 % predicted (r = −0.39, P < .01). C, EBC urea concentrations (measured after lyophilization) were assessed as a surrogate marker of dilution available in the entire cohort. Values were highly variable but modestly lower in subjects with COPD relative to healthy control subjects. *P < .05 by Mann-Whitney. See Figure 1 and 2 legends for expansion of abbreviations.
Calculated dilution factors within this subset varied widely among the samples and were modestly but not significantly higher in COPD (median, 5,200; interquartile range, [IQR], 3,000-9,000) relative to healthy control subjects (median, 4,300; IQR, 2,000-7,500 in NS plus S groups, P = .33). To explore dilution of airway secretions in EBC in all subjects, we examined EBC urea as a surrogate measure of dilution. Interestingly, we observed a modest trend toward decreased EBC urea in subjects with COPD (P = .08 by ANOVA), which became statistically significant with both healthy control groups considered together (median, 45 μM; IQR, 25-83 μM urea healthy control subjects; median, 36 μM; IQR, 21-55 μM urea COPD; P < .05 by Mann-Whitney) (Fig 4A).
Discussion
This study demonstrated that purine concentrations on airway surfaces, as measured through analysis of EBC, are elevated in COPD and correlated with markers of disease severity. These results are consistent with previous human4,5 and animal studies9,13 that link adenosine and airway inflammation and suggest that this purine plays a pathophysiologic role in COPD.10,23,24 Although indirect evidence of elevated airway adenosine in COPD has previously been reported,11 this study represents the first direct measurement of elevated airway adenosine in COPD.
Although the observed differences in airway purine concentrations were modest, they were similar in magnitude to those reported for EBC adenosine in asthma5,7 and for other EBC biomarkers in COPD.25 Differences in adenosine may have been masked by treatment effects, since use of inhaled corticosteroids (common within our COPD cohorts) has been associated with reduced EBC adenosine.5 In addition, smoking has been shown to alter airway purines4,26 and may have made it more difficult to distinguish COPD from healthy smokers, although the strong relationship between COPD and smoking limited our ability to directly assess the impact of smoking in this study. Indeed, our findings support a model in which inflammatory responses to smoking result in increased airway purines (adenosine and AMP), which intensify as chronic inflammation develops and worsens in COPD. This mechanism is consistent with a recently published study demonstrating increased ATP in BAL fluid of smokers and subjects with COPD.26
Our findings suggest that purinergic signaling pathways play a role in the pathophysiology of COPD, differing from other reports suggesting that airway adenosine (measured in sputum)14 and ATP (measured in EBC)27 are not elevated in this disease. These contradictory findings may reflect issues associated with different methods of collecting and analyzing airway secretions. For example, the sputum derived from the larger airways28 may accumulate adenosine deaminase that lowers adenosine concentrations, whereas adenosine concentrations could remain elevated in the smaller airways from which airways secretions in EBC are generated.29 Alternatively, sputum collection and processing may alter adenosine concentrations, perhaps by osmotic or mechanically stimulated purine release or cellular activity/necrosis during sample preparation.30 Such issues may also affect purine measures in BAL.4,26
Although EBC collection does not introduce chemical gradients or expose airway surfaces to mechanical forces,16,22 we cannot exclude the possibility that patient selection, storage time, or the particular EBC collection device used in this study influenced measured concentrations. Furthermore, the very low and highly variable fraction of airway secretions within the condensate posed considerable analytic challenges. We observed a nearly two orders of magnitude variation in dilution factors, consistent with several previous studies.27,31,32 Controlling for dilution using ratios to urea or dilution factors provided a more accurate assessment of airway surface concentrations. Furthermore, decreased EBC urea in subjects with COPD suggests that EBC from these subjects may contain a smaller fraction of airway secretions than normal, although these decreases were modest and may have been difficult to detect in smaller studies.22 Overall, these findings reinforce the need to control for dilution when reporting nonvolatile biomarker concentrations in EBC and complicate interpretation of studies in which EBC dilution was not measured, including the previous analysis of EBC ATP.27 Our analyses suggest that a simple ratio to urea is sufficient to control for dilution, and our mass spectrometry method offers the simplicity of measuring urea simultaneously with several biomarkers with the potential of adding novel biomarkers.
Dilution factor measurements allowed us to estimate adenosine concentrations of ∼1 to 2 μM in healthy subjects. These values are much lower than the 60-μM concentrations reported in BAL fluid,4 although not dissimilar to values measured in cultured airway epithelia33 and our own previous studies.6 Given the likelihood that mechanical and osmotic forces may alter concentrations of purines measured in lavage or sputum,30 estimates from EBC may more accurately reflect the unperturbed airway, although potentially limited by experimental and treatment effects noted previously. Nevertheless, our measured concentrations remain above the submicromolar EC50 values of the A1, A2A, and A3 adenosine receptors found on airway epithelial and inflammatory cells.34 Although signaling via these receptors triggers complex responses, activation of the A2A receptor (and possibly the A1 receptor) generally suppresses inflammatory responses.1,10 Thus, our results suggest that airway adenosine may be antiinflammatory at the concentrations observed in the healthy airway. In contrast, higher adenosine concentrations in COPD may increase A2B receptor activation, which has a higher EC5034 and has been associated with more proinflammatory responses.1 Although these hypotheses are appealing, the relationships reflect complex relationships between various receptor subtypes and inflammatory cascades. Furthermore, localized elevations of adenosine (eg, from metabolism of AMP at cell surfaces35) could trigger signaling events without significant alteration of adenosine concentrations in airway secretions as measured by EBC or other methods.
In summary, our data indicate that airway purines are elevated in subjects with COPD and can be assessed simply and noninvasively by mass spectrometric analysis of EBC. These findings suggest that purinergic signaling pathways may be an appropriate target for therapeutic intervention. With further study to better define variability and reproducibility, evaluation of EBC purines (appropriately corrected for dilution) could serve as biomarkers of disease severity in COPD.
Acknowledgments
Author contributions: Dr Esther had full access to the data and vouches for the integrity of the manuscript.
Dr Esther: contributed to performing mass spectrometry, data analyses, and wrote and critically reviewed the manuscript.
Dr Lazaar: contributed to providing samples and critically reviewed the manuscript.
Dr Bordonali: contributed to providing biostatistical support and critically reviewed the manuscript.
Dr Qaqish: contributed to providing biostatistical support and critically reviewed the manuscript.
Dr Boucher: contributed to supervising the project and critically reviewed the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Esther received research funding from GlaxoSmithKline for this project. Dr Lazaar is employed by and holds stock in GlaxoSmithKline. Drs Bordonali, Qaqish, and Boucher have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: GlaxoSmithKline funded the clinical studies from which the samples were obtained and the mass spectrometric analyses but had no role in conduct of this study, data extraction, or interpretation of the data. The National Institutes of Health, which provided grants, had no role in the design of the study, the collection and analysis of the data, or in the preparation of the manuscript.
Other contributions: We thank Bart Celli, MD; Reynold Panettieri, MD; and Sandi VanBuren, RN, for their dedicated efforts, along with all patients and investigators who participated in RES19044 or ECLIPSE.
Abbreviations
- AMP
adenosine monophosphate
- ANOVA
analysis of variance
- EBC
exhaled breath condensate
- ECLIPSE
Evaluation of COPD Longitudinally to Identify Predictive Surrogate End-points
- GOLD
Global Initiative for Chronic Obstructive Lung Disease
- NS
nonsmoker
- S
smoker
Funding/Support: This study was supported by the National Institutes of Health [Grants 1K23HL089708, SCCOR HL34322 MTCC] and GlaxoSmithKline.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.Spicuzza L, Di Maria G, Polosa R. Adenosine in the airways: implications and applications. Eur J Pharmacol. 2006;533(1-3):77–88. doi: 10.1016/j.ejphar.2005.12.056. [DOI] [PubMed] [Google Scholar]
- 2.Mann JS, Holgate ST, Renwick AG, Cushley MJ. Airway effects of purine nucleosides and nucleotides and release with bronchial provocation in asthma. J Appl Physiol. 1986;61(5):1667–1676. doi: 10.1152/jappl.1986.61.5.1667. [DOI] [PubMed] [Google Scholar]
- 3.Vizi E, Huszár E, Csoma Z, et al. Plasma adenosine concentration increases during exercise: a possible contributing factor in exercise-induced bronchoconstriction in asthma. J Allergy Clin Immunol. 2002;109(3):446–448. doi: 10.1067/mai.2002.121955. [DOI] [PubMed] [Google Scholar]
- 4.Driver AG, Kukoly CA, Ali S, Mustafa SJ. Adenosine in bronchoalveolar lavage fluid in asthma. Am Rev Respir Dis. 1993;148(1):91–97. doi: 10.1164/ajrccm/148.1.91. [DOI] [PubMed] [Google Scholar]
- 5.Huszár E, Vass G, Vizi E, et al. Adenosine in exhaled breath condensate in healthy volunteers and in patients with asthma. Eur Respir J. 2002;20(6):1393–1398. doi: 10.1183/09031936.02.00005002. [DOI] [PubMed] [Google Scholar]
- 6.Esther CR, Jr, Boysen G, Olsen BM, et al. Mass spectrometric analysis of biomarkers and dilution markers in exhaled breath condensate reveals elevated purines in asthma and cystic fibrosis. Am J Physiol Lung Cell Mol Physiol. 2009;296(6):L987–L993. doi: 10.1152/ajplung.90512.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Csoma Z, Huszár E, Vizi E, et al. Adenosine level in exhaled breath increases during exercise-induced bronchoconstriction. Eur Respir J. 2005;25(5):873–878. doi: 10.1183/09031936.05.00110204. [DOI] [PubMed] [Google Scholar]
- 8.Chunn JL, Molina JG, Mi T, Xia Y, Kellems RE, Blackburn MR. Adenosine-dependent pulmonary fibrosis in adenosine deaminase-deficient mice. J Immunol. 2005;175(3):1937–1946. doi: 10.4049/jimmunol.175.3.1937. [DOI] [PubMed] [Google Scholar]
- 9.Chunn JL, Young HW, Banerjee SK, Colasurdo GN, Blackburn MR. Adenosine-dependent airway inflammation and hyperresponsiveness in partially adenosine deaminase-deficient mice. J Immunol. 2001;167(8):4676–4685. doi: 10.4049/jimmunol.167.8.4676. [DOI] [PubMed] [Google Scholar]
- 10.Mohsenin A, Blackburn MR. Adenosine signaling in asthma and chronic obstructive pulmonary disease. Curr Opin Pulm Med. 2006;12(1):54–59. doi: 10.1097/01.mcp.0000199002.46038.cb. [DOI] [PubMed] [Google Scholar]
- 11.Varani K, Caramori G, Vincenzi F, et al. Alteration of adenosine receptors in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;173(4):398–406. doi: 10.1164/rccm.200506-869OC. [DOI] [PubMed] [Google Scholar]
- 12.Polosa R. Adenosine-receptor subtypes: their relevance to adenosine-mediated responses in asthma and chronic obstructive pulmonary disease. Eur Respir J. 2002;20(2):488–496. doi: 10.1183/09031936.02.01132002. [DOI] [PubMed] [Google Scholar]
- 13.Blackburn MR, Volmer JB, Thrasher JL, et al. Metabolic consequences of adenosine deaminase deficiency in mice are associated with defects in alveogenesis, pulmonary inflammation, and airway obstruction. J Exp Med. 2000;192(2):159–170. doi: 10.1084/jem.192.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Versluis M, ten Hacken N, Postma D, et al. Adenosine receptors in COPD and asymptomatic smokers: effects of smoking cessation. Virchows Arch. 2009;454(3):273–281. doi: 10.1007/s00428-009-0727-9. [DOI] [PubMed] [Google Scholar]
- 15.Esther CR, Jr, Jasin HM, Collins LB, Swenberg JA, Boysen G. A mass spectrometric method to simultaneously measure a biomarker and dilution marker in exhaled breath condensate. Rapid Commun Mass Spectrom. 2008;22(5):701–705. doi: 10.1002/rcm.3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Effros RM, Biller J, Foss B, et al. A simple method for estimating respiratory solute dilution in exhaled breath condensates. Am J Respir Crit Care Med. 2003;168(12):1500–1505. doi: 10.1164/rccm.200307-920OC. [DOI] [PubMed] [Google Scholar]
- 17.Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS, GOLD Scientific Committee Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001;163(5):1256–1276. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 18.Sims MW, Tal-Singer RM, Kierstein S, et al. Chronic obstructive pulmonary disease and inhaled steroids alter surfactant protein D (SP-D) levels: a cross-sectional study. Respir Res. 2008;9:13. doi: 10.1186/1465-9921-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vestbo J, Anderson W, Coxson HO, et al. ECLIPSE investigators Evaluation of COPD Longitudinally to Identify Predictive Surrogate End-points (ECLIPSE) Eur Respir J. 2008;31(4):869–873. doi: 10.1183/09031936.00111707. [DOI] [PubMed] [Google Scholar]
- 20.Lazarowski ER, Boucher RC, Harden TK. Constitutive release of ATP and evidence for major contribution of ecto-nucleotide pyrophosphatase and nucleoside diphosphokinase to extracellular nucleotide concentrations. J Biol Chem. 2000;275(40):31061–31068. doi: 10.1074/jbc.M003255200. [DOI] [PubMed] [Google Scholar]
- 21.Esther CR, Jr, Alexis NE, Clas ML, et al. Extracellular purines are biomarkers of neutrophilic airway inflammation. Eur Respir J. 2008;31(5):949–956. doi: 10.1183/09031936.00089807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Effros RM, Peterson B, Casaburi R, et al. Epithelial lining fluid solute concentrations in chronic obstructive lung disease patients and normal subjects. J Appl Physiol. 2005;99(4):1286–1292. doi: 10.1152/japplphysiol.00362.2005. [DOI] [PubMed] [Google Scholar]
- 23.Vass G, Horváth I. Adenosine and adenosine receptors in the pathomechanism and treatment of respiratory diseases. Curr Med Chem. 2008;15(9):917–922. doi: 10.2174/092986708783955392. [DOI] [PubMed] [Google Scholar]
- 24.Livingston M, Heaney LG, Ennis M. Adenosine, inflammation and asthma—a review. Inflamm Res. 2004;53(5):171–178. doi: 10.1007/s00011-004-1248-2. [DOI] [PubMed] [Google Scholar]
- 25.Borrill ZL, Roy K, Singh D. Exhaled breath condensate biomarkers in COPD. Eur Respir J. 2008;32(2):472–486. doi: 10.1183/09031936.00116107. [DOI] [PubMed] [Google Scholar]
- 26.Lommatzsch M, Cicko S, Müller T, et al. Extracellular adenosine triphosphate and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;181(9):928–934. doi: 10.1164/rccm.200910-1506OC. [DOI] [PubMed] [Google Scholar]
- 27.Lázár Z, Huszár É, Kullmann T, et al. Adenosine triphosphate in exhaled breath condensate of healthy subjects and patients with chronic obstructive pulmonary disease. Inflamm Res. 2008;57(8):367–373. doi: 10.1007/s00011-008-8009-6. [DOI] [PubMed] [Google Scholar]
- 28.Alexis NE, Hu SC, Zeman K, Alter T, Bennett WD. Induced sputum derives from the central airways: confirmation using a radiolabeled aerosol bolus delivery technique. Am J Respir Crit Care Med. 2001;164(10 pt 1):1964–1970. doi: 10.1164/ajrccm.164.10.2104051. [DOI] [PubMed] [Google Scholar]
- 29.Johnson GR, Morawska L. The mechanism of breath aerosol formation. J Aerosol Med Pulm Drug Deliv. 2009;22(3):229–237. doi: 10.1089/jamp.2008.0720. [DOI] [PubMed] [Google Scholar]
- 30.Picher M, Burch LH, Boucher RC. Metabolism of P2 receptor agonists in human airways: implications for mucociliary clearance and cystic fibrosis. J Biol Chem. 2004;279(19):20234–20241. doi: 10.1074/jbc.M400305200. [DOI] [PubMed] [Google Scholar]
- 31.Effros RM, Hoagland KW, Bosbous M, et al. Dilution of respiratory solutes in exhaled condensates. Am J Respir Crit Care Med. 2002;165(5):663–669. doi: 10.1164/ajrccm.165.5.2101018. [DOI] [PubMed] [Google Scholar]
- 32.Horváth I, Hunt J, Barnes PJ, et al. ATS/ERS Task Force on Exhaled Breath Condensate Exhaled breath condensate: methodological recommendations and unresolved questions. Eur Respir J. 2005;26(3):523–548. doi: 10.1183/09031936.05.00029705. [DOI] [PubMed] [Google Scholar]
- 33.Lazarowski ER, Tarran R, Grubb BR, van Heusden CA, Okada S, Boucher RC. Nucleotide release provides a mechanism for airway surface liquid homeostasis. J Biol Chem. 2004;279(35):36855–36864. doi: 10.1074/jbc.M405367200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fredholm BB, Irenius E, Kull B, Schulte G. Comparison of the potency of adenosine as an agonist at human adenosine receptors expressed in Chinese hamster ovary cells. Biochem Pharmacol. 2001;61(4):443–448. doi: 10.1016/s0006-2952(00)00570-0. [DOI] [PubMed] [Google Scholar]
- 35.Eltzschig HK, Weissmüller T, Mager A, Eckle T. Nucleotide metabolism and cell-cell interactions. Methods Mol Biol. 2006;341:73–87. doi: 10.1385/1-59745-113-4:73. [DOI] [PubMed] [Google Scholar]