Abstract
Cough is a common symptom of diseases such as asthma and COPD and also presents as a disease in its own right. Treatment options are limited; a recent meta-analysis concluded that over-the-counter remedies are ineffective, and there is increasing concern about their use in children. Transient receptor potential cation channel, subfamily A, member 1 (TRPA1) channels are nonselective cation channels that are activated by a range of natural products (eg, allyl isothiocyanate), a multitude of environmental irritants (eg, acrolein, which is present in air pollution, vehicle exhaust, and cigarette smoke), and inflammatory mediators (eg, cyclopentenone prostaglandins). TRPA1 is primarily expressed in small-diameter, nociceptive neurons where its activation probably contributes to the perception of noxious stimuli. Inhalational exposure to irritating gases, fumes, dusts, vapors, chemicals, and endogenous mediators can lead to the development of cough. The respiratory tract is innervated by primary sensory afferent nerves, which are activated by mechanical and chemical stimuli. Recent data suggest that activation of TRPA1 on these vagal sensory afferents by these irritant substances could lead to central reflexes, including dyspnea, changes in breathing pattern, and cough, which contribute to the symptoms and pathophysiology of respiratory diseases.
Airway Inflammatory Disease and Cough
Cough is the most frequent reason for consultation with a family doctor1 or with a general or respiratory physician. Patients with chronic cough probably account for 10% to 38% of respiratory outpatient practice in the United States.2 Chronic cough, of various causes, is a common presentation to specialist respiratory clinics and is reported as a troublesome symptom by 7% of the population.3 Treatment options are limited. A recent meta-analysis concluded that over-the-counter cough remedies are ineffective,4 and there is increasing concern about the use of therapies in children.5 Despite its importance, our understanding of the mechanisms that provoke cough are poor.
Asthma and COPD are inflammatory diseases of the airway characterized by airflow limitation. A common symptom of both these diseases is chronic cough. Currently, the majority of patients with inflammatory diseases of the airway are treated with a combination of long-acting β2-agonists and corticosteroids; however, significant safety issues exist with these therapies. Although long- and short-acting β2-agonists help to provide patients with short-term relief from airflow limitation, they do little to treat the underlying pathology and many of the symptoms (including cough). Clearly these conditions represent a large unmet medical need that should be addressed urgently by the development of novel disease-modifying therapies.6,7
Transient Receptor Potential Channels
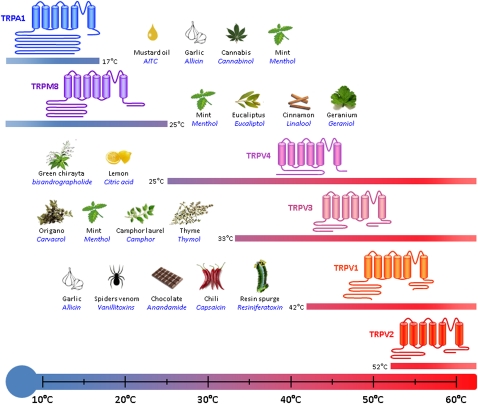
The transient receptor potential (TRP) cation channel, subfamily A, member 1 (TRPA1; formerly ANKTM1) is a Ca2+-permeant nonselective channel with 14 ankyrin repeats in its amino terminus belonging to the TRP family, which in mammals is a superfamily of at least 28 TRP channels.8,9 TRPs are cation-selective channels that exhibit varying degrees of calcium permeability and respond to a wide range of stimuli (eg, temperature, mechanical, osmolarity, chemical). A subset of TRP channels is activated by different temperatures (eg, TRPV1-4, TRPM8, and TRPA1). TRPV1 and TRPV2 are activated by heat in the noxious range (>42°C and >52°C, respectively), whereas TRPA1 is reported to be activated by noxious cold (<17°C), and TRPV3, TRPV4, and TRPM8 are activated by innocuous warm and cool stimuli.10,11 But perhaps of greater interest, in the context of this review, is that these channels are also expressed in small-diameter sensory neurons whose cell bodies are located in sensory ganglia (eg, jugular, trigeminal, dorsal root) with projections to the periphery (eg, tongue, skin, and visceral organs, including the lung).12-14
The TRPA1 gene encodes a protein that has six putative transmembrane domains with a proposed pore region between transmembrane domains five and six and with cytoplasmic N and C termini. The native, functional channel is believed to form tetramers, which operate as nonselective cation channels in mammalian cells.15 TRPA1 was first cloned from cultured human lung fibroblasts,16 but recent studies suggest that TRPA1 is highly expressed in the sensory nerve cell bodies within the trigeminal, dorsal root, vagal jugular, and vagal nodose ganglia. Both the vagal jugular and vagal nodose ganglia project TRPA1-expressing C-fibers to the airways and lungs. Interestingly, single-cell reverse transcriptase-polymerase chain reaction (PCR) analysis revealed that TRPA1 mRNA, but not TRPM8, is uniformly expressed in lung-labeled TRPV1-expressing vagal sensory neurons. Neither TRPA1 nor TRPM8 mRNA was expressed in TRPV1-negative neurons.17
Activators and Mechanism of Activation of TRP Channels
TRPA1 has been characterized as a thermoreceptor that is activated by cold temperature.8 In addition, TRPA1 channels are also activated by a wide range of chemical stimuli (Fig 1).15
Figure 1.
TRP channels act as thermosensors in sensory nerves. Transient receptor cation channels expressed in sensory neurons are activated by ambient changes. TRPA1 is activated by noxious cold from 17°C and colder temperatures. TRPM8, TRPV4, and TRPV3 are activated by warmer temperatures, with a similar threshold of 25°C for TRPM8 (which senses chilling) and TRPV4. TRPV3 is activatedy by hotter temperature (33°C threshold) creating a sensory link with TRPV1 and TRPV2, which are activated by noxious heat with respective thresholds of 42°C and 52°C. All channels can also be activated by a wide variety of agonists present in the environment. Natural stimulants are indicated on the side of each channel with their most common natural source. TRP = transient receptor potential; TRPA1 = transient receptor potential cation channel, subfamily A, member 1.
Electrophilic Agents
Many of the TRPA1 agonists described are reactive electrophiles, including a range of natural products, such as allyl isothiocyanate and allicin found in mustard oil and garlic.17-19 The channel is also activated by a multitude of environmental irritants, such as ozone, isothiocyanate, cinnamaldehyde, acrolein, and nicotine, many of which are present in air pollution, vehicle exhaust, and cigarette smoke.20-30 However, in addition to the exogenous electrophilic agents, endogenous electrophilic agents have been found to activate TRPA1, including some α,β-unsaturated aldehydes, cyclopentenone prostaglandin metabolites, and products of nitrative stress, such as 4-hydroxy-2-nonenal and nitro-oleic acid.24,27 Point mutation studies have identified that electrophile-mediated activation of the TRPA1 channel occurs via the covalent modification of three cysteine residues and one lysine residue found on the intracellular N-terminal ankyrin repeat sequence.
Nonelectrophilic Agents
TRPA1 can also be activated by a range of nonelectrophilic agonists, including certain anesthetics (eg, isoflurane), menthol, Δ9-tetrahydrocannabinol, nonsteroidal antiinflammatories (eg, flufenamic, niflumic and mefenamic acid, diclofenac, flurbiprofen, indomethacin).31 It is believed that these agents activate TRPA1 via an alternative mechanism, given that they are not believed to be reactive enough to form covalent bonds.
Indirect Activating Agents
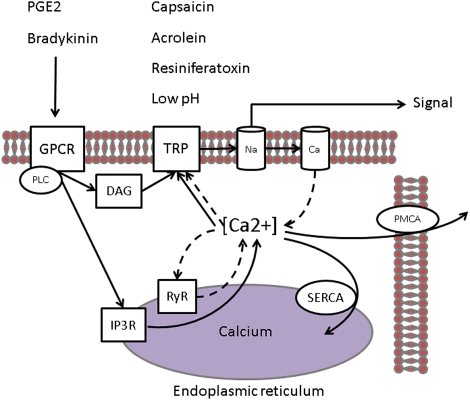
TRPA1 can be activated by elevation in intracellular calcium in the local environment, which suggests that it can be activated via the activation of other TRP channels in the locality or that calcium influx by TRPA1 itself increases intracellular calcium leading to a positive feedback loop.32,33 In fact, Ca2+ is a key regulator of TRPA1 activity, both potentiating and subsequently inactivating it.34 G protein-coupled receptor agonists, such as prostaglandin E2 or bradykinin, can also activate TRPV1 and TRPA1 channels via hydrolysis of phosphatidylinositol 4,5-bisphosphate and by production of diacylglycerol by the associated phospholipase C. The inositol 1,4,5-triphosphate produced in parallel will release calcium from intracellular stores by binding to the inositol 1,4,5-triphosphate receptor located on the endoplasmic reticulum membrane. In addition, as TRP channels activate, the resulting inward currents depolarizes the membrane, triggering the opening of low-threshold voltage-gated sodium channels, which will further depolarize the membrane triggering the opening of voltage-gated calcium channels. Calcium influx through voltage-gated calcium channels and the ryanodine receptor will induce elevation of intracellular calcium ([Ca2+]i). This significant [Ca2+]i elevation could be another mechanism regulating the activity of TRP channels, thereby inhibiting TRPV1 and activating TRPA1.33,35 This cycled regulation of [Ca2+]i by the membrane potential and of membrane potential by [Ca2+]i is modulated by the capacity of the cell to clamp intracellular level of calcium, mainly via endoplasmic calcium ATPase and plasma membrane calcium ATPase (Fig 2).
Figure 2.
Calcium flux is associated with TRPV1 and TRPA1 activation in the cell. TRPV1 and TRPA1 produce a depolarizing inward current, mainly calcium, when opened by the binding of their respective agonists, such as capsaicin (V1), acrolein (A1), resiniferatoxin (V1), or H+ protons (V1). GPCR agonists, such as PGE2 or bradykinin, can also activate TRPV1 and TRPA1 channels via hydrolysis of phosphatidylinositol 4,5-bisphosphate and by production of diacylglycerol by the associated PLC. The inositol 1,4,5-triphosphate produced in parallel will release calcium from intracellular stores by binding to the inositol IP3R located on the endoplasmic reticulum membrane. In addition, as TRP channels activate, the resulting inward current depolarizes the membrane, triggering the opening of low-threshold voltage-gated sodium channels, which will further depolarize the membrane triggering the opening of voltage-gated calcium channels (VGCC). Calcium influx through VGCC and the RyR will induce elevation of intracellular calcium ([Ca2+]i). This significant [Ca2+]i elevation could be another mechanism regulating the activity of TRP channels, thereby inhibiting TRPV1 and activating TRPA1.33,35 This cycled regulation of [Ca2+]i by the membrane potential (Vm) and of Vm by [Ca2+]i is modulated by the capacity of the cell to clamp intracellular level of calcium, mainly via SERCA and PMCA. Ca = voltage-gated calcium channel; DAG = diacylglycerol; GPCR = G protein-coupled receptor; IP3R =1,4,5-triphosphate receptor; Na = voltage-gated sodium channels; PGE2 = prostaglandin E2; PLC = phospholipase C; PMCA = plasma membrane calcium ATPase; RyR = ryanodine receptor; SERCA = endoplasmic reticulum Ca2+-ATPase. See Figure 1 legend for expansion of other abbreviations.
Antagonists
Despite the increased interest in TRPA1 as a therapeutic target, there have been few TRPA1 inhibitors reported in the patent literature.36 However, although the development of antagonists has been slow, the number of identified agonists is continuing to increase.37 Fortunately tool compounds that block this channel have existed for several years. One of these is the nonselective cation channel blocker ruthenium red, which, although it is described to be a potent blocker, is not selective and blocks several of the TRP channels, including TRPV1. Identification of toxicity issues has prevented the development of this compound.36
Interestingly, (±) camphor and related compounds have also been reported to be weak TRPA1 antagonists. However, the high doses needed to block TRPA1 in vivo, interaction with other TRP channels (eg, TRPV3), and toxicity issues preclude these being either useful tools or development compounds.15 AP-18 ([Z]-4-[4-clorophynyl]-3-methylbut-3-en-2-oxime) is a more selective, competitive TRPA1 antagonist, which is believed to act via the displacement of ligands such as cinnamaldehyde from its binding site.38 Interestingly, this compound has been used in vivo, and the data generated have contributed to the evidence presented on the role of TRPA1 in the development of mechanical and cold hypersensitivity characteristic of inflammatory processes.38 Unfortunately, not much is known about the in vivo oral bioavailability, pharmacokinetic profile, and selectivity of this compound, but some companies (eg, Abbott Laboratories) have used this as a starting point and used this pharmacophore to inform the development of their own molecules. In fact, Abbott laboratories have disclosed information on one of their lead compounds, (1E, 3E)-1-(4-fluorophenyl)-2-methyl-pent-1,-3-one oxime, which is a potent compound, with good oral bioavailability and anti-hyperalgesic activity in an in vivo model of osteoarthritis.36 However, it is not clear whether this compound is progressing to clinical trials.
More recently, HC-030031 has been developed by Hydra Biosciences and is currently commercially available. This compound has demonstrated in vivo activity in a range of models23 and has contributed to the wealth of data generated in the last 2 years on the role of TRPA1 in hyperalgesia and in certain models of inflammatory pain.39
TRPA1 and Cough
Recently, ion channels of the TRP class have been implicated in the afferent sensory loop of the cough reflex40-42 and in the heightened cough sensitivity seen in disease.43 Agonists of the TRPV1 capsaicin receptor, such as vanilloids and protons, are among the most effective chemical stimuli that cause cough in awake animals/humans.40-42 It has long been established that TRPV1 receptor activation can elicit a cough response in both animal models and in humans.40-42 This receptor is polymodal and activated, by vanilloids (eg, capsaicin), noxious heat (≥ 42°C), extracellular protons (pH ≤ 5.9), endogenous lipids (eg, anandamide), and eicosanoids (eg, leukotriene B4, 12-[S]-HPETE, 15-[S]-HPETE). However, it does not respond to many irritants known to initiate cough. The mechanism whereby a range of seemingly diverse irritants initiate acute and chronic cough has remained a mystery until an alternative “cough receptor,” which responds to known environmental irritants, was discovered.
Another TRP receptor, which is not activated by capsaicin, the TRPA1 channel, has been shown to bind ligands such as acrolein, which is present in air pollution and the acrid smoke from organic material.20 A number of studies have demonstrated that stimulation of TRPA1 channels activates the vagal bronchopulmonary C-fibers in guinea pig and rodent lungs.14,35,44 In addition, acrolein and cinnamaldehyde have been shown to activate guinea pig isolated vagus nerve with the selective TRPA1 antagonists, AP-18 and HC-030031, able to cause a concentration-dependent inhibition of acrolein-induced nerve activation. In addition, AP-18 was able to inhibited acrolein-induced depolarization of isolated human vagus nerve.45 Indeed, recent data from our laboratory have shown that the TRPA1-selective agonists acrolein and cinnamaldehyde induce coughing in guinea pigs and human volunteers, respectively.45 This finding has been confirmed by Andrè and colleagues,46 who stimulated coughing in guinea pigs with aerosolized TRPA1-selective agonists and subsequently demonstrated inhibition of these responses with both selective and nonselective TRPA1 blockers. In addition, cigarette smoke-induced cough in guinea pigs can also be partially (∼50%) inhibited by HC-030031.46 It may be that the tussive response remaining may be mediated by activation of neuronal nicotinic acetylcholine receptors, as a role for these receptors has been identified in cigarette smoke-induced cough in normal volunteers.47 That TRPA1 has been identified as a pro-tussive receptor in both clinical trials and a guinea pig model, and that this effect can be blocked by selective antagonists, are extremely significant findings. This opens up a whole new field of research for potential antitussive remedies that could help to alleviate cough, not only associated with the common cold and seasonal flu, but more importantly for those who suffer from excessive coughing associated with chronic inflammatory diseases.
TRPA1, Sensory Hyperresponsiveness, Asthma, and COPD
In addition to directly activating a cough reflex, TRPA1 ligands may also be involved in the hypersensitization of cough and other sensory reflexes. In healthy individuals, stimulation of sensory nerves may be protective; however, in individuals with airway disease, the heightened response to sensory irritants may exacerbate disease and have a deleterious health impact. For example, individuals with allergic asthma are more sensitive to pollutants than healthy individuals.48,49 Neither the mechanisms through which irritants stimulate sensory nerves nor the mechanisms responsible for the heightened irritant responsiveness in airway disease are known, but upregulation of TRP channel activity is a likely suspect. In fact, chronic inflammation in a mouse asthma model was shown to lead to changes in transcriptional patterns of TRP channels, inducing expression of TRPV1 in myelinated airway fibers.50 Consistent with this observation were data demonstrating an increase in TRPV1 expression in nociceptive-like primary afferent neurons in patients with chronic cough compared with normal volunteers43 and the observation of TRPV1 expression at nonneuronal sites in patients with chronic cough.51 More recently, the association of a functional single-nucleotide polymorphism, TRPV1-I585V, with childhood asthma has been identified. The loss of function of TRPV1 genetic variant is associated with a lower risk of active childhood asthma.52
Exposures to high levels of TRPA1 agonists (eg, chlorine and aldehydes) often induces reactive airways dysfunction syndrome (RADS).53-55 RADS is characterized by asthma-like symptoms, including cough.53 The broad chemical sensitivity of TRPA1 may explain the sensitivity observed in patients with RADS to multiple agents. An initial chemical sensory challenge and tissue injury may sensitize TRPA1 channels through inflammatory signaling pathways, thereby establishing long-lasting hypersensitivity to multiple reactive chemicals.13,18,20,56 Patients with RADS are only partially responsive to current asthma therapies. Interestingly, exposure to the environmental toxins ozone and toluene diisocyanate,29,30 which are now known to activate TRPA1, have been reported to evoke symptoms such as cough, dyspnea, and wheezing.57,58 Therefore, TRPA1 antagonists may be useful for inhibiting the increased chemosensory responses accompanying these conditions.
In addition to the role in sensory nerve hyperresponsiveness, TRPA1 has also been implicated in the symptoms and pathophysiology of asthma. Sensitization and subsequent airways challenge of rodents with ovalbumin (OVA) is a commonly used experimental model system to recapitulate characteristic features of allergic asthma, including lung tissue inflammation, increased eosinophils, and elevated levels of T helper (Th) 2-derived cytokines in BAL fluid, increased mucus in the airways, airways hyperreactivity, and elevated serum IgE. In some models (eg, Brown Norway rat model of allergic inflammation) early asthmatic response and late asthmatic response (LAR) can also be observed.59 In models of OVA-induced airway inflammation, HC-030031 has been shown to inhibit eosinophilia and airway hyperresponsiveness.60 The role of TRPA1 in this model was then confirmed using TRPA1−/− mice.60 In order to assess TRPA1 expression and the possible contribution of immune cells to the allergic mouse phenotype, Taqman quantitative PCR was used to compare TRPA1 transcript levels in cDNA derived from spleen containing a large variety of leukocyte precursors, Th2 lymphocytes, whole mouse lung and BAL fluid leukocytes of OVA-challenged mice, and dorsal root ganglia. Relative transcript quantities in spleen, Th2 cells, whole lung, and leukocytes were minimal, with dorsal root ganglia expression several hundred-fold higher. Additional quantitative PCR experiments using cDNA prepared from primary leukocytes and leukocyte cell lines failed to detect the presence of TRPA1 cDNA. These results point to a key role for sensory neuronal TRPA1 channels in allergic airway inflammation. Taken together these data suggest that TRPA1 has an important role in allergen-induced airways inflammation, mucus production, and airways hyperreactivity in an animal model that recapitulates characteristic features of allergic asthma.
In another study conducted by Raemdonck et al,61 HC-030031 significantly attenuated the LAR measured by whole-body plethysmography in an OVA-sensitized and -challenged rat model. A marked inhibitory effect on the LAR was also observed with the muscarinic receptor antagonist tiotropium. One could therefore hypothesize that the TRPA1 blocker may be inhibiting sensory nerve activation and as a consequence the generation of central reflex events and activation of parasympathetic nerves. In summary, these data suggest that TRPA1 blockers may have a therapeutic benefit in the treatment of allergic asthma.
COPD is characterized by airflow obstruction, which may be accompanied by airways inflammation, mucus hypersecretion, and cough. The most important risk factor in the development of COPD is cigarette smoking, and oxidative stress is believed to play an important role in the lung tissue damage observed in the airways of patients with COPD. In addition, cigarette smoke extract (CSE) or aldehydes increased Ca2+ influx in TRPA1-transfected cells, but not in control HEK293 cells, and promoted neuropeptide release from isolated guinea pig airway tissue. Furthermore, using isolated guinea pig bronchial rings, Andrè et al25 demonstrated that CSE as well as acrolein or crotonaldehyde produced a contraction of bronchial rings that was inhibited by HC-030031, but not by the TRPV1 antagonist capsazepine or reactive oxygen scavengers. Lastly, instillation of CSE into the trachea of wild-type mice and TRPA1−/− mice only induced plasma protein extravasation in the wild-type mice. These data suggest that targeting TRPA1 may have therapeutic potential in diseases caused by cigarette smoke, such as COPD.
Acetaminophen (paracetamol, N-acetyl-p-aminophenol [APAP]) is one of the most popular analgesic/antipyretic medicines worldwide. In overdose, APAP causes severe liver damage via a toxic metabolite, N-acetyl-p-benzo-quinoneimine, but it is safe at therapeutic doses. However, in the past 10 years a wide series of epidemiologic studies has shown that exposure to therapeutic doses of APAP is one of the risk factors for asthma and COPD.62 Interestingly, N-acetyl-p-benzo-quinoneimine, like other electrophilic molecules, is a TRPA1 activator, which can evoke neurogenic inflammation through the release of neuropeptides from sensory nerve endings. These TRPA1-mediated events may contribute to the increased risk of asthma and COPD associated with the therapeutic use of paracetamol.
Therapeutic Implications
An emerging role for TRPA1 has become apparent in the inflammatory response in animal models of allergic airways inflammation60,61 and in the cough reflex.45,46 It is still unclear whether there is cooperation between TRPV1 and TRPA1 channels. Both are activated, for example, by tussive agents, and so it could be possible that these TRP channels act in concert to elicit functional responses. The dependence of TRPA1 on Ca2+ may result in the activation of TRPA1 channels by an overflow of Ca2+ in the locale of other activated channels (eg, TRPV1) without ever being modified by a reactive ligand. Furthermore, TRPA1 channels may also act as an amplifier of other Ca2+ mobilizing pathways.63 However, whether this sort of cooperation exists in generating effects such as the cough reflex has yet to be determined. It is clear that blockade of both TRPA1 and TRPV1 can inhibit or partially inhibit responses to a range of tussive agents.64 As such, it is difficult to predict whether a TRPA1 antagonist would be more effective than a TRPV1 in heightened sensory nerve responsive disease states or whether a combination drug would be better. In fact, pharmaceutical companies may choose to initiate drug discovery programs to develop dual inhibitors based on the fact that both channels may well play a role in respiratory disease and cough. Unfortunately, the hyperthermic effects of TRPV1 antagonists reveal that TRPV1 is tonically active in thermoregulatory pathways. This role of TRPV1 has been identified as a potential confounding factor in the clinical development of drugs of this class. At this stage, it remains unclear if hyperthermia can be separated from efficacy in pain, although this path is being actively pursued.65 Information to date would suggest that TRPA1 has a more restricted expression profile, which may make for a more optimal safety profile, although this remains to be seen with the increased testing of TRPA1 antagonists in both preclinical and clinical studies.
Conclusions
In conclusion, the identification of a role for TRPA1 in airway inflammatory responses and in the cough reflex has opened up a new area of research. This novel and exciting finding could have major implications for understanding the pathogenesis of respiratory diseases and for the treatment of cough, which presents as a significant unmet medical need. Because of their central role and activation by a wide range of irritant and chemical substances, either by exogenous agents, endogenously produced mediators during inflammation, or oxidant stress, we suggest TRPA1 channels should be considered as one of the most promising targets currently identified for the development of novel antitussive drugs and possibly (if early reports are substantiated) a broader antiinflammatory role in airway disease.
Acknowledgments
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsors had no role in the design of the study, the collection and analysis of the data, or in the preparation of the manuscript.
Abbreviations
- APAP
N-acetyl-p-aminophenol
- CSE
smoke extract
- OVA
ovalbumin
- PCR
polymerase chain reaction
- RADS
reactive airways dysfunction syndrome
- Th
T helper
- TRP
transient receptor potential
- TRPA1
transient receptor potential cation channel, subfamily A, member 1
Footnotes
Funding/Support: Dr Burrell was funded by a grant from the Medical Research Council, England [Grant G0800196], and Dr Dubuis by a project grant from the Wellcome Trust [Grant 089301/Z/09/Z].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.McCormick A, Fleming DM, Charlton J. London, England: HMSO; 1995. Office of Population Censuses and Surveys. Morbidity Statistics from General Practice, Fourth National Study 1991-1992. Series MB5 no 3. [Google Scholar]
- 2.Irwin RS, Corrao WM, Pratter MR. Chronic persistent cough in the adult: the spectrum and frequency of causes and successful outcome of specific therapy. Am Rev Respir Dis. 1981;123(4 pt 1):413–417. doi: 10.1164/arrd.1981.123.4.413. [DOI] [PubMed] [Google Scholar]
- 3.Ford AC, Forman D, Moayyedi P, Morice AH. Cough in the community: a cross sectional survey and the relationship to gastrointestinal symptoms. Thorax. 2006;61(11):975–979. doi: 10.1136/thx.2006.060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schroeder K, Fahey T. Systematic review of randomised controlled trials of over the counter cough medicines for acute cough in adults. BMJ. 2002;324(7333):329–331. doi: 10.1136/bmj.324.7333.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith SM, Henman M, Schroeder K, Fahey T. Over-the-counter cough medicines in children: neither safe or efficacious? Br J Gen Pract. 2008;58(556):757–758. doi: 10.3399/bjgp08X342642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization Asthma. Fact Sheet No. 307. World Health Organization Web site. http://www.who.int/mediacentre/factsheets/fs307/en/index.htmlAccessed May 2011.
- 7.World Health Organization Chronic Obstructive Pulmonary Disease (COPD). Fact Sheet No. 315. World Health Organization Web site. http://www.who.int/mediacentre/factsheets/fs315/en/index.htmlAccessed February 2011.
- 8.Clapham DE. TRP channels as cellular sensors. Nature. 2003;426(6966):517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- 9.Patapoutian A, Tate S, Woolf CJ. Transient receptor potential channels: targeting pain at the source. Nat Rev Drug Discov. 2009;8(1):55–68. doi: 10.1038/nrd2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999;398(6726):436–441. doi: 10.1038/18906. [DOI] [PubMed] [Google Scholar]
- 11.McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416(6876):52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- 12.Anand U, Otto WR, Facer P, et al. TRPA1 receptor localisation in the human peripheral nervous system and functional studies in cultured human and rat sensory neurons. Neurosci Lett. 2008;438(2):221–227. doi: 10.1016/j.neulet.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 13.Story GM, Peier AM, Reeve AJ, et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112(6):819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 14.Nassenstein C, Kwong K, Taylor-Clark T, et al. Expression and function of the ion channel TRPA1 in vagal afferent nerves innervating mouse lungs. J Physiol. 2008;586(6):1595–1604. doi: 10.1113/jphysiol.2007.148379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rech JC, Eckert WA, Maher MP, Banke T, Bhattacharya A, Wickenden AD. Recent advances in the biology and medicinal chemistry of TRPA1. Future Med Chem. 2010;2(5):843–858. doi: 10.4155/fmc.10.29. [DOI] [PubMed] [Google Scholar]
- 16.Jaquemar D, Schenker T, Trueb B. An ankyrin-like protein with transmembrane domains is specifically lost after oncogenic transformation of human fibroblasts. J Biol Chem. 1999;274(11):7325–7333. doi: 10.1074/jbc.274.11.7325. [DOI] [PubMed] [Google Scholar]
- 17.Jordt SE, Bautista DM, Chuang HH, et al. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427(6971):260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- 18.Bandell M, Story GM, Hwang SW, et al. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41(6):849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- 19.Macpherson LJ, Geierstanger BH, Viswanath V, et al. The pungency of garlic: activation of TRPA1 and TRPV1 in response to allicin. Curr Biol. 2005;15(10):929–934. doi: 10.1016/j.cub.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 20.Bautista DM, Jordt SE, Nikai T, et al. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124(6):1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 21.Facchinetti F, Amadei F, Geppetti P, et al. Alpha,beta-unsaturated aldehydes in cigarette smoke release inflammatory mediators from human macrophages. Am J Respir Cell Mol Biol. 2007;37(5):617–623. doi: 10.1165/rcmb.2007-0130OC. [DOI] [PubMed] [Google Scholar]
- 22.Macpherson LJ, Dubin AE, Evans MJ, et al. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature. 2007;445(7127):541–545. doi: 10.1038/nature05544. [DOI] [PubMed] [Google Scholar]
- 23.McNamara CR, Mandel-Brehm J, Bautista DM, et al. TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci U S A. 2007;104(33):13525–13530. doi: 10.1073/pnas.0705924104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trevisani M, Siemens J, Materazzi S, et al. 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc Natl Acad Sci U S A. 2007;104(33):13519–13524. doi: 10.1073/pnas.0705923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andrè E, Campi B, Materazzi S, et al. Cigarette smoke-induced neurogenic inflammation is mediated by alpha,beta-unsaturated aldehydes and the TRPA1 receptor in rodents. J Clin Invest. 2008;118(7):2574–2582. doi: 10.1172/JCI34886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brône B, Peeters PJ, Marrannes R, et al. Tear gasses CN, CR, and CS are potent activators of the human TRPA1 receptor. Toxicol Appl Pharmacol. 2008;231(2):150–156. doi: 10.1016/j.taap.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 27.Andersson DA, Gentry C, Moss S, Bevan S. Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. J Neurosci. 2008;28(10):2485–2494. doi: 10.1523/JNEUROSCI.5369-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Talavera K, Gees M, Karashima Y, et al. Nicotine activates the chemosensory cation channel TRPA1. Nat Neurosci. 2009;12(10):1293–1299. doi: 10.1038/nn.2379. [DOI] [PubMed] [Google Scholar]
- 29.Taylor-Clark TE, Kiros F, Carr MJ, McAlexander MA. Transient receptor potential ankyrin 1 mediates toluene diisocyanate-evoked respiratory irritation. Am J Respir Cell Mol Biol. 2009;40(6):756–762. doi: 10.1165/rcmb.2008-0292OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor-Clark TE, Undem BJ. Ozone activates airway nerves via the selective stimulation of TRPA1 ion channels. J Physiol. 2010;588(pt 3):423–433. doi: 10.1113/jphysiol.2009.183301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu H, Tian J, Zhu Y, et al. Activation of TRPA1 channels by fenamate nonsteroidal anti-inflammatory drugs. Pflugers Arch. 2010;459(4):579–592. doi: 10.1007/s00424-009-0749-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doerner JF, Gisselmann G, Hatt H, Wetzel CH. Transient receptor potential channel A1 is directly gated by calcium ions. J Biol Chem. 2007;282(18):13180–13189. doi: 10.1074/jbc.M607849200. [DOI] [PubMed] [Google Scholar]
- 33.Zurborg S, Yurgionas B, Jira JA, Caspani O, Heppenstall PA. Direct activation of the ion channel TRPA1 by Ca2+ Nat Neurosci. 2007;10(3):277–279. doi: 10.1038/nn1843. [DOI] [PubMed] [Google Scholar]
- 34.Wang YY, Chang RB, Waters HN, McKemy DD, Liman ER. The nociceptor ion channel TRPA1 is potentiated and inactivated by permeating calcium ions. J. Biol Chem. 2008;283(47):32691–32703. doi: 10.1074/jbc.M803568200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vyklický L, Nováková-Tousová K, Benedikt J, Samad A, Touska F, Vlachová V. Calcium-dependent desensitization of vanilloid receptor TRPV1: a mechanism possibly involved in analgesia induced by topical application of capsaicin. Physiol Res. 2008;57(suppl 3):S59–S68. doi: 10.33549/physiolres.931478. [DOI] [PubMed] [Google Scholar]
- 36.Viana F, Ferrer-Montiel A. TRPA1 modulators in preclinical development. Expert Opin Ther Pat. 2009;19(12):1787–1799. doi: 10.1517/13543770903393771. [DOI] [PubMed] [Google Scholar]
- 37.Bessac BF, Jordt SE. Breathtaking TRP channels: TRPA1 and TRPV1 in airway chemosensation and reflex control. Physiology (Bethesda) 2008;23:360–370. doi: 10.1152/physiol.00026.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petrus M, Peier AM, Bandell M, et al. A role of TRPA1 in mechanical hyperalgesia is revealed by pharmacological inhibition. Mol Pain. 2007;3:40. doi: 10.1186/1744-8069-3-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eid SR, Crown ED, Moore EL, et al. HC-030031, a TRPA1 selective antagonist, attenuates inflammatory- and neuropathy-induced mechanical hypersensitivity. Mol Pain. 2008;4:48. doi: 10.1186/1744-8069-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laude EA, Higgins KS, Morice AH. A comparative study of the effects of citric acid, capsaicin and resiniferatoxin on the cough challenge in guinea-pig and man. Pulm Pharmacol. 1993;6(3):171–175. doi: 10.1006/pulp.1993.1023. [DOI] [PubMed] [Google Scholar]
- 41.Lalloo UG, Fox AJ, Belvisi MG, Chung KF, Barnes PJ. Capsazepine inhibits cough induced by capsaicin and citric acid but not by hypertonic saline in guinea pigs. J Appl Physiol. 1995;79(4):1082–1087. doi: 10.1152/jappl.1995.79.4.1082. [DOI] [PubMed] [Google Scholar]
- 42.Trevisani M, Milan A, Gatti R, et al. Antitussive activity of iodo-resiniferatoxin in guinea pigs. Thorax. 2004;59(9):769–772. doi: 10.1136/thx.2003.012930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Groneberg DA, Niimi A, Dinh QT, et al. Increased expression of transient receptor potential vanilloid-1 in airway nerves of chronic cough. Am J Respir Crit Care Med. 2004;170(12):1276–1280. doi: 10.1164/rccm.200402-174OC. [DOI] [PubMed] [Google Scholar]
- 44.Taylor-Clark TE, McAlexander MA, Nassenstein C, et al. Relative contributions of TRPA1 and TRPV1 channels in the activation of vagal bronchopulmonary C-fibres by the endogenous autacoid 4-oxononenal. J Physiol. 2008;586(14):3447–3459. doi: 10.1113/jphysiol.2008.153585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Birrell MA, Belvisi MG, Grace M, et al. TRPA1 agonists evoke coughing in guinea pig and human volunteers. Am J Respir Crit Care Med. 2009;180(11):1042–1047. doi: 10.1164/rccm.200905-0665OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Andrè E, Gatti R, Trevisani M, et al. Transient receptor potential ankyrin receptor 1 is a novel target for pro-tussive agents. Br J Pharmacol. 2009;158(6):1621–1628. doi: 10.1111/j.1476-5381.2009.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee LY, Burki NK, Gerhardstein DC, Gu Q, Kou YR, Xu J. Airway irritation and cough evoked by inhaled cigarette smoke: role of neuronal nicotinic acetylcholine receptors. Pulm Pharmacol Ther. 2007;20(4):355–364. doi: 10.1016/j.pupt.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 48.Thurston GD, Bates DV. Air pollution as an underappreciated cause of asthma symptoms. JAMA. 2003;290(14):1915–1917. doi: 10.1001/jama.290.14.1915. [DOI] [PubMed] [Google Scholar]
- 49.Leikauf GD. Hazardous air pollutants and asthma. Environ Health Perspect. 2002;110(suppl 4):505–526. doi: 10.1289/ehp.02110s4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang G, Lin RL, Wiggers M, Snow DM, Lee LY. Altered expression of TRPV1 and sensitivity to capsaicin in pulmonary myelinated afferents following chronic airway inflammation in the rat. J Physiol. 2008;586(pt 23):5771–5786. doi: 10.1113/jphysiol.2008.161042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mitchell JE, Campbell AP, New NE, et al. Expression and characterization of the intracellular vanilloid receptor (TRPV1) in bronchi from patients with chronic cough. Exp Lung Res. 2005;31(3):295–306. doi: 10.1080/01902140590918803. [DOI] [PubMed] [Google Scholar]
- 52.Cantero-Recasens G, Gonzalez JR, Fandos C, et al. Loss of function of transient receptor potential vanilloid 1 (TRPV1) genetic variant is associated with lower risk of active childhood asthma. J Biol Chem. 2010;285(36):27532–27535. doi: 10.1074/jbc.C110.159491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brooks SM, Weiss MA, Bernstein IL. Reactive airways dysfunction syndrome (RADS). Persistent asthma syndrome after high level irritant exposures. Chest. 1985;88(3):376–384. doi: 10.1378/chest.88.3.376. [DOI] [PubMed] [Google Scholar]
- 54.Prezant DJ, Levin S, Kelly KJ, Aldrich TK. Upper and lower respiratory diseases after occupational and environmental disasters. Mt Sinai J Med. 2008;75(2):89–100. doi: 10.1002/msj.20028. [DOI] [PubMed] [Google Scholar]
- 55.Shakeri MS, Dick FD, Ayres JG. Which agents cause reactive airways dysfunction syndrome (RADS)? A systematic review. Occup Med (Lond) 2008;58(3):205–211. doi: 10.1093/occmed/kqn013. [DOI] [PubMed] [Google Scholar]
- 56.Dai Y, Wang S, Tominaga M, et al. Sensitization of TRPA1 by PAR2 contributes to the sensation of inflammatory pain. J Clin Invest. 2007;117(7):1979–1987. doi: 10.1172/JCI30951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Coffey MJ, Wheeler CS, Gross KB, Eschenbacher WL, Sporn PH, Peters-Golden M. Increased 5-lipoxygenase metabolism in the lungs of human subjects exposed to ozone. Toxicology. 1996;114(3):187–197. doi: 10.1016/s0300-483x(96)03487-7. [DOI] [PubMed] [Google Scholar]
- 58.Moller DR, McKay RT, Bernstein IL, Brooks SM. Persistent airways disease caused by toluene diisocyanate. Am Rev Respir Dis. 1986;134(1):175–176. doi: 10.1164/arrd.1986.134.1.175. [DOI] [PubMed] [Google Scholar]
- 59.Birrell MA, Hardaker E, Wong S, et al. Ikappa-B kinase-2 inhibitor blocks inflammation in human airway smooth muscle and a rat model of asthma. Am J Respir Crit Care Med. 2005;172(8):962–971. doi: 10.1164/rccm.200412-1647OC. [DOI] [PubMed] [Google Scholar]
- 60.Caceres AI, Brackmann M, Elia MD, et al. A sensory neuronal ion channel essential for airway inflammation and hyperreactivity in asthma. Proc Natl Acad Sci U S A. 2009;106(22):9099–9104. doi: 10.1073/pnas.0900591106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Raemdonck K, De Alba J, Birrell MA, Belvisi MG. A role for airway sensory nerves in the allergen induced late asthmatic response in a rat model of allergic asthma. Am J Respir Crit Care Med. 2010;181:A5542. [Google Scholar]
- 62.Nassini R, Materazzi S, Andrè E, et al. Acetaminophen, via its reactive metabolite N-acetyl-p-benzo-quinoneimine and transient receptor potential ankyrin-1 stimulation, causes neurogenic inflammation in the airways and other tissues in rodents. FASEB J. 2010;24(12):4904–4916. doi: 10.1096/fj.10-162438. [DOI] [PubMed] [Google Scholar]
- 63.Cavanaugh EJ, Simkin D, Kim D. Activation of transient receptor potential A1 channels by mustard oil, tetrahydrocannabinol and Ca2+ reveals different functional channel states. Neuroscience. 2008;154(4):1467–1476. doi: 10.1016/j.neuroscience.2008.04.048. [DOI] [PubMed] [Google Scholar]
- 64.Maher SA, Grace MS, Birrell MA, Belvisi MG. Prostaglandin E2-induced sensory nerve activation is mediated by TRPA1 and TRPV1. Am J Respir Crit Care Med. 2010;181:A5541. [Google Scholar]
- 65.Lehto SG, Tamir R, Deng H, et al. Antihyperalgesic effects of (R,E)-N-(2-hydroxy-2,3-dihydro-1H-inden-4-yl)-3-(2-(piperidin-1-yl)-4-(trifluoromethyl)phenyl)-acrylamide (AMG8562), a novel transient receptor potential vanilloid type 1 modulator that does not cause hyperthermia in rats. J Pharmacol Exp Ther. 2008;326(1):218–229. doi: 10.1124/jpet.107.132233. [DOI] [PubMed] [Google Scholar]